Abstract
Context
Anthocyanins are phenolic compounds found in berries. They exhibit promising health benefits in humans, but no accurate biomarkers of berry intake have been identified thus far.
Objective
The aim of this systematic review is to propose a biomarker of anthocyanin-rich berry intake in human plasma and urine.
Data Sources
PubMed and Cochrane databases were searched from January 2008 to January 2019.
Study Selection
Databases were searched for human intervention studies that assessed the presence of anthocyanins in human body fluids using high-throughput techniques. Non-English articles and studies publishing targeted analyses were excluded.
Data Extraction
Ten clinical trials, in which 203 phenolic compounds were identified, were included and assessed qualitatively. The following criteria were used to identify biomarkers of berry intake: frequency, plausibility, dose-response, time response, robustness, reliability, stability, analytical performance, and reproducibility. Sensitivity and specificity of potential biomarkers were determined by the receiver operating characteristic curve.
Results
Of the 203 phenolic compounds identified in human samples, the anthocyanin cyanidin-3-glucoside was the molecule found most frequently in urine (58.06%) and plasma (69.49%). Cyanidin-3-glucoside fulfills the essential criterion of plausibility as well as the dose-response, time response, stability, and analytical performance criteria. Its positive predictive value is 74% (P = 0.210) in plasma, which is acceptable, and 61.7% (P = 0.402) in urine.
Conclusions
Current evidence suggests that cyanidin-3-glucoside is a potential biomarker of anthocyanin-rich berry intake in plasma and urine of healthy humans.
PROSPERO registration number
CRD42018096796.
Keywords: anthocyanins, berry, biomarker, cyanidin-3-glucoside, plasma, urine
INTRODUCTION
In recent years, interest in the implementation of healthy diets high in fruits and vegetables,1–6 shown to have positive effects on disease prevention7–9 and life expectancy, has been increasing.10 Some of the health benefits attributed to the frequent consumption of fruits and vegetables can be attributed, at least in part, to phenolic compounds, which are naturally produced biomolecules contained in a variety of plants.11,12 Phenolic compounds, however, are classified into widely varying families of biomolecules, and not all families have the same effects on maintenance of health and prevention of disease.13,14 One of the most important classes of phenolic compounds are the flavonoids, particularly the anthocyanins, which have shown promising potential in the prevention of diseases and conditions such as obesity,15–17 type 2 diabetes,17–19 hypertension,20 prostate cancer,21–23 lung cancer,24–26 heart failure,27 renal failure,28–30 Alzheimer disease, and Parkinson disease.31–35 Anthocyanins are water-soluble pigments responsible for the red or blue coloration of certain flowers, seeds, fruits, and plants.36 They are most commonly found in great concentrations in both the flesh and the skin of red-fleshed apples37 and in most berries.38 They can be further divided into parent anthocyanins or anthocyanin metabolites, depending on their chemical structure or their metabolization.39 Parent anthocyanins are cyanidin-3-glucoside (C3G), peonidin-3-glucoside, and malvidin-3-glucoside, while anthocyanin metabolites are cyanidin-3-glucuronide, peonidin-3-O-arabinoside, and malvidin-3-galactoside.39
Despite the important role of fruits and vegetables in human health, only a few biochemical markers that can assess individual intake of specific food items have been described.40,41 The identification of biomarkers of anthocyanin-rich foods such as berries42 could help further elucidate the specific health benefits of anthocyanins.
It is possible, however, that a biomarker of anthocyanin intake could have low specificity. One possible explanation for the lack of specific biomarkers described thus far might be the heterogeneous composition of foods, which hinders the identification of viable biomarker candidates. Hence, there is a need to identify specific biomarkers to assess the consumption of berries. Recently, guidelines for addressing the complex assessment and validation of biomarkers of food intake have been proposed.42,43 Dragsted et al43 were the first to propose a comprehensive guideline for the validation of biomarkers of food intake after conducting a systematic review of the literature. They proposed a novel process, based on the analysis of different criteria, for the identification of possible biomarkers of food intake. Therefore, the aim of the present systematic review of clinical trials, conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, is to propose a possible biomarker of phenolic compounds present in human body fluids after the ingestion of anthocyanin-rich berries.
METHODS
Search strategy and selection criteria
The present systematic review of human clinical trials of berry consumption was conducted in accordance with the PRISMA methodology44 and was previously registered in University of York’s PROSPERO (International Prospective Register of Systematic Reviews) with the registration number CRD42018096796.
A Web-based search of the PubMed and Cochrane Library databases was performed using with the following terms, which included all berries with high anthocyanin content: (blackcurrant OR “blackcurrant extract” OR blackberry OR “blackberry extract” OR raspberry OR “raspberry extract” OR blueberry OR “blueberry extract” OR cherry OR “cherry extract” OR redcurrant OR “redcurrant extract” OR grape) AND (biomarker* OR marker* OR metabolite* OR pharmacokinetics OR biokinetics) AND (intake OR ingestion OR consumption OR eating OR diet) AND (human OR men OR women OR patient) AND (urine OR blood OR plasma OR serum OR faeces OR feces) NOT (animal OR mouse OR mice OR rat OR pig OR juice OR drink OR cell OR in vitro OR questionnaire OR self-reported OR systematic review).
Published articles were screened on the basis of their titles, abstracts, and full texts, with the following inclusion criteria applied: (1) intervention studies performed in humans; (2) healthy male or female participants; (3) participant age between 18 and 90 years; (4) parallel studies; (5) untargeted analytical approach; (6) diet free of phenolic compounds for ≥ 24 hours before the start of the study; (7) analysis of body fluids (blood, plasma or urine, feces, or saliva) performed; (8) body fluids analyzed at baseline; (9) dose characterization performed; (10) use of a controlled, polyphenol-free diet during the intervention; and (11) studies published from January 1, 2008, to January 10, 2019. The following exclusion criteria were applied: (1) articles not published in English; (2) crossover washout period of less than 48 hours; (3) administration of phenolic compound–rich fruits in conjunction with other nutritional elements; (4) presence of gastrointestinal pathologies such as inflammatory bowel disease or chronic malabsorption syndrome; (5) participants under any kind of medication or treatment; (6) targeted (ie, nongeneral) analytical approach; (7) nonhealthy individuals; and (8) any inclusion criterion not fulfilled. Further information about the PICOS (Population, Intervention, Comparison, Outcomes, and Study design) criteria is detailed in Table 1.
Table 1.
PICOS criteria for inclusion and exclusion of studies
| Criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Healthy men and women | Participants receiving any kind of medication or treatment; nonhealthy individuals; individuals with gastrointestinal pathologies such as inflammatory bowel disease or chronic malabsorption syndrome |
| Intervention | Oral or intravenous administration of an anthocyanin-rich berry; analysis of body fluid (blood, plasma, urine, feces, or saliva) performed | Administration of fruits rich in phenolic compounds in conjunction with other nutritional elements |
| Comparison | None | None |
| Outcome | Bioavailability of phenolic compounds in plasma and urine | None |
| Study design | Human intervention studies, randomized controlled trials, and randomized controlled crossover trials | Observational studies, studies without a washout period, studies that used a targeted analytical approach |
Process for selecting a biomarker candidate
In the present systematic review, the following process is proposed for selecting a biomarker candidate. First, a systematic review was performed to search for all studies of possible phenolic compounds present or absent in human body fluids. Second, the risk of bias of all included clinical trials was assessed to determine study quality. Third, of the phenolic compounds found in human body fluids, the one described most frequently was identified. Fourth, the 8 criteria proposed by Dragsted et al43 for validating biomarkers of food consumption were applied to the biomarker candidates found most frequently. This was done to identify the most viable candidates. Special emphasis was placed on the plausibility criterion, which is an essential criterion for any biomarker of food intake.43 Fifth, to determine which phenolic compounds identified would be most viable as biomarker candidates, further statistical analysis was conducted to determine the specificity and sensitivity of each compound by calculating the area under the curve (AUC) receiver operating characteristic (ROC) curve. Finally, results were discussed in relation to the bibliographic references available, and conclusions were drawn.
Data extraction and analysis
Using the PRISMA criteria,44 2 authors (Ú.C. and B.A.S-R.) independently extracted published data from the main text and tables. Any differences were resolved by a third author (R.S.). The following information was extracted from all included articles: (1) study characteristics, including type (postprandial, parallel, or crossover) and length of study, type of samples analyzed, sources of anthocyanins, routes of administration used, and number and size of doses used; (2) number and characteristics of participants, including age, sex, and health status; and (3) total or specific amounts of phenolic compounds reported. The PRISMA checklist is provided as Table S1 in the Supporting Information online.
Risk-of-bias assessment
Two authors (B.A.S-R. and Ú.C.) independently assessed the potential risk of bias of the studies that met the inclusion criteria, using the methodological index for nonrandomized studies (MINORS).45 Items were scored with 0 if no data were reported; with 1 when data were reported but were inadequate; and with 2 when data were both reported and adequate. If, after applying the MINORS criteria, a study received a score of 13 or above, with 24 being the maximum score, risk of bias was considered low.45 Additionally, in accordance with the PRISMA guidelines,44 2 authors (B.A.S-R. and Ú.C.) reached a consensus, and a third author (R.S.) independently resolved any discrepancies between the other 2 authors. The potential risk of bias of the present systematic review was assessed by AMSTAR 2, a tool for the critical appraisal of systematic reviews that include randomized or nonrandomized studies of healthcare interventions.46 AMSTAR 2 is based on the evaluation of 16 items by means of simple response categories. The results of the systematic review can then be rated as being of high, moderate, low, or critically low quality.
Criteria for assessment of biomarkers of berry intake
The assessment and validation of biomarkers of food intake was performed according to Praticò et al42 and Dragsted et al43 and included the 8 criteria proposed by Dragsted et al43: (1) plausibility or specificity, to determine if the biomarker was specific or could be attributed to a food or food group. If the phenolic compound composition of the berries was not reported in the included studies, it was retrieved from the Phenol-Explorer database47; (2) dose-response, to determine if the concentration of the biomarker in the sample(s) analyzed increased or decreased in agreement with the dose of molecule used; (3) time response, to determine if the description of the kinetics of the molecule is adequate to enable prudent choices about sample type and time window; (4) robustness, to determine if the biomarker remains robust even after the ingestion of different types of complex meals; (5) reliability, to determine if the biomarker compares well with other biomarkers or other data following intake of the same food or food group; (6) stability, to determine if the marker is stable during collection, processing, and storage; (7) analytical performance, to determine if the specificity and sensitivity of the molecule are adequate, and if the molecule can be measured with accuracy; and (8) reproducibility, to determine if analysis of the molecule has been successfully reproduced in another laboratory.43 To evaluate each of the 8 criteria, the researcher could answer as follows: (a) yes, which indicates the phenolic compound was investigated and meets the criterion completely; (b) no, which indicates the phenolic compound was investigated but did not meet the minimum requirements for the criterion to be met; or (c) undetermined, which indicates the phenolic compound has not yet been researched or the results obtained were uncertain. However, a molecule could be considered a specific biomarker of food intake even if not all these criteria were answered with “yes” responses. If a phenolic compound candidate fulfills all criteria except the plausibility criterion, it must be discarded as a potential biomarker. Moreover, each phenolic compound should be analyzed in accordance with the objectives of its specific use. For instance, an unstable biomarker might be useful for short-term, but not long-term, assessment of exposure.
Statistical analyses
The frequency with which each phenolic compound appeared in human body fluids was calculated and expressed as a percentage. The dose of anthocyanin-rich berries consumed was expressed as the mean and standard deviation (SD). Since quantitative information about the concentration of each phenolic compound in each sample could not be obtained from the studies included in the review, the concentrations that were reported have been translated into a qualitative dichotomic variable expressed as the presence or absence of any given phenolic compound molecule in plasma and urine. A cutoff point of phenolic compound metabolites that appeared in more than 40% of the plasma or urine samples of all individuals analyzed was established. The χ2 test was used to compare the presence of the most frequent phenolic compound metabolites in the 2 matrices. Sensitivity (true positive) and specificity (false positive) values were calculated using the AUC ROC, which was obtained from the calculation of the logistic regression model that was constructed for phenolic compounds deemed most promising on the basis of the trapezoidal rule. Thus, AUC values close to 100% indicate better discriminatory power. Results from the qualitative variables were expressed as percentages. It is possible that biomarker candidates could be composed of 2 or more less-specific phenolic compounds, and therefore the bivariate Pearson correlation was used to calculate correlations between phenolic compounds found in urine or plasma. All statistical analyses were performed using IBM SPSS software, version 25.0. All P values less than 0.05 were considered statistically significant.
RESULTS
Literature search and study selection
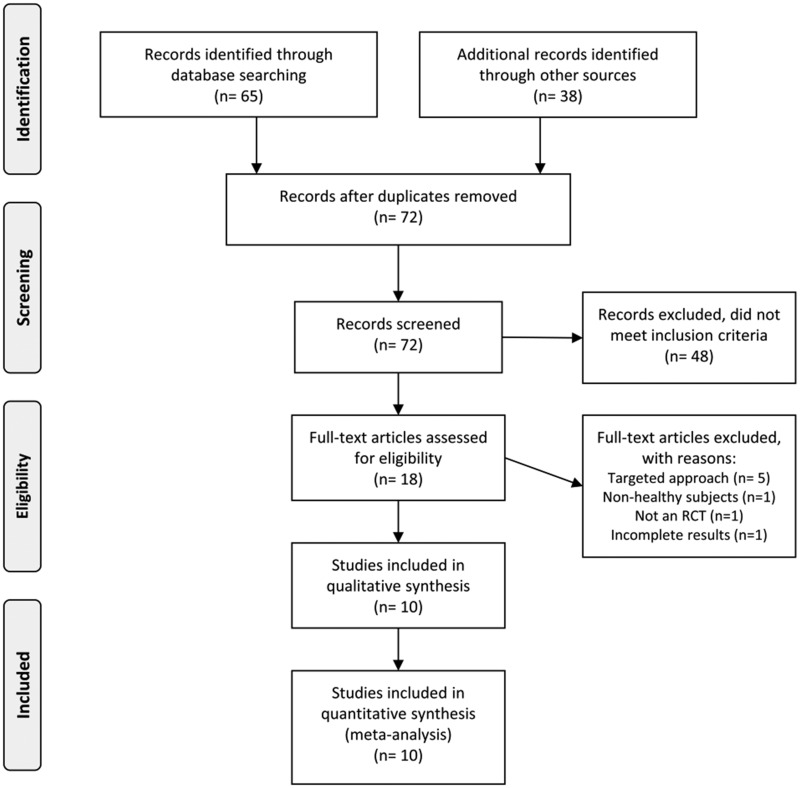
The initial screening identified 103 trials published between January 1, 2008, and January 10, 2019. After the further analysis, 79 studies were excluded for not meeting the inclusion criteria, and 6 were excluded as duplicate publications. As a result, 18 publications met the inclusion criteria and were examined further. Eight of these 18 publications were excluded for other reasons, shown in Figure 1; thus, 10 articles were included in the review.48–57 One of the 10 articles performed both short- and long-term exposure experiments.49 Therefore, these experiments were included separately in the review, resulting in a total of 11 studies. No study had a control group. The complete selection process is shown in Figure 1.
Figure 1.
Flow diagram of the literature search process. Abbreviation: RCT, randomized controlled trial.
Risk-of-bias assessment
The MINORS methodology was used to determine the risk of bias in all 10 articles included. Six studies had no conflict of interest,48,49,51,53,54,57 3 did not report any information about possible conflicts of interest,50,55,56 which led to a lower score, and 1 study reported conflicts of interest for receiving funding from the alcoholic beverage industry, though the funding did not influence the study results.52 All 10 articles received a score above 13 points (mean ± SD, 18.10 ± 1.45 points), which was considered a low risk of bias. The MINORS scores for all studies that met the inclusion criteria, along with the results of further analysis, are provided in Table S2 in the Supporting Information online.
The AMSTAR 2 tool was used to assess the nonrandomized clinical trials included in the present review. The results show this review to be of high quality for its low risk of bias (see Appendix S1 in the Supporting Information online).
Overview of findings
Consumption of the following berries was reported in the 10 articles included: red raspberry,48 raspberry,51 wild blueberry,49,53 black raspberry,50 grape pomace,52 mixed berry puree,54 blackberry,55,57 elderberry, and lowbush blackberry.56
Since none of the included studies reported the composition of the different berries administered, the Phenol-Explorer database47 was used to determine the polyphenol content of berries. The daily oral dose of anthocyanins ranged between 12 g of elderberry extract56 and 500 mL of mixed berry puree, which was described as being equivalent to 500 g.54 The mean dose of anthocyanins administered was 181 ± 130 g/d. A total of 105 participants were included in the analysis (39 men, 36 women, and 30 individuals whose sex was not reported).
The following methods were used for the detection of phenolic compounds: high-performance liquid chromatography (HPLC) in 5 studies50–52,56,57 HPLC coupled with quadrupole high-resolution time of flight mass spectrometry (HPLC-QTOF-MS) in 2 studies,49,53 HPLC coupled with a diode-array detector (HPLC-DAD) in 1 study,54 HPLC–electrospray ionization tandem mass spectroscopy (HPLC-ESI-MS/MS) in 1 study,55 and ultra HPLC-QTOF-MS (UHPLC-QTOF-MS) in 1 study.48
Of the 10 studies included in this review, 1 evaluated phenolic compounds in plasma only,53 5 evaluated phenolic compounds in urine only,50,52,54–56 and 4 evaluated phenolic compounds in both urine and plasma48,49,51,57; of the last 4 studies, 1 included short-term and long-term experiments in a single study.49 None of the included studies assessed the presence of parent anthocyanins or anthocyanin metabolites in matrices other than plasma or urine, such as in saliva, blood, or feces.
With regard to the period of berry consumption, 7 studies were conducted as short-term trials, which included periods ranging between 2 hours and 48 hours,49,51–57 2 studies were performed as long-term trials, which ranged between 7 days and 32 days,48,50 and 1 study performed both long-term and short-term experiments.49 The mean (± SD) length of the long-term interventions was 23 ± 11.34 days. Complete information about the included studies is reported in Table 2.48–57
Table 2.
Characteristics of included studies in which parent anthocyanin or anthocyanin metabolites were assessed in human body fluids after ingestion of anthocyanin-rich berries
| Type of study | Reference | Source of anthocyanins | No. of doses | Dose given | Duration of intervention | Study participants |
Sample |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex |
Age (y) | No. | |||||||||
| M (no.) | F (no.) | Plasma | Urine | ||||||||
| Long-term | |||||||||||
| Zhang et al (2018)48 | Red raspberry | 32 | 125 g | 32 d | NR | NR | NR | 2 | X | X | |
| Feliciano et al (2016)49 | Wild blueberry | 60 | 100 g | 30 d | 18 | – | 18–70 | 18 | X | X | |
| Tian et al (2006)50 | Black raspberry | 7 | 40 g | 7 d | NR | NR | NR | 10 | NP | X | |
| Short-term | |||||||||||
| Ludwig et al (2015)51 | Raspberry | 1 | 300 g | 48 h | 4 | 5 | 22–44 | 9 | X | X | |
| Feliciano et al (2016)49 | Wild blueberry | 1 | 100 g | 24 h | 19 | – | 18–71 | 18 | X | X | |
| Sasot et al (2017)52 | Grape pomace extract | 1 | 200 mL | 24 h | 6 | 6 | 24–40 | 12 | NP | X | |
| Zhong et al (2017)53 | Wild blueberry beverage | 1 | 150 g | 24 h | 6 | 6 | 20–45 | 12 | X | NP | |
| Pimpão et al (2014)54 | Mixed berry puree | 1 | 500 mL | 24 h | 3 | 6 | 23–54 | 9 | NP | X | |
| Felgines et al (2005)55 | Blackberry | 1 | 200 g | 24 h | 02 | 03 | NR | 5 | NP | X | |
| Wu et al (2002)56 | Elderberry extract | 1 | 12 g | 24 h | – | 04 | 60–70 | 4 | NR | X | |
| Lowbush blueberry | 1 | 189 g | 24 h | – | 06 | 60–70 | 6 | NR | X | ||
| Marques et al (2016)57 | Blackberry | 1 | 250 g | 2 h | NR | NR | NR | 18 | X | X | |
Abbreviations and symbols: NR, not reported; NP, not performed; X, analysis performed.
Phenolic compounds detected in plasma and urine
Phenolic compounds detected most frequently
A total of 203 different phenolic compounds were detected in urine and plasma after consumption of anthocyanin-rich berries in the human studies included in the present review (see Table S3 in the Supporting Information online). Ninety-seven phenolic compounds were found in both plasma and urine, 21 were found only in plasma, and 85 were found only in urine. Of these, those that appeared in more than 40% of samples (the cutoff value established to designate the most frequently detected phenolic compounds, since a higher cutoff significantly limited the number of candidate phenolic compounds) of human plasma, urine, or both were selected, resulting in 19 different phenolic compounds (Table 3).
Table 3.
Phenolic compounds found in more than 40% of urine or plasma samples, as indicated by gray shading.
| Metabolite | Urine (N = 93) | Plasma (N = 59) |
|---|---|---|
| No. (%) | No. (%) | |
| Cyanidin-3-glucoside | 54 (58.06) | 41 (69.49) |
| 3,4-Dihydroxyphenylacetic acid | 41 (44.09) | 29 (49.15) |
| 4-Hydroxybenzoic acid | 41 (44.09) | 30 (50.85) |
| Caffeic acid | 41 (44.09) | 30 (50.85) |
| Hippuric acid | 41 (44.09) | 32 (54.24) |
| 4-Hydroxyhippuric acid | 39 (41.94) | 27 (45.76) |
| Gallic acid | 41 (44.09) | 2 (3.39) |
| 2-Methylpyrogallol-O-sulfate | 39 (41.94) | 18 (30.51) |
| Dihydrocaffeic acid | 39 (41.94) | 18 (30.51) |
| Protocatechuic acid | 39 (41.94) | 12 (20.34) |
| Cyanidin-3-glucuronide | 25 (26.88) | 41 (69.49) |
| 4-Hydroxyphenylacetic acid | 32 (34.41) | 32 (54.24) |
| Ferulic acid | 29 (31.18) | 32 (54.24) |
| p-Coumaric acid | 14 (15.05) | 32 (54.24) |
| Syringic acid | 30 (32.26) | 30 (50.85) |
| Vanillic acid | 29 (31.18) | 30 (50.85) |
| Ferulic acid 4-O-glucuronide | 9 (9.68) | 27 (45.76) |
| Ferulic acid 4-sulfate | 30 (32.26) | 27 (45.76) |
| Isoferulic acid 3-O- β-D-glucuronide | 18 (19.35) | 27 (45.76) |
The following 10 phenolic compounds, in decreasing order, were detected most frequently in urine: C3G (58.06%); 3,4-dihydroxyphenylacetic acid (44.09%); 4-hydroxybenzoic acid (44.09%); caffeic acid (44.09%); gallic acid (44.09%); hippuric acid (44.09%); 2-methylpyrogallol-O-sulfate (41.94%); 4-hydroxyhippuric acid (41.94%); dihydrocaffeic acid (41.94%); and protocatechuic acid (41.94%).
In plasma, the following 15 phenolic compounds, in decreasing order, were detected in more than 40% of samples: C3G (69.49%); cyanidin-3-glucuronide (69.49%); 4-hydroxyphenylacetic acid (54.24%); ferulic acid (54.24%); hippuric acid (54.24%); p-coumaric acid (54.24%); 4-hydroxybenzoic acid (50.85%); caffeic acid (50.85%); syringic acid (50.85%); vanillic acid (50.85%); 3,4-dihydroxyphenylacetic acid (49.15%); 4-hydroxyhippuric acid (45.76%); ferulic acid 4-O-glucuronide (45.76%), ferulic acid 4-sulfate (45.76%); and isoferulic acid 3-O-β-D-glucuronide (45.76%).
The phenolic compounds C3G, 3,4-dihydroxyphenylacetic acid, 4-hydroxybenzoic acid, caffeic acid, hippuric acid, and 4-hydroxyhippuric acid were present in high frequencies in either plasma or urine samples. In particular, C3G was the phenolic compound most frequently detected in both urine (58.3%, n = 54 of 93 samples) and plasma (69.49%, n = 41 of 59 samples). Cyanidin-3-glucuronide was frequent in plasma (69.49%, n = 41 of 59 samples), but not in urine (26.88%, n = 25 of 93 samples). Complete information on the 19 most frequently detected phenolic compounds is shown in Table 3.
Correlation between phenolic compounds found most frequently
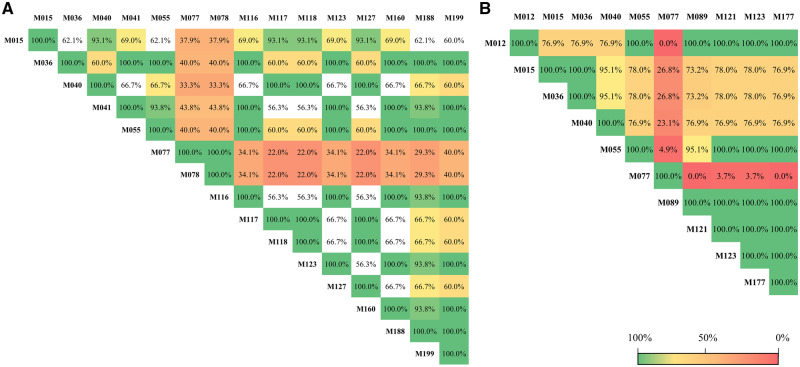
The 19 phenolic compounds found most frequently in this systematic review (Table 3) were used to create a correlation matrix to determine whether any correlations exist between the phenolic compounds. In plasma, 15 phenolic compounds were found to be significantly correlated (P < 0.05), whereas in urine, 10 phenolic compounds were correlated (P < 0.05). Cyanidin-3-glucoside was correlated with cyanidin-3-glucuronide in plasma (r = 1.00; P < 0.001), but not in urine. The correlation matrix of the most frequent metabolites found in plasma or in urine is presented in Figure 2.
Figure 2.
Correlation matrix of the most frequent metabolites found in plasma or urine. (A) Correlation between the significant metabolites found in plasma. (B) Correlation between the significant metabolites found in urine. All percentages of correlation values are statistically significant (P < 0.05) unless otherwise indicated; asterisks (*) indicate nonsignificant values. Metabolites: M012, 2-methylpyrogallol-O-sulfate; M015, 3,4-dihydroxyphenylacetic acid; M036, 4-hydroxybenzoic acid; M040, 4-hydroxyhippuric acid; M041, 4-hydroxyphenylacetic acid; M055, caffeic acid; M077, cyanidin-3-glucoside; M078, cyanidin-3-glucuronide; M089, dihydrocaffeic acid; M116, ferulic acid; M117, ferulic acid 4-O-glucuronide; M118, ferulic acid 4-sulfate; M121, gallic acid; M123, hippuric acid; M127, isoferulic acid 3-O-β-D-glucuronide; M160, p-coumaric acid; M177, protocatechuic acid; M188, syringic acid; M199, vanillic acid.
Assessment of a candidate biomarker of anthocyanin-rich berry intake
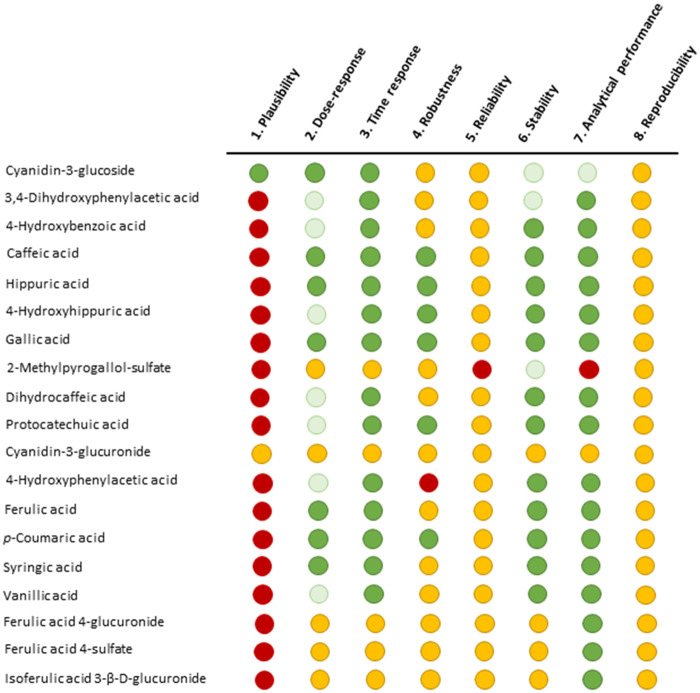
The 19 phenolic compounds detected most frequently in this systematic review (Table 3) were assessed as potential biomarkers of anthocyanin-rich berry intake, using the 8 criteria proposed by Dragsted et al43 (Figure 3). Cyanidin-3-glucoside fulfills the plausibility criterion, which is an essential criterion for any biomarker of food intake, as well as the dose-response, time response, stability, and analytical performance criteria. Cyanidin-3-glucuronide, a metabolite of C3G, also fulfills the plausibility criterion, but its presence in plasma and urine has scarcely been studied. The other 17 phenolic compounds do not fulfill the plausibility criteria and thus were excluded as candidate biomarkers of berry intake.
Figure 3.
Criteria for validating biomarkers of food intake, applied to molecules that appeared in more than 40% of samples. Dark green: yes; the criterion is fulfilled for at least some use of the biomarker; light green: partial yes; the criterion is fulfilled, but more information is needed for complete validation; yellow: undetermined; information is insufficient to validate the criterion; red: no; the criterion has been investigated but is not fulfilled.
Sensitivity and specificity analyses of C3G as a biomarker of anthocyanin-rich berry intake
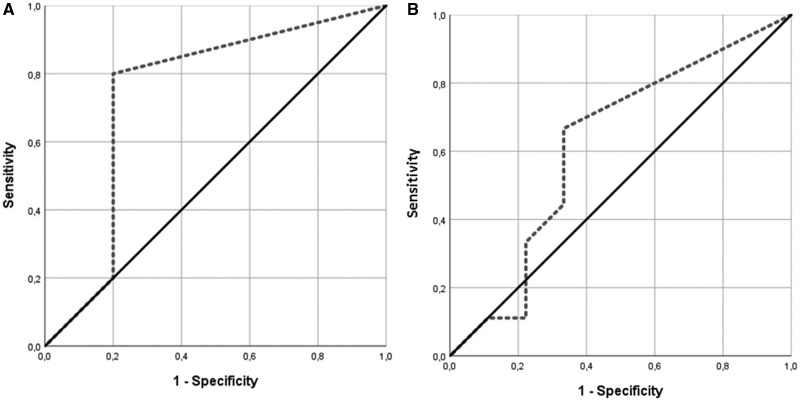
To test the sensitivity and specificity of C3G as a biomarker of berry intake, the AUC ROC of C3G in plasma and urine was calculated. The AUC was 74% (P = 0.210) in plasma and 61.7% (P = 0.402) in urine (Figure 4).
Figure 4.
Results of the receiver operating characteristic (ROC) analysis, performed to determine the sensitivity and specificity of C3G in (A) plasma (AUC 74%; P = 0.210) or (B) urine (AUC 61.7%; P = 0.402).
DISCUSSION
Of the 203 phenolic compounds identified in the present review, 2 anthocyanins are detected most frequently after consumption of anthocyanin-rich berries. Cyanidin-3-glucoside is the phenolic compound and anthocyanin detected most frequently in both urine and plasma, while cyanidin-3-glucuronide is the second most frequent anthocyanin in plasma, but not in urine.
As noted in the section “Assessment of a Candidate Biomarker of Anthocyanin-Rich Berry Intake” C3G is one of two phenolic compounds that fulfill the criterion of plausibility. In order for a molecule to be considered a biomarker of intake, it must meet the plausibility criterion.43 Since the other phenolic compounds did not fulfill the plausibility criterion, they were not considered viable candidates as biomarkers of anthocyanin-rich berry intake. Cyanidin-3-glucoside also met several other criteria. Consequently, each of the 8 Dragsted et al43 criteria is analyzed and discussed below, but only for C3G.
Criteria for validation of C3G as a biomarker of anthocyanin-rich berry intake
Plausibility
The plausibility of a specific molecule within a certain food or food group to become a biomarker requires consideration of different confounding factors, such as the molecule’s presence in other foods as well as whether the molecule is used as an additive or is generated endogenously from the metabolization of other compounds.43 As a result, plausibility demonstrates a causal relationship between the ingestion of an anthocyanin-rich berry and the presence of the anthocyanin in biological samples. According to Phenol-Explorer, C3G is found in the anthocyanin-rich berries consumed in the studies included in this review.47 The results show that C3G was present in 69.49% of the plasma samples and 58.06% of the urine samples obtained from participants after intake of anthocyanin-rich berries in clinical trials.
Cyanidin-3-glucoside is a relatively infrequent molecule in nature, present mostly in red- or blue-pigmented fruits such as berries but also in certain vegetables such as red cabbage and olives.47 Additionally, C3G has been found in several organs, such as the brain, liver, vascular endothelium, kidney, and lungs, in different animal models following ingestion of diverse anthocyanin-rich foods.58–60
Cyanidin-3-glucoside is not a derivative of other chemical substances commonly present in mammalian organs, such as 3,4-dihydroxyphenylacetic acid, a derivative of dopamine that can be found in animal brains,61–63 or ferulic acid, which can be obtained from the metabolization of caffeic acid and is also present in diverse foods such as some fermented alcoholic beverages,64 fruit,65 cereals,66 vegetables,67,68 vegetable oils,69 and seeds.67,68 Moreover, C3G is not used as an additive for the preservation of processed foods.70 Cyanidin-3-glucuronide, a metabolite of C3G, also fulfills the plausibility criterion, but information about its metabolization and presence in food is scarce.
Dose-response
The dose-response criterion refers to the relationship between the dose of anthocyanins ingested and the presence of anthocyanins in organs or tissues. The dose response depends on the bioavailability of C3G, taking into account the limits/levels of saturation and the saturation kinetics of C3G.43 After a single oral ingestion of 150 g of wild blueberry puree, the mean Cmax of C3G was 2.4 ± 0.2 nmol/L.53 After oral ingestion of 300 g of raspberries, the mean Cmax was 0.2 ± 0.1 nmol/L.51 After a single oral ingestion of 250 g of blackberries, the mean Cmax was 47 ± 8 ng/mL.57 An oral dose of 500 mg of 13C-labeled C3G resulted in a maximal C3G concentration of 141nM ± 70nM in human serum.71 Several aspects determine the bioavailability of C3G. First, C3G is chemically composed of a benzopyran core, which, in turn, is integrated by a phenolic ring, a pyran ring, and a benzoyl ring.72 Second, it has a molecular weight of 449.4 g/mol, a polar surface area of log P = 0.39, a partition coefficient of Å = 191, and known hydrophobic/hydrophilic characteristics.72 Third, it can undergo glycosylation and methylation, both of which affect its pharmacokinetics.73 For instance, a second glycosylation in the fifth carbon is known to increase the hydrophilic properties of C3G, thereby increasing its bioavailability, while an extra malonyl group makes it more hydrophobic, thereby decreasing its bioavailability.72,74,75 Fourth, C3G’s absorption saturation is observed around an ingested dose of 200nM, primarily because C3G binds and interacts with other molecules (eg, cellulose) while being absorbed.72 On the basis of these findings about the bioavailability of C3G, it seems likely that C3G meets the dose-response criterion to become a suitable biomarker of berry intake.
Time response
The time response criterion refers to the time required for the maximum C3G concentration to be reached in plasma and urine as well as to the range of time that C3G concentrations are measurable,43 which in turn is influenced by the absorption and elimination of C3G. When evaluating whether the time response criterion is met, the specific purpose of the biomarker should be considered. In the case of C3G, time-response kinetic parameters have been determined through the present systematic review. In human plasma, the mean (± SD) Tmax of C3G was 1.8 ± 0.3 hours after a single oral dose of 150 g of wild blueberry puree, and the AUC was 62.2 ± 10.3 nmol/h/L.53 The rapid absorption of C3G has also been confirmed by the following values in human plasma: Tmax of 66 ±15 minutes after ingestion of 250 g of blackberries,57 and Tmax of 1 hour after ingestion of 300 g of raspberries.51 In human serum, the Tmax of C3G was 1.8 ± 0.2 hours after a single oral dose of 500 mg of 13C-labeled C3G.71 In another study, C3G was found to have a minimum mean bioavailability of 12.38% ± 1.38% in humans.73
Several animal studies have reported different peak C3G levels, depending on the animal, the specimen, or the organs investigated.39 In mice plasma, the oral administration of C3G extract at 500 mg/kg resulted in an elimination rate constant of 0.43 h−1, and the intravenous administration of 1 mg/kg resulted in an elimination rate constant of 1.84 h−1,60 with the half-life of C3G being 1.6 hours after oral ingestion and 0.4 hour after intravenous administration.60 In addition, Marczylo et al60 reported AUC values of 25.1 nmol/h/mL after C3G oral intake and 3.01 nmol/h/mL after intravenous administration in mice. Moreover, the maximum peak concentration of C3G in the heart tissue of mice, 1.40 × 104 pmol/g, was reached 10 minutes after oral administration of C3G extract at a dosage of 500 mg/kg.39,60
In brain tissue of mice, the maximum peak concentration of 44.98 pmol/g was reached 15 seconds after intravenous administration of 668 nmol of C3G extract.39,59 In brain tissue of pigs, the concentration of C3G was 3.17 pmol/g after long-term (3 weeks) oral administration of bilberry extract at 82.5 mg/kg/d.39,76
Stability
The stability criterion refers to the best practices needed for collection and storage of biological specimens to avoid degradation of C3G, thereby permitting C3G concentrations to be measured in such specimens.43 Like other anthocyanins, C3G is unstable at high temperatures.77 For example, microwave heating with 300 W at high temperatures reduces the content of C3G in pomegranate juice.72 Similarly, longer exposures to lower baking temperatures (30 minutes at 140°C and 15 minutes at 240°C) also reduce the amount of C3G found in muffins enriched with raspberry and cranberry pomace powder.78 Storage temperature can significantly affect the stability of C3G in foods kept at 5°C, 20°C, and 35°C, even if phenolic acids, procyanidins, and flavone glycosides are added to protect the structure of C3G from degradation.79 The stability of C3G is also affected by pH.78 At low pH values (pH ≤ 4.0), C3G exists as a flavylium cation and, as pH rises, transforms into either its carbinol (pH = 5.2) or its quinoidal (pH = 5.5–6.0) form, whereas at higher pH values (pH ≥ 6.0), C3G reaches equilibrium and acquires a cis-chalcone conformation.80
Analytical performance
The analytical performance criterion for C3G refers to the reliability of its chemical analysis, ie, whether analytical variability, accuracy, sensitivity, and specificity are adequate.43 The minimum requirement for this criterion to be fulfilled by C3G is the ability to measure C3G at different concentrations, a so-called semiquantitative analysis.43 In the case of C3G, high-throughput analysis techniques such as HPLC allow identification and quantification of C3G in a wide range of different concentrations in human body fluids52,81,82 and in diverse animal organs and tissues.39,59,76,83
For instance, Czank et al73 demonstrated that 13C-marked C3G molecules could be identified and measured in human blood, urine, breath, feces, and plasma, with maximum concentrations in plasma ranging between 1.4 and 592 nmol/L.84 Moreover, the analytical performance of C3G has also been evaluated by liquid chromatography-tandem mass spectrometry (LC-MS/MS), which has a precision that ranges from 1.2% to 14.5% and an intraday and interday accuracy of 5.1% to 13.6% and 11.5% to 10.9%, respectively. As a consequence, LC-MS/MS was found to be a reproducible, reliable, and accurate method for measurement of C3G.85
In order for C3G to meet the criterion of analytical performance and be fully validated as a suitable biomarker, a stable isotope-labeled standard would need to be present in every sample.43 The gold standard of this isotope-labeled molecule for C3G has not been described to date.
Reproducibility, robustness, and reliability
Reproducibility is achieved only when the analysis of C3G detection has been performed identically in at least 2 different laboratories and evaluated by interlaboratory comparison tests. Such standardized analysis is not commonly performed and represents an area of uncertainty.
Another criterion not fulfilled by C3G is robustness, which evaluates the behavior of C3G in complex diets. Like most putative biomarkers, C3G has been identified in a limited number of well-designed, highly controlled intervention studies.60 To date, however, it has not been evaluated when ingested along with other nutrients as a part of a complex meal.
Lastly, the purpose of the reliability criterion is to compare C3G as a new biomarker against the current gold-standard methodology, HPLC, in a controlled setting with supervised food intake and then validate the results via direct comparison using methods such as Passing-Bablok regression.86 Since this has not yet been done, the reliability criterion has not yet been met.
C3G as a useful biomarker of anthocyanin-rich berry consumption
Of all the possible phenolic compounds that were found to be viable biomarkers of berry intake, C3G is the molecule found most frequently in plasma and urine. It therefore fulfills the key criterion of plausibility because its presence has been demonstrated in anthocyanin-rich berries,47 supporting C3G as a biomarker of anthocyanin-rich berry intake. Moreover, it also fulfills 4 additional criteria for use as a biomarker, ie, dose-response, time response, stability, and analytical performance.
Since C3G is a relatively rare anthocyanin in plants, and because it is not produced by the metabolization of other compounds, the presence of C3G in human body fluids after the consumption of food is caused directly by C3G ingestion. The positive predictive value of C3G as a biomarker of anthocyanin-rich berry intake is 74% in plasma and 61.7% in urine, although these values are not significant. Predictive values between 70% and 80% are considered acceptable,87 and thus a 74% predictive value of C3G in plasma is acceptable, suggesting that, in plasma, C3G could be a suitable biomarker of anthocyanin-rich berry intake, although further confirmatory studies are warranted.
C3G and human health
The ability to detect C3G might play an important role in human health, particularly since epidemiological studies have shown that the consumption of more than 2 cups of blueberries per week is associated with a significantly slower rate of lung function loss.88
Cyanidin-3-glucoside has also shown anticancer properties in cell and animal experiments in which it was isolated and administered intravenously at relatively high doses of 250nM and 500nM.60,89,90 Among the acknowledged activities of C3G, its roles in DNA protection,91 in reduction of body weight and amelioration of insulin resistance in mice with diet-induced obesity, in reduction of hepatic steatosis,92 and in activation of fatty acid metabolism93 are worth highlighting, along with its anti-inflammatory89 antimicrobial, and protective epigenetic effects in cancer and neurological diseases.72,93–95
Limitations
One of the main limitations of this systematic review is the design of the studies originally screened, which were nonrandomized clinical trials. Such studies often use untargeted analysis to assess the presence of a specific biomarker in a single anthocyanin-rich food, usually berries such as strawberries or blueberries, or other fruits like apples. Thus, authors commonly place special emphasis on the specific phenolic compound found in a single anthocyanin-rich food when reporting their results, while complete reports of the phenolic compounds found in all samples analyzed are usually not described.48–57 On the other hand, studies that used a targeted analytical approach were not included in the present review, and only a few studies met the inclusion criteria for use of an untargeted analytical approach in plasma and urine. The systematic review methodology for identifying biomarkers of food intake by untargeted analysis might not have detected or measured all compounds in a specimen, and therefore it is possible that another biomarker of anthocyanin-rich berry intake might exist. However, at this time, and with the information available, C3G remains the best candidate as a biomarker of anthocyanin-rich berry intake. Moreover, although C3G is a good biomarker of berry intake, it might also be suitable as a biomarker for intake of other foods that contain C3G, but this requires further study.
Another important limitation of the present review is the variation in the sample size of the clinical trials included. To account for the small sample size of some studies, a qualitative meta-analysis of the data was performed. As a result, all studies of different sizes contributed equally to a pool of information, which was further analyzed to reach conclusions.
Another objective of the present review was to identify an anthocyanin biomarker in different human body fluids. Thus far, however, there is insufficient information about the presence of anthocyanins in body fluids other than plasma and urine, such as saliva, which can be collected easily and noninvasively to obtain biological information.
The methodological heterogeneity of anthocyanin identification and quantification in human plasma and urine also represents a critical limitation of this qualitative review. As result, quantitative data could not be analyzed because of differences in the doses used for experimentation, the methods used for quantification, and the sources of anthocyanins administered.
The dose of anthocyanin-rich berries used in most of the included studies varied widely, from 12 g/d to 300 g/d orally, but the complete chemical composition of the ingested berries was not described. Furthermore, the time between administration of the dose and collection of body fluid samples also presented an important variable.
CONCLUSION
Up to 203 different phenolic compounds have been reported in plasma and urine samples from healthy humans. The phenolic compound identified most frequently after consumption of anthocyanin-rich berries is C3G, found in 69.49% of plasma samples and in 58.06% of urine samples. When the process proposed here for the selection of a biomarker candidate is applied, C3G meets the critically important plausibility criterion, as well as the dose-response, time response, stability, and analytical performance criteria. In addition, it has an acceptable positive predictive value of 74% in plasma. Thus, C3G is a promising biomarker of anthocyanin-rich berry consumption in plasma and urine samples of healthy humans.
Supplementary Material
Acknowledgments
Author contributions. B.A.S-R., Ú.C., and R.S. were responsible for study conception and design. B.A.S-R. and Ú.C. acquired the data. B.A.S-R., Ú.C., R.S., and S.F-C. analyzed and interpreted the data. B.A.S-R. and Ú.C. drafted the manuscript. Ú.C., R.S., S.F-C., A.P., and E.L. performed critical revision of the manuscript.
Funding/support. The AppleCOR Project (subproject AGL2016-76943-C2-2-R and subproject AGL2016-76943-C2-1-R) was made possible with the support of the Ministerio de Economía, Indústria y Competitividad, the Agencia Estatal de Investigación, and the European Regional Development Fund. The NFOC-Salut group is a consolidated research group of the Generalitat de Catalunya, Spain (reference no. 2017 SGR 522). The role of the funders was limited to an economic contribution through a competitive call. The funders had no role in the conception, design, performance, or approval of the work.
B.A.S-R. has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement (no. 713679) as well a predoctoral grant from the Universitat Rovira i Virgili and the Fundació Catalunya-La Pedrera (reference no. 2017 MFP-COFUND-30). Ú.C. has received a Pla Estratègic de Recerca i Innovació en Salut (PERIS) postdoctoral grant (no. SLT002/16/00239; Catalunya, Spain) from the Generalitat de Catalunya. A.P. has a Torres Quevedo postdoctoral grant with the Subprograma Estatal de Incorporación, Plan Estatal de Investigación Científica Técnica y de Innovación.
Declaration of interest. The authors have no relevant interests to declare.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Appendix S1 AMSTAR 2 results
Table S1 PRISMA checklist
Table S2 Risk-of-bias assessment of studies screened by means of the MINORS methodology
Table S3 Phenolic compounds detected in urine and plasma in the studies included for analysis
References
- 1. Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4:384S–392S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Romieu I, Dossus L, Barquera S, et al. Energy balance and obesity: what are the main drivers? Cancer Causes Control. 2017;28:247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Volpe R, Sotis G, Gavita R, et al. Healthy diet to prevent cardiovascular diseases and osteoporosis: the experience of the “ProSa”. High Blood Press Cardiovasc Prev. 2012;19:65–71. [DOI] [PubMed] [Google Scholar]
- 4. Priano SM, Hong OS, Chen JL.. Lifestyles and health-related outcomes of U.S. hospital nurses: a systematic review. Nurs Outlook. 2018;66:66–76. [DOI] [PubMed] [Google Scholar]
- 5. Ventura Dde A, Fonseca Vde M, Ramos EG, et al. Association between quality of the diet and cardiometabolic risk factors in postmenopausal women. Nutr J. 2014;13:121.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andersen JR, Søgnen E, Natvig GK.. Diet quality in 116 Norwegian men and women with coronary heart disease. Eur J Cardiovasc Nurs. 2006;5:244–250. [DOI] [PubMed] [Google Scholar]
- 7. Guasch-Ferré M, Merino J, Sun Q, et al. Dietary polyphenols, Mediterranean diet, prediabetes, and type 2 diabetes: a narrative review of the evidence. Oxid Med Cell Longev. 2017;2017:6723931.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi:10.1056/NEJMoa1800389 [DOI] [PubMed] [Google Scholar]
- 9. Georgoulis M, Kontogianni MD, Yiannakouris N.. Mediterranean diet and diabetes: prevention and treatment. Nutrients. 2014;6:1406–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y, Pan A, Wang DD, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. 2018;138:345–355. doi:10.1161/CIRCULATIONAHA.117.032047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang PY. Polyphenols in health and disease. Cell Biochem Biophys. 2015;73:649–664. [DOI] [PubMed] [Google Scholar]
- 12. Khurana S, Venkataraman K, Hollingsworth A, et al. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients. 2013;5:3779–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao D, Simon JE, Wu Q.. A critical review on grape polyphenols for neuroprotection: strategies to enhance bioefficacy. Crit Rev Food Sci Nutr. 2019;1–29. [DOI] [PubMed] [Google Scholar]
- 14. Lutz M, Fuentes E, Ávila F, et al. Roles of phenolic compounds in the reduction of risk factors of cardiovascular diseases. Molecules. 2019;24:366.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reis JF, Monteiro VVS, de Souza Gomes R, et al. Action mechanism and cardiovascular effect of anthocyanins: a systematic review of animal and human studies. J Transl Med. 2016;14:315.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee YM, Yoon Y, Yoon H, et al. Dietary anthocyanins against obesity and inflammation. Nutrients. 2017;9:1089.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo H, Ling W.. The update of anthocyanins on obesity and type 2 diabetes: experimental evidence and clinical perspectives. Rev Endocr Metab Disord. 2015;16:1–13. [DOI] [PubMed] [Google Scholar]
- 18. Hribar U, Ulrih N.. The metabolism of anthocyanins. Curr Drug Metab. 2014;15:3–13. [DOI] [PubMed] [Google Scholar]
- 19. Belwal T, Nabavi SF, Nabavi SM, et al. Dietary anthocyanins and insulin resistance: when food becomes a medicine. Nutrients. 2017;9:1111.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cassidy A, Bertoia M, Chiuve S, et al. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am J Clin Nutr. 2016;104:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pal HC, Sharma S, Strickland LR, et al. Delphinidin reduces cell proliferation and induces apoptosis of non-small-cell lung cancer cells by targeting EGFR/VEGFR2 signaling pathways. PLoS One. 2013;8:e77270.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu JN, Panchanathan R, Lee WS, et al. Anthocyanins from the fruit of Vitis Coignetiae Pulliat inhibit TNF-Augmented cancer proliferation, migration, and invasion in A549 cells. Asian Pacific J Cancer Prev. 2017;18:2919–2923. doi: 10.22034/APJCP.2017.18.11.2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ko H, Jeong M-H, Jeon H, et al. Delphinidin sensitizes prostate cancer cells to TRAIL-induced apoptosis, by inducing DR5 and causing caspase-mediated HDAC3 cleavage. Oncotarget. 2015;6:9970–9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ho ML, Chen PN, Chu SC, et al. Peonidin 3-glucoside inhibits lung cancer metastasis by downregulation of proteinases activities and MAPK pathway. Nutr Cancer. 2010;62:505–516. [DOI] [PubMed] [Google Scholar]
- 25. Kim MH, Jeong YJ, Cho HJ, et al. Delphinidin inhibits angiogenesis through the suppression of HIF-1α and VEGF expression in A549 lung cancer cells. Oncol Rep. 2017;37:777–784. [DOI] [PubMed] [Google Scholar]
- 26. Kausar H, Jeyabalan J, Aqil F, et al. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012;325:54–62. [DOI] [PubMed] [Google Scholar]
- 27. Isaak CK, Wang P, Prashar S, et al. Supplementing diet with Manitoba lingonberry juice reduces kidney ischemia-reperfusion injury. J Sci Food Agric. 2017;97:3065–3076. [DOI] [PubMed] [Google Scholar]
- 28. Wang S, Li B, Li C, et al. Potential renoprotective agents through inhibiting CTGF/CCN2 in diabetic nephropathy. J Diabetes Res. 2015;2015:962383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kang MK, Lim SS, Lee JY, et al. Anthocyanin-rich purple corn extract inhibit diabetes-associated glomerular angiogenesis. PLoS One. 2013;8:e79823.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stefanovic V, Savic V, Vlahovic P, et al. Reversal of experimental myoglobinuric acute renal failure with bioflavonoids from seeds of grape. Ren Fail. 2000;22:255–266. [DOI] [PubMed] [Google Scholar]
- 31. Thummayot S, Tocharus C, Suksamrarn A, et al. Neuroprotective effects of cyanidin against Aβ-induced oxidative and ER stress in SK-N-SH cells. Neurochem Int. 2016;101:15–21. [DOI] [PubMed] [Google Scholar]
- 32. Subash S, Essa MM, Al-Adawi S, et al. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regen Res. 2014;9:1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Airoldi C, La Ferla B, D’Orazio G, et al. Flavonoids in the treatment of Alzheimer’s and other neurodegenerative diseases. Curr Med Chem. 2018;25:3228–3246. [DOI] [PubMed] [Google Scholar]
- 34. Ali T, Kim MJ, Rehman SU, et al. Anthocyanin-loaded PEG-gold nanoparticles enhanced the neuroprotection of anthocyanins in an Aβ1–42 mouse model of Alzheimer’s disease. Mol Neurobiol. 2017;54:6490–6506. [DOI] [PubMed] [Google Scholar]
- 35. Ali T, Kim T, Rehman SU, et al. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer’s disease. Mol Neurobiol. 2018;55:6076–6093. [DOI] [PubMed] [Google Scholar]
- 36. Khoo HE, Azlan A, Tang ST, et al. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61:1361779.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bars-Cortina D, Macià A, Iglesias I, et al. Phytochemical profiles of new red-fleshed apple varieties compared with traditional and new white-fleshed varieties. J Agric Food Chem. 2017;65:1684–1696. [DOI] [PubMed] [Google Scholar]
- 38. Delgado-Vargas F, Jiménez AR, Paredes-López O, et al. Natural pigments: carotenoids, anthocyanins, and betalains—characteristics, biosynthesis, processing, and stability. Crit Rev Food Sci Nutr. 2000;40:173–289. [DOI] [PubMed] [Google Scholar]
- 39. Sandoval-Ramírez BA, Catalán Ú, Fernández-Castillejo S, et al. Anthocyanin tissue bioavailability in animals: possible implications for human health: a systematic review. J Agric Food Chem. 2018;66:11531–11543. [DOI] [PubMed] [Google Scholar]
- 40. Lang R, Lang T, Bader M, et al. High-throughput quantitation of proline betaine in foods and suitability as a valid biomarker for citrus consumption. J Agric Food Chem. 2017;65:1613–1619. [DOI] [PubMed] [Google Scholar]
- 41. Sun T, Rong Y, Hu X, et al. Plasma alkylresorcinol metabolite, a biomarker of whole-grain wheat and rye intake, and risk of type 2 diabetes and impaired glucose regulation in a Chinese population. Diabetes Care. 2018;41:440–445. [DOI] [PubMed] [Google Scholar]
- 42. Praticò G, Gao Q, Scalbert A, et al. Guidelines for Food Intake Biomarker Reviews (FIBRev): how to conduct an extensive literature search for food intake biomarker discovery. Genes Nutr. 2018;13:3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dragsted LO, Gao Q, Scalbert A, et al. Validation of biomarkers of food intake—critical assessment of candidate biomarkers. Genes Nutr. 2018;13:14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 45. Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. [DOI] [PubMed] [Google Scholar]
- 46. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neveu V, Perez-Jimenez J, Vos F, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010;2010:bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang X, Sandhu A, Edirisinghe I, et al. An exploratory study of red raspberry (Rubus idaeus L.) (poly)phenols/metabolites in human biological samples. Food Funct. 2018;9:806–818. [DOI] [PubMed] [Google Scholar]
- 49. Feliciano RP, Istas G, Heiss C, et al. Plasma and urinary phenolic profiles after acute and repetitive intake of wild blueberry. Molecules. 2016;21:1120–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tian Q, Giusti MM, Stoner GD, et al. Urinary excretion of black raspberry (Rubus occidentalis) anthocyanins and their metabolites. J Agric Food Chem. 2006;54:1467–1472. [DOI] [PubMed] [Google Scholar]
- 51. Ludwig IA, Mena P, Calani L, et al. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic Biol Med. 2015;89:758–769. [DOI] [PubMed] [Google Scholar]
- 52. Sasot G, Martínez-Huélamo M, Vallverdú-Queralt A, et al. Identification of phenolic metabolites in human urine after the intake of a functional food made from grape extract by a high resolution LTQ-Orbitrap-MS approach. Food Res Int. 2017;100(pt 3):435–444. [DOI] [PubMed] [Google Scholar]
- 53. Zhong S, Sandhu A, Edirisinghe I, et al. Characterization of wild blueberry polyphenols bioavailability and kinetic profile in plasma over 24-h period in human subjects. Mol Nutr Food Res. 2017;61:1700405. doi:10.1002/mnfr.201700405 [DOI] [PubMed] [Google Scholar]
- 54. Pimpão RC, Dew T, Figueira ME, et al. Urinary metabolite profiling identifies novel colonic metabolites and conjugates of phenolics in healthy volunteers. Mol Nutr Food Res. 2014;58:1414–1425. [DOI] [PubMed] [Google Scholar]
- 55. Felgines C, Talavera S, Texier O, et al. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans. J Agric Food Chem. 2005;53:7721–7727. [DOI] [PubMed] [Google Scholar]
- 56. Wu X, Cao G, Prior RL.. Absorption and metabolism of anthocyanins in elderly women after consumption of elderberry or blueberry. J Nutr. 2002;132:1865–1871. [DOI] [PubMed] [Google Scholar]
- 57. Marques C, Fernandes I, Norberto S, et al. Pharmacokinetics of blackberry anthocyanins consumed with or without ethanol: a randomized and crossover trial. Mol Nutr Food Res. 2016;60:2319–2330. [DOI] [PubMed] [Google Scholar]
- 58. Ziberna L, Tramer F, Moze S, et al. Transport and bioactivity of cyanidin 3-glucoside into the vascular endothelium. Free Radic Biol Med. 2012;52:1750–1759. [DOI] [PubMed] [Google Scholar]
- 59. Fornasaro S, Ziberna L, Gasperotti M, et al. Determination of cyanidin 3-glucoside in rat brain, liver and kidneys by UPLC/MS-MS and its application to a short-term pharmacokinetic study. Sci Rep. 2016;6:22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marczylo TH, Cooke D, Brown K, et al. Pharmacokinetics and metabolism of the putative cancer chemopreventive agent cyanidin-3-glucoside in mice. Cancer Chemother Pharmacol. 2009;64:1261–1268. [DOI] [PubMed] [Google Scholar]
- 61. Kalita J, Kumar S, Vijaykumar K, et al. A study of CSF catecholamine and its metabolites in acute and convalescent period of encephalitis. J Neurol Sci. 2007;252:62–66. [DOI] [PubMed] [Google Scholar]
- 62. Okabe N, Kyoyama H.. 3,4-Dihydroxyphenylacetic acid. Acta Crystallogr E Crystallogr Commun. 2001;57:o715–o716. [Google Scholar]
- 63. Raskind MA, Peskind ER, Holmes C, et al. Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer’s disease. Biol Psychiatry. 1999;46:756–765. [DOI] [PubMed] [Google Scholar]
- 64. Jandera P, Škeříková V, Řehová L, et al. RP-HPLC analysis of phenolic compounds and flavonoids in beverages and plant extracts using a CoulArray detector. J Sep Sci. 2005;28:1005–1022. [DOI] [PubMed] [Google Scholar]
- 65. Zuo Y, Wang C, Zhan J.. Separation, characterization, and quantitation of benzoic and phenolic antioxidants in American cranberry fruit by GC-MS. J Agric Food Chem. 2002;50:3789–3794. [DOI] [PubMed] [Google Scholar]
- 66. Flander L, Rouau X, Morel MH, et al. Effects of laccase and xylanase on the chemical and rheological properties of oat and wheat doughs. J Agric Food Chem. 2008;56:5732–5742. [DOI] [PubMed] [Google Scholar]
- 67. Periago MJ, Martínez-Valverde I, Chesson A, et al. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J Sci Food Agric. 2002;82:323–330. [Google Scholar]
- 68. Haghi G, Hatami A.. Simultaneous quantification of flavonoids and phenolic acids in plant materials by a newly developed isocratic high-performance liquid chromatography approach. J Agric Food Chem. 2010;58:10812–10816. [DOI] [PubMed] [Google Scholar]
- 69. Kalogeropoulos N, Mylona A, Chiou A, et al. Retention and distribution of natural antioxidants (α-tocopherol, polyphenols and terpenic acids) after shallow frying of vegetables in virgin olive oil. LWT Food Sci Technol. 2007;40:1008–1017. [Google Scholar]
- 70.Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants. Thirty-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland: World Health Organization; 1991. WHO Technical Report Series 801. [PubMed] [Google Scholar]
- 71. De Ferrars RM, Czank C, Zhang Q, et al. The pharmacokinetics of anthocyanins and their metabolites in humans. Br J Pharmacol. 2014;171:3268–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Olivas-Aguirre FJ, Rodrigo-García J, Martínez-Ruiz NdR, et al. Cyanidin-3-O-glucoside: physical-chemistry, foodomics and health effects. Molecules. 2016;21:1264.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Czank C, Cassidy A, Zhang Q, et al. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a 13C-tracer study. Am J Clin Nutr. 2013;97:995–1003. [DOI] [PubMed] [Google Scholar]
- 74. Felgines C, Krisa S, Mauray A, et al. Radiolabelled cyanidin 3-O-glucoside is poorly absorbed in the mouse. Br J Nutr. 2010;103:1738–1745. [DOI] [PubMed] [Google Scholar]
- 75. Miyazawa T, Nakagawa K, Kudo M, et al. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3- glucoside and cyanidin-3,5-diglucoside, into rats and humans. J Agric Food Chem. 1999;47:1083–1091. [DOI] [PubMed] [Google Scholar]
- 76. Chen TY, Kritchevsky J, Hargett K, et al. Plasma bioavailability and regional brain distribution of polyphenols from apple/grape seed and bilberry extracts in a young swine model. Mol Nutr Food Res. 2015;59:2432–2447. [DOI] [PubMed] [Google Scholar]
- 77. Tachibana N, Kimura Y, Ohno T.. Examination of molecular mechanism for the enhanced thermal stability of anthocyanins by metal cations and polysaccharides. Food Chem. 2014;143:452–458. [DOI] [PubMed] [Google Scholar]
- 78. Mildner-Szkudlarz S, Bajerska J, Górnaś P, et al. Physical and bioactive properties of muffins enriched with raspberry and cranberry pomace powder: a promising application of fruit by-products rich in biocompounds. Plant Foods Hum Nutr. 2016;71:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pacheco-Palencia LA, Talcott ST.. Chemical stability of açai fruit (Euterpe oleracea Mart.) anthocyanins as influenced by naturally occurring and externally added polyphenolic cofactors in model systems. Food Chem. 2010;118:17–25. [Google Scholar]
- 80. Janeiro P, Brett A.. Redox behavior of anthocyanins present in Vitis vinifera L. Electroanalysis. 2007;19:1779–1786. [Google Scholar]
- 81. Chen TY, Ferruzzi MG, Wu QL, et al. Influence of diabetes on plasma pharmacokinetics and brain bioavailability of grape polyphenols and their phase II metabolites in the Zucker diabetic fatty rat. Mol Nutr Food Res. 2017;61:1700111. doi:10.1002/mnfr.201700111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pimpão RC, Ventura MR, Ferreira RB, et al. Phenolic sulfates as new and highly abundant metabolites in human plasma after ingestion of a mixed berry fruit purée. Br J Nutr. 2015;113:454–463. [DOI] [PubMed] [Google Scholar]
- 83. Ichiyanagi T, Shida Y, Rahman MM, et al. Bioavailability and tissue distribution of anthocyanins in bilberry (Vaccinium myrtillus L.) extract in rats. J Agric Food Chem. 2006;54:6578–6587. [DOI] [PubMed] [Google Scholar]
- 84. Kay CD. Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutr Res Rev. 2006;19:137–146. [DOI] [PubMed] [Google Scholar]
- 85. Yang C, Wang Q, Yang S, et al. An LC–MS/MS method for quantitation of cyanidin-3-O-glucoside in rat plasma: application to a comparative pharmacokinetic study in normal and streptozotocin-induced diabetic rats. Biomed Chromatogr. 2018;32:e4042. doi: 10.1002/bmc.4042 [DOI] [PubMed] [Google Scholar]
- 86. Bland JM, Altman DG.. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–310. [PubMed] [Google Scholar]
- 87. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315–1316. [DOI] [PubMed] [Google Scholar]
- 88. Mehta AJ, Cassidy A, Litonjua AA, et al. Dietary anthocyanin intake and age-related decline in lung function: longitudinal findings from the VA Normative Aging Study. Am J Clin Nutr. 2016;103:542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pratheeshkumar P, Son YO, Wang X, et al. Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-κB signaling pathways in SKH-1 hairless mice skin. Toxicol Appl Pharmacol. 2014;280:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Petroni K, Trinei M, Fornari M, et al. Dietary cyanidin 3-glucoside from purple corn ameliorates doxorubicin-induced cardiotoxicity in mice. Nutr Metab Cardiovasc Dis. 2017;27:462–469. [DOI] [PubMed] [Google Scholar]
- 91. Acquaviva R, Russo A, Galvano F, et al. Cyanidin and cyanidin 3-O-β-D-glucoside as DNA cleavage protectors and antioxidants. Cell Biol Toxicol. 2003;19:243–252. doi: 10.1023/b:cbto.0000003974.27349.4e [DOI] [PubMed] [Google Scholar]
- 92. Guo H, Xia M, Zou T, et al. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J Nutr Biochem. 2012;23:349–360. [DOI] [PubMed] [Google Scholar]
- 93. Guo H, Guo J, Jiang X, et al. Cyanidin-3-O-β-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food Chem Toxicol. 2012;50:3040–3047. [DOI] [PubMed] [Google Scholar]
- 94. Pandey KB, Rizvi SI.. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Min J, Yu SW, Baek SH, et al. Neuroprotective effect of cyanidin-3-O-glucoside anthocyanin in mice with focal cerebral ischemia. Neurosci Lett. 2011;500:157–161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.