Abstract
Introduction
Peripheral artery disease (PAD) is the third most prevalent cardiovascular disease worldwide, with smoking and diabetes being the strongest risk factors. The most prominent symptom is leg pain while walking, known as intermittent claudication. To improve mobility, first-line treatment for intermittent claudication is supervised exercise programmes, but these remain largely unavailable and economically impractical, which has led to the development of structured home-based exercise programmes. This trial aims to determine the effectiveness and cost advantage of TeGeCoach, a 12-month long home-based exercise programme, compared with usual care of PAD. It is hypothesised that TeGeCoach improves walking impairment and lowers the need of health care resources that are spent on patients with PAD.
Methods and analysis
The investigators conduct a prospective, pragmatic randomised controlled clinical trial in a health insurance setting. 1760 patients diagnosed with PAD at Fontaine stage II are randomly assigned to either TeGeCoach or care-as-usual. TeGeCoach consists of telemonitored intermittent walking exercise with medical supervision by a physician and telephone health coaching. Participants allocated to the usual care group receive information leaflets and can access supervised exercise programmes, physical therapy and a variety of programmes for promoting a healthy lifestyle. The primary outcome is patient reported walking ability based on the Walking Impairment Questionnaire. Secondary outcome measures include quality of life, health literacy and health behaviour. Claims data are used to collect total health care costs, healthcare resource use and (severe) adverse events. Outcomes are measured at baseline, 12 and 24 months.
Ethics and dissemination
Ethical approval has been obtained from the Medical Association Hamburg. Findings are disseminated through peer-reviewed journals, reports to the funding body, conference presentations and media press releases. Data from this trial are made available to the public and researchers upon reasonable request.
NCT03496948 (www.clinicaltrials.gov), Pre-results.
Keywords: health economics, health services administration & management, clinical trials, VASCULAR MEDICINE, peripheral artery disease
Strengths and limitations of this study.
To our knowledge, this pragmatic clinical trial conducted in a health insurance-based setting is the first to investigate the clinical effectiveness of a structured home-based exercise programme in patients with peripheral artery disease.
Collecting data on healthcare costs and utilisation allows a comprehensive economic evaluation of a structured home-based exercise programme in patients with peripheral artery disease.
An initial 1 hour baseline assessment based on remote activity monitoring data determines the current walking impairment in order to create a training plan tailored to varying fitness levels; however, the validity of this assessment method has not been established yet.
Since a potentially high attrition may endanger the internal validity of the results, a linear mixed model without any ad hoc imputation is used in order to deal adequately with missing values arising from study dropouts.
The heterogeneity of intervention delivery given the pragmatic trial approach ensures the generalisability of the results applicable to the real-world clinical practice, which yet may lead to a dilution of the treatment effect.
Introduction
Peripheral artery disease (PAD) is the third most prevalent atherosclerotic cardiovascular disease with over 200 million people affected worldwide and has become one of the leading causes of disability and death.1 2 It is characterised by the progressive narrowing of the peripheral arteries resulting in the reduction of blood supply, eventually leading to functional impairment and mobility loss.3 If not intervened sufficiently early, the atherosclerotic processes can lead to ulcer formation and gangrenous necrosis (ie, critical limb ischaemia),4 and may affect other vascular beds with potentially fatal consequences.5 6 PAD is markedly more prevalent in the elderly population, estimating that 5.4% and 18.6% of individuals aged from 45 to 49 and 85 to 89 years are affected, respectively.1 2 The amount of people with PAD has risen rapidly in recent years, with a sharp increase by nearly 25% between 2000 and 2010 in the general population.2 Likewise, in Germany, the amount of PAD-related hospitalisations increased by 20.7% between 2005 and 2009, from 400 928 to 483 961. Meanwhile, hospital reimbursement costs for the treatment of PAD have grown nationwide from €2.14 billion in 2007 to €2.6 billion in 2009, a 21% increase within 2 years.7 Major risk factors are tobacco smoking and diabetes, followed by high cholesterol, hypertension, history of cardiovascular disease (ie, coronary heart disease, stroke) and chronic kidney disease.1 2 8 9
The most common clinical manifestation is leg pain while walking, known as intermittent claudication (IC), which reflects impaired haemodynamics and vascular dysfunction.10 11 IC is associated with diminished mental health and lower quality of life, thus reducing symptom burden is the cornerstone of the comprehensive care for patients with PAD.12–15 Besides pharmacotherapy, risk factor management and surgical revascularisation procedures, exercise-based interventions provide substantial mental and physical health benefits for patients with IC.16–19 Accordingly, formal supervised exercise programmes (SEPs) are shown to be effective in the treatment of PAD with IC and are recommended as first-line therapy with the highest level of evidence in a variety of published clinical guidelines.20–22 SEPs involve the use of intermittent walking exercise and are minimum 3-month commitments, with at least three sessions per week (30–60 min per session) provided in a clinical setting (eg, hospital outpatient setting, outpatient facility or a physician’s office). Although SEPs commonly form part of usual care, its use is hampered by low uptake and adherence rates, possibly due to copayment requirements and lack of reimbursement, lack of available local training centres and the burden of travelling.23 24 These obstacles highlight the need for innovative models of care, which have led to the emergence of structured home-based exercise programmes (HEPs) where SEPs are not available or impractical to deliver.25 According to clinical practice guidelines, structured HEPs can serve as a useful alternative to SEPs20 22 as they improve walking impairment26 and are preferred by patients over SEPs.27 Structured HEPs are performed independently by the patient but follow an exercise regimen similar to that of SEPs, with a duration of 3–6 months. Protocols of structured HEPs show considerable variation with regard to programme duration, form of exercise, exercise frequency and duration, and intervention components used (for an overview, see Hageman et al).28 To achieve benefits, structured HEPs include psychological behaviour change techniques (eg, goal setting, barrier identification and motivational interviewing), regular follow-ups with a healthcare professional or coach (eg, face-to-face and phone), activity monitoring and feedback (eg, wearable activity trackers and logbooks), patient education or any combination thereof.28 Although inferior to SEPs,24 28–30 structured HEPs have been shown to improve performance-based,31–40 patient-reported31–38 40 and cardiorespiratory fitness39 outcomes with high adherence,31 37 whereas unstructured exercise giving merely ‘go home and walk’ advice to patients with symptomatic PAD has proven ineffective.41
Given the promising results demonstrating the efficacy of structured HEPs in previous explanatory trials, pragmatic trials (ie, with high external validity) are urgently warranted to establish the effectiveness of structured HEPs with the goal to inform clinical practice and to shape healthcare policies.42 In response to the lack of effectiveness trials, while drawing on best available evidence and experience with previous telecoaching studies,43 three German statutory health insurance funds (KKH Kaufmännische Krankenkasse, TK Techniker Krankenkasse and mhplus Krankenkasse) launch TeGeCoach, a 12-month long-structured HEP that involves telemonitored intermittent walking exercise using wearable activity trackers with medical supervision by a physician, and motivational interviewing-based telephone health coaching. TeGeCoach provides a streamlined, structured HEP approach based on current evidence using several components that have been shown to be beneficial; telephone health coaching have been shown to be a cost efficient and effective tool in the management of other chronic diseases,44–46 supporting physical activity and dietary behaviour change.47 Therefore, structured HEPs involving telephone health coaching may also offer great potential for patients with PAD, although the frequency of coaching conversations may play a critical role in whether telephone health coaching is beneficial.48 With regard to the mode of exercise, intermittent walking exercise has been proven to be effective in patients with IC, which involves repeated bouts of exercise to maximally tolerable claudication pain alternated with recovery breaks.49 Likewise, the use of activity trackers alone or as an intervention modality are considered a convenient way for facilitating physical activity50 51 with long-term health benefits,52 while remote activity monitoring (eg, by a coach) may improve walking impairment and significantly lower the costs of healthcare in PAD patients.53 Among older adults, the use of activity trackers is well accepted and may be effective to encourage physical activity,54 55 with behavioural change techniques such as social support and motivating feedback facilitating their (long-term) use.56 57 Furthermore, adding some kinds of counselling to the use of wearable activity tracker (eg, activity monitor-based counselling) could allow the health coach to deliver behaviour change techniques and to support sustained exercise. For example, using an activity tracker with regular feedback combined with access to SEPs has proven to improve functional walking performance and quality of life in PAD patients.58 Similarly, telephone health coaching combined with activity monitoring was found to increase physical activity and reduce sedentary behaviour in elderly people.59
The aim of this study is to explore the effectiveness of TeGeCoach, a structured HEP for patients with PAD. A randomised controlled trial of 1760 patients with PAD is conducted to determine whether TeGeCoach improves patient-reported walking impairment while lowering healthcare costs at 12-month and 24-month follow-up, compared with the usual care of PAD (care-as-usual, CAU). It is hypothesised that TeGeCoach improves walking impairment and lowers the costs of healthcare that are spent on patients with PAD. Given the size and remote nature of the study (ie, no personal contact to research staff), as well as the pragmatic trial approach (ie, measurement of outcomes should be patient relevant and should not interfere with the usual care),60 it was opted to use only patient-reported outcome measures (PROMs), while collecting healthcare utilisation and costs from claims data. PROMs emphasise the patient perspective by collecting information that are directly relevant to the patients; with growing interest in comparative effectiveness research, PROMs are commonly used in clinical trials to measure treatment effects.61 If effective, TeGeCoach could be widely integrated into PAD usual care with the potential to provide health benefits for patients with PAD while reducing healthcare costs.
Methods
Trial design
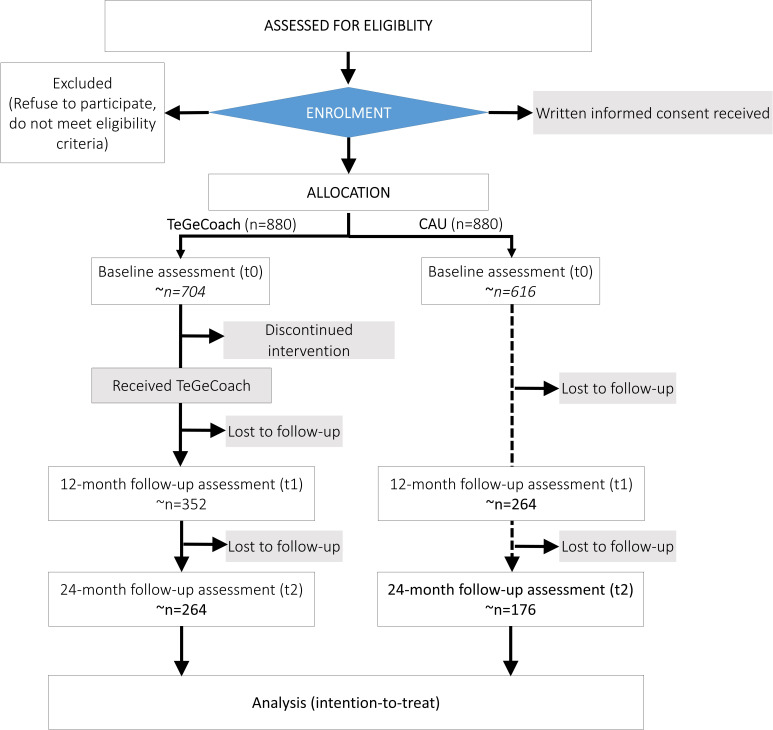
This is a two-arm, parallel-group, open-label, pragmatic, randomised, controlled superiority trial embedded within three German statutory health insurance funds (KKH Kaufmännische Krankenkasse, TK Techniker Krankenkasse and mhplus Krankenkasse). It is designed to compare the effects of TeGeCoach (intervention arm) to the CAU, conducted in a health insurance system-based setting (figure 1). Trial initiation was in 4/2018 and ends in 2/2021. The recruitment period was 9 months (4/2018 to 12/2018; table 1). The study is conducted in full compliance with Good Clinical Practice quality standards and in accordance with the Declaration of Helsinki of 2008. It is expected that final results are reported after study completion in 2021.
Figure 1.
Prospective flow chart of the study design. CAU, usual care of PAD; PAD, peripheral artery disease; TeGeCoach, telemonitored intermittent walking exercise with medical supervision by a physician and telephone health coaching.
Table 1.
Trial registration data
| Data category | Information |
| Primary registry and trial identifying number | ClinicalTrials.gov (NCT03496948) |
| Date of registration in primary registry | 23 March 2018 |
| Source(s) of monetary or material support | Innovation Fund, Federal Joint Committee (G-BA) |
| Trial sponsor | KKH Kaufmännische Krankenkasse |
| Contact for public queries | FB (frank.bienert@kkh.de), KKH Kaufmännische Krankenkasse |
| Contact for scientific queries | FR (f.rezvani@uke.de), University Medical Center Hamburg-Eppendorf |
| Public title | TeGeCoach—a home-based exercise programme using telephone health coaching with telemonitoring for patients with peripheral artery disease |
| Scientific title | Telephone health coaching with exercise monitoring using wearable activity trackers (TeGeCoach) for improving walking impairment in peripheral artery disease: study protocol for a randomised controlled trial |
| Countries of recruitment | Germany |
| Health condition(s) or problem(s) studied | Peripheral artery disease (PAD) |
| Intervention(s) | Active comparator: telemonitored intermittent walking exercise with medical supervision by a physician and telephone health coaching (TeGeCoach) |
| Active comparator: care-as-usual (CAU) | |
| Key inclusion and exclusion criteria | Ages eligible for study: ≥35 years and ≤80 years Sexes eligible for study: both Accepts healthy volunteers: no |
| Inclusion criteria: ≥35 years and ≤80 years, insured at one of the participating statutory health insurance funds, access to a telephone, primary or secondary diagnosis of PAD at Fontaine stage IIa/b within the last 36 months, no primary or secondary diagnosis of PAD at Fontaine stage I within the last 12 months, no diagnosis of Fontaine stage III/IV within the last 36 months | |
| Exclusion criteria: immobility that goes beyond claudication, inability to carry out intervention (chronic) physical conditions that interfere with the intervention, cognitive disorders, severe and persistent mental disorders, suicidality, life-threatening illnesses, active or recent participation in any other PAD intervention trial, ongoing hospitalisation, alcoholism and/or other drug dependency, heart failure graded NYHA classes III and IV | |
| Study type | Interventional |
| Allocation: randomised Intervention model: parallel assignment masking: analysis blinding | |
| Primary purpose: prevention | |
| Date of first enrolment | April 2018 |
| Target sample size | 1760 |
| Recruitment status | Completed |
| Primary outcome(s) |
PROM: walking impairment Time points: baseline, 12 and 24 months |
| Key secondary outcomes |
PROMs: generic health-related quality of life, PAD-specific quality of life, depression, generalised anxiety disorder, alcohol use, nicotine dependence, health literacy, patient activation Claims data: total healthcare costs, healthcare resource use, (severe) adverse events Time points: baseline, 12 and 24 months |
NYHA, New York Heart Association; PROM, patient-reported outcome measures.
This study protocol is reported in accordance with the CONsolidated Standards Of Reporting Trials (CONSORT) statement;62 the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement;63 the SPIRIT Patient-Reported Outcome (PRO) extension64 and the Template for Intervention Description and Replication checklist.65
Patient and public involvement statement
This research was planned without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Participants
Participants have to meet the following criteria: registered with one of the participating statutory health insurance funds (KKH Kaufmännische Krankenkasse, TK Techniker Krankenkasse and mhplus Krankenkasse); aged between 35 and 80; German-speaking; access to a telephone (landline or mobile) and a primary or secondary diagnosis of PAD at Fontaine stage IIa (> 200 m, Fontaine stage IIa) or IIb (< 200 m, Fontaine stage IIb) within the last 36 months (corresponding ICD-10-German Modification codes I70.21, I70.22 and I73.9). Participants should have no primary or secondary diagnosis of PAD at Fontaine stage I (asymptomatic) within the last 12 months, and no diagnosis of Fontaine stage III (ischaemic rest pain) or IV (ulcer, gangrene) within the last 36 months to increase diagnostic accuracy (corresponding ICD-10-German Modification codes I70.23, I70.24 and I73.25).
Exclusion criteria for participants are immobility that goes beyond claudication (Fontaine stage III or IV; inability to carry out intervention); (chronic) physical conditions that interfere with the intervention (eg, COPD); cognitive disorders (inability to carry out intervention); severe and persistent mental disorders (adherence reasons); suicidality (safety reasons); life-threatening illnesses (safety reasons); active or recent participation in any other PAD intervention trial; ongoing hospitalisation; (self-reported) alcoholism and/or other drug dependency (adherence reasons) and heart failure-graded NYHA classes III and IV (inability to carry out intervention and competing risks).
Recruitment
Participants
Recruitment of participants is managed by three statutory health insurance funds in Germany: KKH Kaufmännische Krankenkasse, TK Techniker Krankenkasse and mhplus Krankenkasse. Eligible participants are retrospectively identified using ICD-10-GM diagnosis codes from inpatient and outpatient encounters, which are routinely collected for reimbursement purposes (claims data). Due to the high number of diagnostic errors and poor coding habits in outpatient settings, exclusion criteria are only checked using inpatient diagnosis codes.
An iterative recruitment process was developed, as substantial challenges to the recruitment of clinical trials have been shown in the PAD population.66 Eligible participants are contacted by their health insurance fund to explain the purpose of the study and to confirm their PAD diagnosis by questioning them about their symptoms. Eligible participants receive a study information letter that is supplemented with consent and permission forms (ie, authorisation for release of medical reports by the contracted physician to the health coach). If interested to participate, they are asked to sign all documents and send them back to their health insurance fund. Eligible non-responders who are still interested in the study but have not given written consent are followed up by phone to be reminded of the trial. Once the written consent has been received, a query is submitted to the data warehouse of the respective health insurance fund which automatically assigns a pseudonym to the participant. No participant will be enrolled without full, written informed consent.
Physicians
Each participant allocated to TeGeCoach must be medically supervised by a physician, which is a prerequisite for receiving the TeGeCoach intervention; participants can elect their preferred physician prior to programme start, or are alternatively referred to an already contracted physician by their health coach. To encourage physicians to participate, they enter into an integrated care contract with the respective health insurance fund that provides financial incentives for the delivery of special medical services throughout the intervention.67 The enrolment and reimbursement of contracted physicians is coordinated by medicalnetworks (Kassel, Germany), a company that is specialised on the management of integrated care programmes within the §140a volume V of the German Social Security Code. If the physician of choice refuses to participate, the participant is referred to a nearby contracted physician who has already entered into the integrated care contract. Once enrolled, the health coach contacts the contracted physician to discuss their tasks during the course of the study. Due to recruitment barriers, it is possible that no suitable physician can be found for the patient by the end of the recruitment phase. For safety reasons, participants for whom no physician can be appointed do not receive TeGeCoach.
Treatment allocation and blinding
Participants are allocated in a 1:1 ratio to either the TeGeCoach or CAU group, stratified by health coaching centre using a permuted block method within each stratum. In order to prevent selection bias and to eliminate any predictability (allocation concealment), participants are randomly allocated via Sealed Envelope (London, UK), a secure internet-based randomisation service including concealment, stratification and blocking for each health coaching site.
Blinding of care providers (health coaches and contracted physicians) and participants is not possible because of obvious differences between the TeGeCoach intervention and CAU. However, as supported by the CONSORT guidelines, blinding of the analysis is achieved by engaging an independent data analyst and by withholding information about how the groups were coded before analytical decisions have been completed.68
Interventions
TeGeCoach
TeGeCoach is a 12-month long-structured HEP that is designed to inspire healthy habits in patients with PAD based on the transtheoretical model of behaviour change. The main strategies used to improve health outcomes include patient-centred motivational interviewing, shared decision making and active listening, aiming to help patients to enhance their individual motivation for exercise and receive the support needed to improve their condition.
Telemonitored intermittent walking exercise
Patients are instructed to continuously wear an activity tracker device (ie, from getting up to going to bed; not while showering, bathing and swimming). Two different brands of activity tracker are used that record the number of steps (KKH Krankenkasse and mhplus Krankenkasse: AS 95 Pulse by Beurer; TK Techniker Krankenkasse: Mi Band 2 by Xiaomi). The data from the activity tracker are transmitted automatically to the health coaching platform once per day over the internet using a SIM card modem (econnect, IEM GmbH). A 60-min baseline assessment is initially taken to evaluate the patient’s individual walking capacity whereby patients are instructed to walk at a brisk pace (defined as >50 steps/min) until maximal tolerable claudication pain is reached, followed by breaks and continued walking when the pain subsides (intermittent walking). The net brisk walking time (>50 steps/min) during the 60 min baseline assessment is used to assign patients to one of three intermittent walking plans of increasing duration; the patient is assigned to level A (15-min exercise, including breaks) if he/she is able to walk less than 15 min during the baseline assessment, level B (30-min exercise, including breaks) if he/she is able to walk 15–30 min, and level C (60-min exercise, including breaks) if he/she is able to walk 30–60 min. Patients are instructed to walk intermittently at a brisk pace (>50 steps/min) on at least 5 days per week. The assignment to one of the training levels is not conclusive; the coach regularly reviews, and if necessary, adjusts the walking plan after every coaching session. The goal is to progressively increase walking intervals and shortening breaks until painless walking exercise (or bearable pain) without breaks needed has been achieved by the patient, suggesting to switch to the next training level. In addition to exercise sessions, the health coach also sees the absolute number of steps per day as a measure of overall physical activity. To ensure patient safety, the contracted physician initially reviews the proposed exercise plan, checks if any contraindications to exercise exist and whether all important comorbidities such as high blood pressure, diabetes and coronary heart disease are sufficiently treated. Furthermore, they receive three health reports from the health coach during the course of the programme, which are important for the joint exchange of information to provide collaborative care.
Telephone health coaching
Over the course of 12 months, patients regularly receive health information leaflets and have up to nine structured 30–60 min phone calls with their health coach. During these structured phone calls, the health coach and the patient jointly discuss the progress towards exercise goals and review the activity tracker data to check whether the patient adheres to the walking plan. For this purpose, exercise sessions (ie, intermittent walking represented as changes between walking and break intervals) are visualised and automatically identified as an exercise session by the health coaching platform. Additional phone calls are warranted when no data have been received, no steps were taken or when coaches are alerted that the amount of exercise days has fallen below an individual threshold. During these calls, barriers like lack of motivation, exercise intolerance or technical issues are discussed and how they can be overcome through behavioural support. Along with the walking exercise, patient-tailored topics of interest that are relevant to the management of PAD are discussed in order to improve health literacy, to facilitate patient empowerment and to adopt a proactive stance in dealing with their disease. The health coaching curriculum includes knowledge of PAD, PAD medication, comorbidities of PAD and other related health topics (eg, tobacco use and nutrition). The health coaches use an electronic documentation system to monitor the coaching process (KKH Kaufmännische Krankenkasse and mhplus Krankenkasse: Picama Managed Care, Trustner GmbH; TK Techniker Krankenkasse: Philips GmbH Market DACH). The telephone health coaching is carried out by three health coaching centres that are located throughout Germany, each affiliated to one of the three statutory health insurance funds (Health Coaching Center of KKH Kaufmännische Krankenkasse, Telemedical Center at Robert-Bosch-Hospital on behalf of TK Techniker Krankenkasse, Health Coaching Center of mhplus Krankenkasse) and are staffed with licensed health workers (eg, nurses, physical therapists and medical assistants). To ensure high-quality health coaching, health coaches are regularly supervised by a team of experts and receive 51 hours of training, including 19 hours of programme training, 7 hours of medical training, 8 hours of group supervision and 1 hour of individual supervision. Compliance to coaching guidelines are continuously monitored and reviewed. In addition to the structured TeGeCoach intervention, participants have regular access to CAU as described below.
After 12 months, there is an additional 12 months of unstructured follow-up in which patients have no interaction with their health coach but still have access to their activity tracker device, which they may continue to use to self-monitor their physical activity.
Care as usual
Patients allocated to CAU receive usual medical care through the regular statutory healthcare system. Additionally, participants receive PAD patient information leaflets from their statutory health insurance fund, with each health insurance fund providing its own leaflets. These leaflets provide information about course offerings of the respective health insurance fund to encourage regular exercise and to promote lifestyle changes, including SEPs (vascular and cardio exercise), physical therapy, nutritional assistance programmes, smoking cessation programmes, weight loss programmes, as well as patient education programmes for obesity and diabetes. It is thereby ensured that participants allocated to CAU receive genuine usual care as supplied in everyday practice.
Outcome measures
Outcome measures are listed in table 2 along with timing of assessment; the effectiveness of TeGeCoach is measured based on PROMs, claims data and activity tracker data. PROMs are collected at baseline (t0), at 12 (t1) and 24 (t2) months.
Table 2.
Participant timeline: time schedule of enrolment (eligibility screen, informed consent, pseudonymisation and allocation), study arms (TeGeCoach or CAU) and measurements (questionnaires and claims data)
| Study period | ||||
| Enrolment | Allocation | Postallocation | ||
| Time point* | t1 | t0 | t1 | t2 |
| Enrolment | ||||
| Eligibility screening (claims data) | X | |||
| Informed consent | X | |||
| Pseudonymisation | X | |||
| Allocation | X | |||
| Study arms | ||||
| TeGeCoach (intervention) | ♦—————♦---------♦ | |||
| CAU (control) | --------------------------> | |||
| Measurements | ||||
| Intervention and control arm | ||||
| PROMs (questionnaires)† | X | X | X | |
| Cost and medical outcomes (claims data)‡ | ♦—————♦———♦ | |||
| Intervention arm only | ||||
| ZAPA questionnaire | X | |||
| Walking exercise parameters (activity tracker data)§ | ♦—————♦ | |||
*t1, ~1 month before patient in; t0, baseline; t1, 12-month follow-up; t2, 24-month follow-up.
†WIQ, EQ5D-5L, SF-12, VascuQoL-25, PHQ-9, GHD-7, AUDIT-C, FTND, HLQ, PAM-13.
‡Healthcare costs, healthcare resource use (severe) adverse events.
§Exercise adherence, amount of steps/net walking time (>50 steps/min) per day/week.
AUDIT-C, Alcohol Use Disorders Identification Test; CAU, care-as-usual; EQ5D-5L,; FTND,; GAD-7, Generalized Anxiety Disorder-7; HLQ, Health Literacy Questionnaire; PAM-13, Patient Activation Measure; PHQ-9, Patient Health Questionnaire-9; PROMs, patient-reported outcome measures; SF-12, 12-Item Short Form Health Survey; VascuQoL-25, 25-item Vascular Quality of Life Questionnaire; WIQ, Walking Impairment Questionnaire; ZAPA, Satisfaction with Outpatient Care with Focus on Patient Participation.
Primary outcomes
PROM: walking impairment (WIQ).69–72
Secondary outcomes
PROMs: generic health-related quality of life (EQ5D-5L and SF-12 questionnaires);73–76 PAD-specific quality of life (VascuQoL-25 questionnaire);77 78 depression (PHQ-9 questionnaire);79 generalised anxiety disorder (GAD-7 questionnaire);80 81 alcohol use (AUDIT-C questionnaire);82–84 nicotine dependence (FTND);85 health literacy (HLQ);86 87 patient activation (PAM-13 questionnaire).88 89
Claims data: Total healthcare costs, that is, inpatient hospital care costs; outpatient (ambulatory) services and primary care costs, costs for drugs and other medical supplies, sick pay costs; healthcare resource use, that is, time period until hospitalisation, probability of hospitalisation, number and duration of inpatient hospitalisation, outpatient medical treatment, drug dose; (severe) adverse events, that is, death, amputation and revascularisation (see online supplementary file).
bmjopen-2019-032146supp001.pdf (48.6KB, pdf)
Additional outcomes (intervention arm only)
PROM: patient satisfaction (ZAPA questionnaire).90
Activity tracker data: exercise adherence, for example, number of alerts and corresponding phone calls made when step frequency or the duration of exercise sessions fall below an individual threshold range; amount of steps/net walking time (>50 steps/min) per day/week.
Sample size
To find a ‘meaningful’ effect that is clinically relevant, practicable and economically feasible, the sample size is calculated based on the distribution-based minimal clinically relevant difference (MCID) for small changes on the WIQ following 3 months of a structured HEP that have been determined in previous studies (WIQ speed MCID: 6%; WIQ distance MCID: 5%; WIQ stair climbing MCID: 5%).91 As TeGeCoach is more intensive and longer, a small-to-moderate group difference was estimated (f=0.15), while accounting for the inherited heterogeneity of this pragmatic trial that could lead to a dilution of the treatment effect. Assuming a response rate of 30% (TeGeCoach) and 20% (CAU) from baseline 24-month follow-up (t2),43 92 a sample size of 1760 (880 per group) is required to have 176 and 264 participants at t2 in the CAU and intervention arm, respectively, which is sufficient to detect the estimated small-to-moderate effect with 80% power and a 5% level of significance (Gpower V.3.1.9.2).
Data collection and management
Data management and storage are carried out in compliance with the General Data Protection Regulation in the European Union and Good Scientific Practice guidelines by the German Research Foundation.93 To ensure confidentiality, all data are collected, processed, analysed and stored in deidentified form by replacing personally identifying information of each participant with a unique patient identification number (ie, by pseudonymisation), which allows to combine data from multiple sources and to merge longitudinal data. Linkage to an identity (depseudonymization) is not possible without a separately stored pseudonymisation key, which is protected by technical and organisational measures.
At each study point, the data coordinators of the health insurance funds send out a set of paper-based questionnaires (PROMs) to the participants. Participants are asked to send them back to the Department of Medical Psychology at the University Medical Center Hamburg-Eppendorf. To maximise response rates, participants who have not send their questionnaire back in time receive a postal reminder after 2–4 weeks. All participants are followed up at t1 and t2, irrespective of whether questionnaires have been returned at previous study points. Questionnaire data are entered into an electronic database, with only authorised personnel being allowed to retrieve, enter or change data. For data quality and monitoring purposes, validation checks regarding out of range data, illogical and invalid responses and data entry errors are performed.
Claims data are routinely collected for the purpose of billing and contains information on all contacts with the healthcare system including ICD codes, operations and procedure key codes (the German equivalent to the American procedure coding system), medication prescriptions and amount of sick leaves. After study completion, the health insurance funds assemble and pseudonymise the claims data and send it to the study team (University Medical Center Hamburg-Eppendorf). No individual insurance information can be identified from this data.
Activity tracker data are automatically uploaded to the electronic documentation system via SIM card modem (econnect, IEM GmbH) once per day. The statutory health insurance funds share the activity tracker data with the study team in pseudonymised form.
All data are stored for a maximum of 10 years, securely locked in cabinets and saved on password-protected computers in areas with restricted access. Personally identifiable information of participants and pseudonymisation keys are only accessible to the data coordinators at each health insurance fund. The pseudonymisation keys are deleted 2 years after study completion so that virtually from this point all data are fully anonymised. Regarding dissemination, all publicly available data are fully anonymised and do not disclose identities. Participants have the right to be informed about their data. If a participant decides to withdraw from the trial prematurely, the data already collected may be used, unless revoking their informed consent. Deletion of the data cannot be requested if the data have already been anonymised.
Statistical analysis
Analyses are by intention-to-treat in accordance with the CONSORT guidelines, that is, participants who do not adhere to or withdraw from the prescribed TeGeCoach intervention and for whom no doctor could be appointed (see the ‘Recruitment’ section) are included in the analyses as randomised. For questionnaire data, changes from baseline to follow-up measurements are compared between study arms using linear mixed models.94 Single imputation using the Expected-Maximisation algorithm are applied for item-level missing data. Scale-level imputation of missing data is not necessary since this is fully handled by estimating mixed models with full information maximum likelihood.95 96 In order to take correlation between the observations into account, models are adjusted for participant and health coaching centre characteristics. For claims data, changes over time between groups are compared between study arms using random-effects regression models (difference-in-differences method) after eliminating differences in observable baseline characteristics between groups with the use of entropy balancing.97 Entropy balancing allows a better balancing compared with conventional processes such as propensity matching. Tests of treatment effects are conducted at a two-sided significance level of 0.05. In order to check the robustness of the results, subgroup analyses are performed to determine the influence of baseline characteristics (eg, degree of walking impairment), health insurance fund (ie, KKH Kaufmännische Krankenkasse, TK Techniker Krankenkasse and mhplus Krankenkasse) and type of analysis (ie, intention-to-treat and per-protocol).
Data monitoring and harms
This trial is not monitored by a data monitoring committee, and no interim analyses are performed as TeGeCoach is a low risk, non-invasive intervention with no identifiable risks. Over the course of the intervention, participants allocated to TeGeCoach are medically monitored by their treating physician while having regular access to the usual care of PAD. The risks from the use of wearable activity trackers is low; all devices have been certified and conform to health, safety and environmental protection standards for products sold within the European Union (CE certificate).
Ethics and dissemination
The informed consent forms and all other documents that are handed out to the participants have been reviewed and approved by the ethical review bodies (Medical Association Hamburg; reference number: PV5708). The ethics committee will be informed in case of any amendments made to the study protocol or informed consent forms.
Findings are disseminated widely through peer-reviewed manuscripts published in scientific journals, reports to the funding body, international conference presentations and media press releases. Furthermore, the study team realises the value of open science and feels committed to information exchange through data being accessible to the research community. Therefore, in an attempt to tackle the problem of hidden data, deidentified participant data from this trial are made available to the public and the medical research community on reasonable request to the corresponding author.
Supplementary Material
Acknowledgments
Special thanks to our research assistants for their support of this study: Mara Pelt, Anastasia Izotova and Sarah Willen.
Footnotes
Funding: This clinical trial receives funding from the German Innovation Fund (01NVF17013) of the Federal Joint Committee (G-BA), the highest decision-making body of the joint self-government of physicians, dentists, hospitals and health insurance funds in Germany. The use of grant funds is monitored by the German Aerospace Center (Deutsches Zentrum für Luft- und Raumfahrt; DLR). Neither the G-BA nor the DLR is involved in the actual conduct of this work (ie, execution, data analysis, interpretation of data and dissemination).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Ethical approval has been obtained at the ethics committee of the Medical Association Hamburg (Ärztekammer Hamburg; reference number: PV5708).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: None.
References
- 1.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509–26. 10.1161/CIRCRESAHA.116.303849 [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. The Lancet 2013;382:1329–40. 10.1016/S0140-6736(13)61249-0 [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA 2004;292:453–61. 10.1001/jama.292.4.453 [DOI] [PubMed] [Google Scholar]
- 4.Nehler MR, Duval S, Diao L, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg 2014;60:686–95. 10.1016/j.jvs.2014.03.290 [DOI] [PubMed] [Google Scholar]
- 5.Criqui MH, McClelland RL, McDermott MM, et al. The Ankle-brachial index and incident cardiovascular events in the MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 2010;56:1506–12. 10.1016/j.jacc.2010.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Criqui MH, Ninomiya JK, Wingard DL, et al. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J Am Coll Cardiol 2008;52:1736–42. 10.1016/j.jacc.2008.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malyar N, Fürstenberg T, Wellmann J, et al. Recent trends in morbidity and in-hospital outcomes of in-patients with peripheral arterial disease: a nationwide population-based analysis. Eur Heart J 2013;34:2706–14. 10.1093/eurheartj/eht288 [DOI] [PubMed] [Google Scholar]
- 8.Eraso LH, Fukaya E, Mohler ER, et al. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol 2014;21:704–11. 10.1177/2047487312452968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA 2012;308:1660–7. 10.1001/jama.2012.13415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamburg NM, Creager MA. Pathophysiology of intermittent claudication in peripheral artery disease. Circ J 2017;81:281–9. 10.1253/circj.CJ-16-1286 [DOI] [PubMed] [Google Scholar]
- 11.Hiatt WR, Armstrong EJ, Larson CJ, et al. Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circ Res 2015;116:1527–39. 10.1161/CIRCRESAHA.116.303566 [DOI] [PubMed] [Google Scholar]
- 12.Gardner AW, Montgomery PS, Wang M, et al. Predictors of health-related quality of life in patients with symptomatic peripheral artery disease. J Vasc Surg 2018;68:1126–34. 10.1016/j.jvs.2017.12.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maksimovic M, Vlajinac H, Marinkovic J, et al. Health-Related quality of life among patients with peripheral arterial disease. Angiology 2014;65:501–6. 10.1177/0003319713488640 [DOI] [PubMed] [Google Scholar]
- 14.Regensteiner JG, Hiatt WR, Coll JR, et al. The impact of peripheral arterial disease on health-related quality of life in the peripheral arterial disease awareness, risk, and treatment: new resources for survival (partners) program. Vasc Med 2008;13:15–24. 10.1177/1358863X07084911 [DOI] [PubMed] [Google Scholar]
- 15.Smolderen KG, Hoeks SE, Pedersen SS, et al. Lower-leg symptoms in peripheral arterial disease are associated with anxiety, depression, and anhedonia. Vasc Med 2009;14:297–304. 10.1177/1358863X09104658 [DOI] [PubMed] [Google Scholar]
- 16.Haas TL, Lloyd PG, Yang H-T, et al. Exercise training and peripheral arterial disease. Compr Physiol 2012;2:2933–3017. 10.1002/cphy.c110065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 2011;123:87–97. 10.1161/CIRCULATIONAHA.109.881888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidon M, McGee H. Exercise-based interventions and health-related quality of life in intermittent claudication: a 20-year (1989–2008) review. Eur J Cardio Prevention Rehabilitation 2010;17:140–54. 10.1097/HJR.0b013e3283377f08 [DOI] [PubMed] [Google Scholar]
- 19.Lane R, Harwood A, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev 2017;12:CD000990. 10.1002/14651858.CD000990.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: Executive summary: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Circulation 2017;135:e686–725. 10.1161/CIR.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawall H, Huppert P, Espinola-Klein C, et al. The diagnosis and treatment of peripheral arterial vascular disease. Dtsch Arztebl Int 2016;113:729–36. 10.3238/arztebl.2016.0729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aboyans V, Ricco J-B, Bartelink M-LEL, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). European Heart Journal 2017;39:763–816. [DOI] [PubMed] [Google Scholar]
- 23.Harwood A-E, Smith GE, Cayton T, et al. A systematic review of the uptake and adherence rates to supervised exercise programs in patients with intermittent claudication. Ann Vasc Surg 2016;34:280–9. 10.1016/j.avsg.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 24.Makris GC, Lattimer CR, Lavida A, et al. Availability of supervised exercise programs and the role of structured home-based exercise in peripheral arterial disease. Eur J Vasc Endovasc Surg 2012;44:569–75. 10.1016/j.ejvs.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 25.McDermott MM, Polonsky TS. Home-Based exercise: a therapeutic option for peripheral artery disease. Circulation 2016;134:1127–9. 10.1161/CIRCULATIONAHA.116.023691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golledge J, Singh TP, Alahakoon C, et al. Meta-Analysis of clinical trials examining the benefit of structured home exercise in patients with peripheral artery disease. Br J Surg 2019;106:319–31. 10.1002/bjs.11101 [DOI] [PubMed] [Google Scholar]
- 27.Harwood AE, Hitchman LH, Ingle L, et al. Preferred exercise modalities in patients with intermittent claudication. Journal of Vascular Nursing 2018;36:81–4. 10.1016/j.jvn.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 28.Hageman D, Fokkenrood HJ, Gommans LN, et al. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev 2018;4:CD005263. 10.1002/14651858.CD005263.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Jundi W, Madbak K, Beard JD, et al. Systematic review of home-based exercise programmes for individuals with intermittent claudication. Eur J Vasc Endovasc Surg 2013;46:690–706. 10.1016/j.ejvs.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 30.Bäck M, Jivegård L, Johansson A, et al. Home-Based supervised exercise versus hospital-based supervised exercise or unsupervised walk advice as treatment for intermittent claudication: a systematic review. J Rehabil Med 2015;47:801–8. 10.2340/16501977-2012 [DOI] [PubMed] [Google Scholar]
- 31.McDermott MM, Liu K, Guralnik JM, et al. Home-Based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 2013;310:57–65. 10.1001/jama.2013.7231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDermott MM, Guralnik JM, Criqui MH, et al. Home‐Based walking exercise in peripheral artery disease: 12‐Month Follow‐up of the goals randomized trial. J Am Heart Assoc 2014;3:e000711 10.1161/JAHA.113.000711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins TC, Lunos S, Carlson T, et al. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized controlled trial. Diabetes Care 2011;34:2174–9. 10.2337/dc10-2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham MA, Swanson V, O'Carroll RE, et al. Randomized clinical trial of a brief psychological intervention to increase walking in patients with intermittent claudication. Br J Surg 2012;99:49–56. 10.1002/bjs.7714 [DOI] [PubMed] [Google Scholar]
- 35.Cunningham MA, Swanson V, Holdsworth RJ, et al. Late effects of a brief psychological intervention in patients with intermittent claudication in a randomized clinical trial. Br J Surg 2013;100:756–60. 10.1002/bjs.9100 [DOI] [PubMed] [Google Scholar]
- 36.Mays RJ, Hiatt WR, Casserly IP, et al. Community-Based walking exercise for peripheral artery disease: an exploratory pilot study. Vasc Med 2015;20:339–47. 10.1177/1358863X15572725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardner AW, Parker DE, Montgomery PS, et al. Step‐Monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc 2014;3:e001107 10.1161/JAHA.114.001107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardner AW, Parker DE, Montgomery PS, et al. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication. Circulation 2011;123:491–8. 10.1161/CIRCULATIONAHA.110.963066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duscha BD, Piner LW, Patel MP, et al. Effects of a 12-week mHealth program on FunctionalCapacity and physical activity in patients with PeripheralArtery disease. Am J Cardiol 2018;122:879–84. 10.1016/j.amjcard.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 40.Tew GA, Humphreys L, Crank H, et al. The development and pilot randomised controlled trial of a group education programme for promoting walking in people with intermittent claudication. Vascular Medicine 2015;20:348–57. 10.1177/1358863X15577857 [DOI] [PubMed] [Google Scholar]
- 41.Mays RJ, Rogers RK, Hiatt WR, et al. Community walking programs for treatment of peripheral artery disease. J Vasc Surg 2013;58:1678–87. 10.1016/j.jvs.2013.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ware JH, Hamel MB. Pragmatic trials — guides to better patient care? N Engl J Med 2011;364:1685–7. 10.1056/NEJMp1103502 [DOI] [PubMed] [Google Scholar]
- 43.Dwinger S, Dirmaier J, Herbarth L, et al. Telephone-based health coaching for chronically ill patients: study protocol for a randomized controlled trial. Trials 2013;14:337 10.1186/1745-6215-14-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Härter M, Dirmaier J, Dwinger S, et al. Effectiveness of Telephone-Based health coaching for patients with chronic conditions: a randomised controlled trial. PLoS One 2016;11:e0161269 10.1371/journal.pone.0161269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Härter M, Dwinger S, Seebauer L, et al. Evaluation of telephone health coaching of German health insurants with chronic conditions. Health Educ J 2013;72:622–34. 10.1177/0017896912453990 [DOI] [Google Scholar]
- 46.Dennis SM, Harris M, Lloyd J, et al. Do people with existing chronic conditions benefit from telephone coaching? a rapid review. Australian Health Review 2013;37:381–8. 10.1071/AH13005 [DOI] [PubMed] [Google Scholar]
- 47.Eakin EG, Lawler SP, Vandelanotte C, et al. Telephone interventions for physical activity and dietary behavior change. Am J Prev Med 2007;32:419–34. 10.1016/j.amepre.2007.01.004 [DOI] [PubMed] [Google Scholar]
- 48.McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease. JAMA 2018;319:1665–76. 10.1001/jama.2018.3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parmenter BJ, Dieberg G, Smart NA. Exercise training for management of peripheral arterial disease: a systematic review and meta-analysis. Sports Med 2015;45:231–44. 10.1007/s40279-014-0261-z [DOI] [PubMed] [Google Scholar]
- 50.Lewis ZH, Lyons EJ, Jarvis JM, et al. Using an electronic activity monitor system as an intervention modality: a systematic review. BMC Public Health 2015;15:585 10.1186/s12889-015-1947-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using Pedometers to increase physical activity and improve health. JAMA 2007;298:2296–304. 10.1001/jama.298.19.2296 [DOI] [PubMed] [Google Scholar]
- 52.Harris T, Limb ES, Hosking F, et al. Effect of pedometer-based walking interventions on long-term health outcomes: prospective 4-year follow-up of two randomised controlled trials using routine primary care data. PLoS Med 2019;16:e1002836 10.1371/journal.pmed.1002836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haveman ME, Kleiss SF, Ma KF, et al. Telemedicine in patients with peripheral arterial disease: is it worth the effort? Expert Rev Med Devices 2019;16:777–86. 10.1080/17434440.2019.1649595 [DOI] [PubMed] [Google Scholar]
- 54.O'Brien T, Troutman-Jordan M, Hathaway D, et al. Acceptability of wristband activity trackers among community dwelling older adults. Geriatr Nurs 2015;36:S21–5. 10.1016/j.gerinurse.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 55.Cadmus-Bertram LA, Marcus BH, Patterson RE, et al. Randomized trial of a Fitbit-based physical activity intervention for women. Am J Prev Med 2015;49:414–8. 10.1016/j.amepre.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehn M, Eriksson LC, Åkerberg N, et al. Activity monitors as support for older persons’ physical activity in daily life: qualitative study of the users’ experiences. JMIR mHealth and uHealth 2018;6:e34 10.2196/mhealth.8345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kononova A, Li L, Kamp K, et al. The use of wearable activity trackers among older adults: focus group study of tracker perceptions, motivators, and barriers in the maintenance stage of behavior change. JMIR mHealth and uHealth 2019;7:e9832 10.2196/mhealth.9832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Normahani P, Kwasnicki R, Bicknell C, et al. Wearable sensor technology efficacy in peripheral vascular disease (wSTEP): a randomized controlled trial. Ann Surg 2018;268:1113–8. 10.1097/SLA.0000000000002300 [DOI] [PubMed] [Google Scholar]
- 59.Lyons EJ, Swartz MC, Lewis ZH, et al. Feasibility and acceptability of a wearable technology physical activity intervention with telephone counseling for mid-aged and older adults: a randomized controlled pilot trial. JMIR Mhealth Uhealth 2017;5:e28 10.2196/mhealth.6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350:h2147 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- 61.Deshpande P, Sudeepthi BLakshmi, Rajan S, et al. Patient-Reported outcomes: a new era in clinical research. Perspect Clin Res 2011;2:137–44. 10.4103/2229-3485.86879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schulz KF, Altman DG, Moher D. Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8:18 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calvert M, Kyte D, Mercieca-Bebber R, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols. JAMA 2018;319:483–94. 10.1001/jama.2017.21903 [DOI] [PubMed] [Google Scholar]
- 65.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 66.Guidon M, McGee H. Recruitment to clinical trials of exercise: challenges in the peripheral arterial disease population. Physiotherapy 2013;99:305–10. 10.1016/j.physio.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 67.Rahman S, Majumder A, Shaban S, et al. Physician participation in clinical research and trials: issues and approaches. Adv Med Educ Pract 2011;2:85 10.2147/AMEP.S14103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Polit DF. Blinding during the analysis of research data. Int J Nurs Stud 2011;48:636–41. 10.1016/j.ijnurstu.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 69.Regensteiner JG, Steiner JF, Panzer RJ, et al. Evaluation of walking impairment by questionnaire in patients with peripheral Arterial-Disease. Clin Res 1990;38:A515–A15. [Google Scholar]
- 70.McDermott MM, Liu K, Guralnik JM, et al. Measurement of walking endurance and walking velocity with questionnaire: validation of the walking impairment questionnaire in men and women with peripheral arterial disease. J Vasc Surg 1998;28:1072–81. 10.1016/S0741-5214(98)70034-5 [DOI] [PubMed] [Google Scholar]
- 71.Sagar SP, Brown PM, Zelt DT, et al. Further clinical validation of the walking impairment questionnaire for classification of walking performance in patients with peripheral artery disease. Int J Vasc Med 2012;2012:1–10. 10.1155/2012/190641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nicolaï SPA, Kruidenier LM, Rouwet EV, et al. The walking impairment questionnaire: an effective tool to assess the effect of treatment in patients with intermittent claudication. J Vasc Surg 2009;50:89–94. 10.1016/j.jvs.2008.12.073 [DOI] [PubMed] [Google Scholar]
- 73.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ware J, Kosinski M, Keller SD. A 12-Item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 75.Gandek B, Ware JE, Aaronson NK, et al. Cross-Validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project. International quality of life assessment. J Clin Epidemiol 1998;51:1171–8. 10.1016/s0895-4356(98)00109-7 [DOI] [PubMed] [Google Scholar]
- 76.Hinz A, Kohlmann T, Stöbel-Richter Y, et al. The quality of life questionnaire EQ-5D-5L: psychometric properties and normative values for the general German population. Qual Life Res 2014;23:443–7. 10.1007/s11136-013-0498-2 [DOI] [PubMed] [Google Scholar]
- 77.Morgan MBF, Crayford T, Murrin B, et al. Developing the vascular quality of life questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg 2001;33:679–87. 10.1067/mva.2001.112326 [DOI] [PubMed] [Google Scholar]
- 78.Mehta T, Venkata Subramaniam A, Chetter I, et al. Assessing the validity and responsiveness of disease-specific quality of life instruments in intermittent claudication. Eur J Vasc Endovasc Surg 2006;31:46–52. 10.1016/j.ejvs.2005.08.028 [DOI] [PubMed] [Google Scholar]
- 79.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann 2002;32:509–15. 10.3928/0048-5713-20020901-06 [DOI] [Google Scholar]
- 80.Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 81.Löwe B, Decker O, Müller S, et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care 2008;46:266–74. 10.1097/MLR.0b013e318160d093 [DOI] [PubMed] [Google Scholar]
- 82.Bush K, Kivlahan DR, McDonell MB, et al. The audit alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. ambulatory care quality improvement project (ACQUIP). alcohol use disorders identification test. Arch Intern Med 1998;158:1789–95. 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- 83.Bradley KA, DeBenedetti AF, Volk RJ, et al. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcoholism: Clinical and Experimental Research 2007;31:1208–17. 10.1111/j.1530-0277.2007.00403.x [DOI] [PubMed] [Google Scholar]
- 84.Dybek I, Bischof G, Grothues J, et al. The reliability and validity of the alcohol use disorders identification test (audit) in a German general practice population sample. J Stud Alcohol 2006;67:473–81. 10.15288/jsa.2006.67.473 [DOI] [PubMed] [Google Scholar]
- 85.Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Addiction 1991;86:1119–27. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- 86.Osborne RH, Batterham RW, Elsworth GR, et al. The grounded psychometric development and initial validation of the health literacy questionnaire (HLQ). BMC Public Health 2013;13:658 10.1186/1471-2458-13-658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nolte S, Osborne RH, Dwinger S, et al. German translation, cultural adaptation, and validation of the health literacy questionnaire (HLQ). PLoS One 2017;12:e0172340 10.1371/journal.pone.0172340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hibbard JH, Mahoney ER, Stockard J, et al. Development and testing of a short form of the patient activation measure. Health Serv Res 2005;40:1918–30. 10.1111/j.1475-6773.2005.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zill JM, Dwinger S, Kriston L, et al. Psychometric evaluation of the German version of the patient activation measure (PAM13). BMC Public Health 2013;13:1027 10.1186/1471-2458-13-1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scholl I, Hölzel L, Härter M, et al. Fragebogen Zur zufriedenheit in Der ambulanten versorgung–schwerpunkt patientenbeteiligung (ZapA). Klinische Diagnostik und Evaluation 2011;4:50–62. [Google Scholar]
- 91.Gardner AW, Montgomery PS, Wang M. Minimal clinically important differences in treadmill, 6-minute walk, and patient-based outcomes following supervised and home-based exercise in peripheral artery disease. Vasc Med 2018;23:349–57. 10.1177/1358863X18762599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol 1997;50:1129–36. 10.1016/S0895-4356(97)00126-1 [DOI] [PubMed] [Google Scholar]
- 93.Deutsche Forschungsgemeinschaft Sicherung guter wissenschaftlicher Praxis. Sicherung Guter Wissenschaftlicher Praxis: Empfehlungen der Kommission “Selbstkontrolle in der Wissenschaft”. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, 2013: 1–109. [Google Scholar]
- 94.Chakraborty H, Gu H. A mixed model approach for intent-to-treat analysis in longitudinal clinical trials with missing values 2009. [PubMed]
- 95.Twisk J, de Boer M, de Vente W, et al. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J Clin Epidemiol 2013;66:1022–8. 10.1016/j.jclinepi.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 96.Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods 2001;6:330–51. 10.1037/1082-989X.6.4.330 [DOI] [PubMed] [Google Scholar]
- 97.Hainmueller J. Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Political Analysis 2012;20:25–46. 10.1093/pan/mpr025 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-032146supp001.pdf (48.6KB, pdf)