Abstract
We report the case of a patient with subarachnoid hemorrhage and three aneurysms arising from the posterior communicating artery (Pcomm)-P1 complex, treated with endovascular coiling and competitive flow diversion. The largest and likely ruptured Pcomm aneurysm was treated with traditional coiling. Two smaller potentially ruptured aneurysms arose from the distal right posterior cerebral artery (PCA) P1 segment. After a failed attempt to treat with conventional flow diversion across the PCA-P1 segment, the P1 aneurysms were successfully treated with competitive flow diversion distal to the PCA-P1 segment from Pcomm to the P2 segment. Over 12 months, competitive flow diversion redirected flow to the right PCA territory via the internal carotid artery-Pcomm-P2, reducing the size of the PCA-P1 segment and obliterating the P1 aneurysms. Competitive flow diversion treatment should be considered for aneurysms occurring at the circle of Willis when traditional methods are not feasible. Herein, we introduce a novel classification for competitive flow diversion treatment.
Keywords: aneurysm, flow diverter, subarachnoid, technique, blood flow
Background
Flow diversion stenting (FDS) is a treatment option for unruptured and ruptured intracranial aneurysms.1 2 Traditional FDS across the ostium of the aneurysm excludes it from the intracranial circulation. Competitive flow diversion is a novel application of FDS, redirecting flow into a normal artery proximal to the aneurysmal parent artery. This reduces flow in the parent artery and causes gradual occlusion of the aneurysm; the risk of stroke is reduced by leptomeningeal collaterals.3 4 We used competitive flow diversion to redirect flow into a normal artery distal to the aneurysm, reducing the contribution of the parent artery to a shared downstream territory.
Case presentation
A 55-year-old woman presented with a World Federation of Neurosurgeons grade 5, Fisher grade 4 subarachnoid hemorrhage (SAH) (figure 1A) and Glasgow Coma Scale (GCS) score of 3. She required cardiopulmonary resuscitation for acute cardiac failure. On transfer to our tertiary care center her clinical examination had improved to GCS 9T.
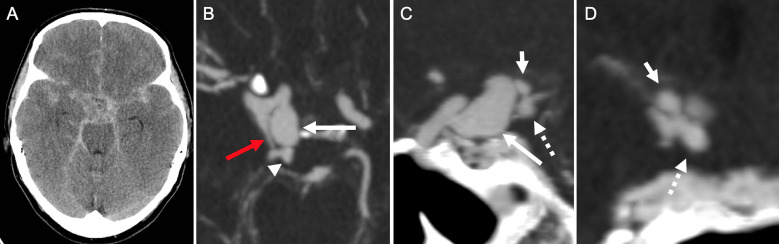
Figure 1.
Non-contrast CT (A) and CT angiography maximum intensity projection in axial (B), sagittal (C), and coronal (D) planes demonstrating diffuse aneurysmal subarachnoid hemorrhage likely associated with a ruptured large 11 mm posteriorly oriented right supraclinoid internal carotid artery aneurysm (long white arrows). Note a prominent right posterior communicating artery (red arrow) arising distal to the right supraclinoid aneurysm. There are additional smaller distal posterior cerebral artery (PCA)-P1 segment aneurysms arising from the distal PCA-P1 segment (arrowhead), one superiorly oriented measuring 3 mm (short arrow) and one inferiorly oriented measuring 4 mm (dotted arrow).
Investigations
CT angiography demonstrated a saccular aneurysm arising at the origin of the posterior communicating artery (Pcomm) measuring 11 mm, as well as two saccular aneurysms arising from the proximal P1/P2 posterior cerebral artery (PCA) junction measuring 3 mm and 4 mm, respectively (figures 1 and 2).
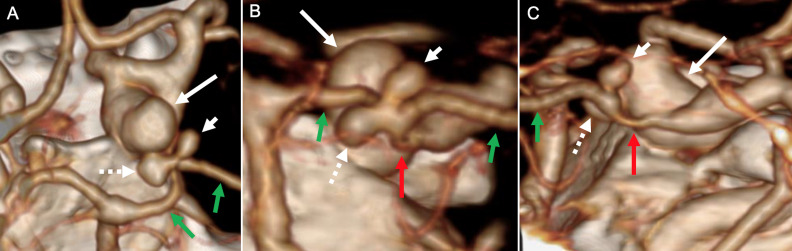
Figure 2.
CT angiography three-dimensional surface-rendered reconstructions in axial (A), coronal oblique (B), and sagittal (C) planes show the large right supraclinoid internal carotid artery ruptured aneurysm (long white arrow) and smaller adjacent posterior cerebral artery (PCA)-P1 segment aneurysms oriented superiorly (short white arrow) and inferomedial (dotted white arrow). The right PCA-P1 and P2 segments are shown by the green arrows and a right dysplastic posterior communicating artery is identified by the red arrows.
Treatment
The patient’s poor cardiac function permitted only a short anesthetic. The 11 mm Pcomm aneurysm was deemed to be the most likely source of SAH and was treated with coil embolization. Seven days post-SAH the patient’s neurological (GCS 15) and cardiac function continued to improve.
The decision was made to secure the smaller P1 aneurysms. The initial treatment plan was to perform conventional FDS from the P1 to P2 segment, across the necks of both P1 aneurysms. This was preferred to coil embolization due to poor working views of the distal P1 segment from the large Pcomm coil mass.
The patient was preloaded with aspirin and clopidogrel and systemically heparinized during the procedure. A Cook 6Fr Shuttle/5Fr Navien system was used to access the left vertebral artery. Several attempts were made to catheterize the right P2 with Phenom 027 and Headway 17 microcatheters, using 0.010 inch and 0.014 inch microwires alone and in tandem. However, it was not possible to advance any microwire far enough into the P2 to track a microcatheter across the sharply angled P1/P2 junction (figure 3A).
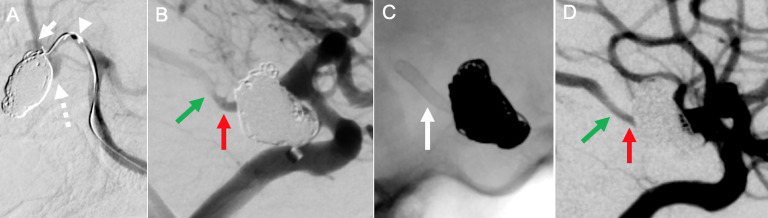
Figure 3.
Digital subtraction angiography in working projection (A) shows superiorly and inferiorly projecting posterior cerebral artery (PCA)-P1 segment aneurysms (arrow and dotted arrow, respectively) adjacent to the coiled ruptured right supraclinoid internal carotid artery (ICA) aneurysm. There was significant difficulty in selecting the right PCA-P2 segment from the right P1 segment with a variety of microwires and microcatheters (arrowhead), and this approach was abandoned in favour of a competitive flow diversion treatment approach. Right ICA angiogram (B) shows the right posterior communicating artery (Pcomm, red arrow) and PCA-P2 segment with washout flow from the right PCA-P1 segment (green arrow). A Silk Baby Vista stent was deployed across from the right Pcomm to the right PCA-P2 segment (C, unsubtracted fluoroscopy, white arrow). Angiogram after stent deployment shows patent flow across the stent with diminished washout flow compared with prior to stent deployment due to the flow diversion effect (D).
A novel form of competitive flow diversion with stent placement from the right Pcomm to the right P2 segment was then considered. This was preferred to traditional stenting of right Pcomm-P2 and coil occlusion of distal P1 due to poor working views of the distal P1 segment from the large Pcomm coil mass and the risk of compromising distal P1 perforators. The Shuttle sheath and support catheter were repositioned in the right ICA. A Silk Vista Baby flow diverting stent (2.75×19 mm) was delivered from the right Pcomm into the right P2 segment with a Headway 17 microcatheter. Angiography demonstrated good wall apposition without stenosis and increased flow through the Pcomm.
Outcome and follow-up
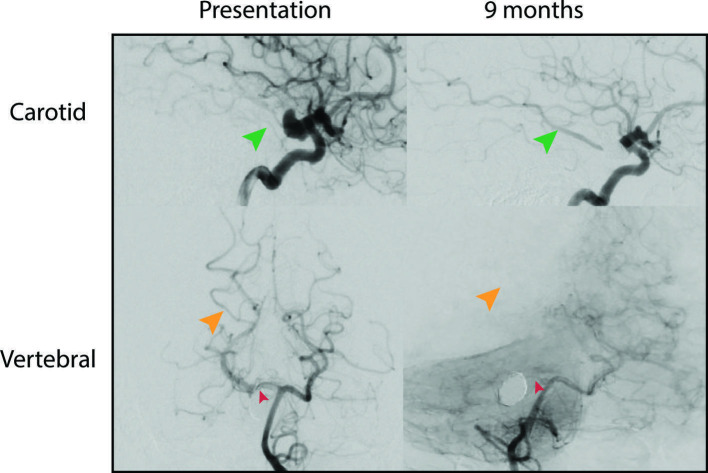
The patient awoke with mild left-sided weakness due to a small thalamic infarct. At 6 months she was neurologically intact and had resumed all normal activities. MR angiography (MRA) on postoperative day 1 demonstrated continued filling of the P1 aneurysms (figure 4A, D). MRA at 3 months demonstrated diminished flow in the right P1 segment and increased flow across the stented Pcomm-P2 segment. One P1 aneurysm was obliterated and the other was diminished in size (figure 4B). A catheter angiogram at 9 months showed similar findings with increased flow from right ICA-Pcomm-P2 and diminised flow from right P1-P2 (figures 4E, F–6). MRA at 12 months showed a further decrease in P1 caliber, increased filling of the P2 segment via the stented Pcomm, and both P1 aneurysms were completely occluded (figure 4C).
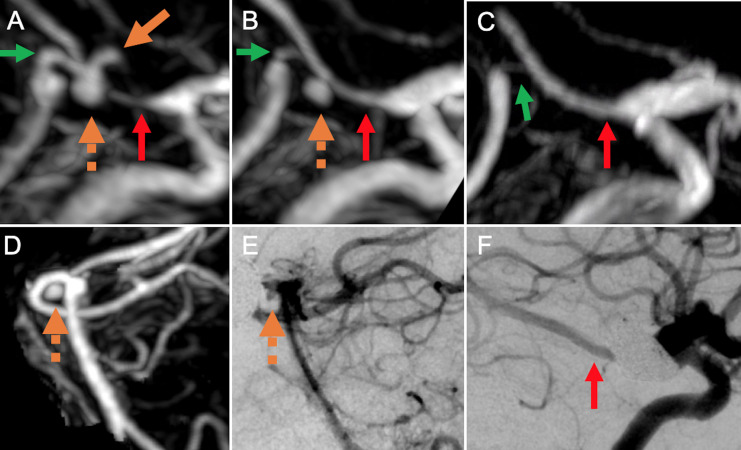
Figure 4.
Contrast-enhanced MR angiography maximum intensity projection images obtained 1 day (A, D), 3 months (B), and 12 months (C) after deployment of flow-diverting stent across from the right posterior communicating artery (Pcomm) to the right posterior cerebral artery (PCA)-P2 segment showing occlusion of a superiorly oriented P1 segment aneurysm at 3 months (orange solid arrow) and progressive occlusion of an inferiorly oriented P1 segment aneurysm (orange dotted arrow) at 12 month follow-up. Note the progressive increase in size of the right Pcomm and PCA-P2 junction (red arrows) and decrease in size of the right PCA-P1 segment (green arrows). Catheter angiogram obtained at 9 months (E, F) after stent deployment shows similar findings.
Figure 5.
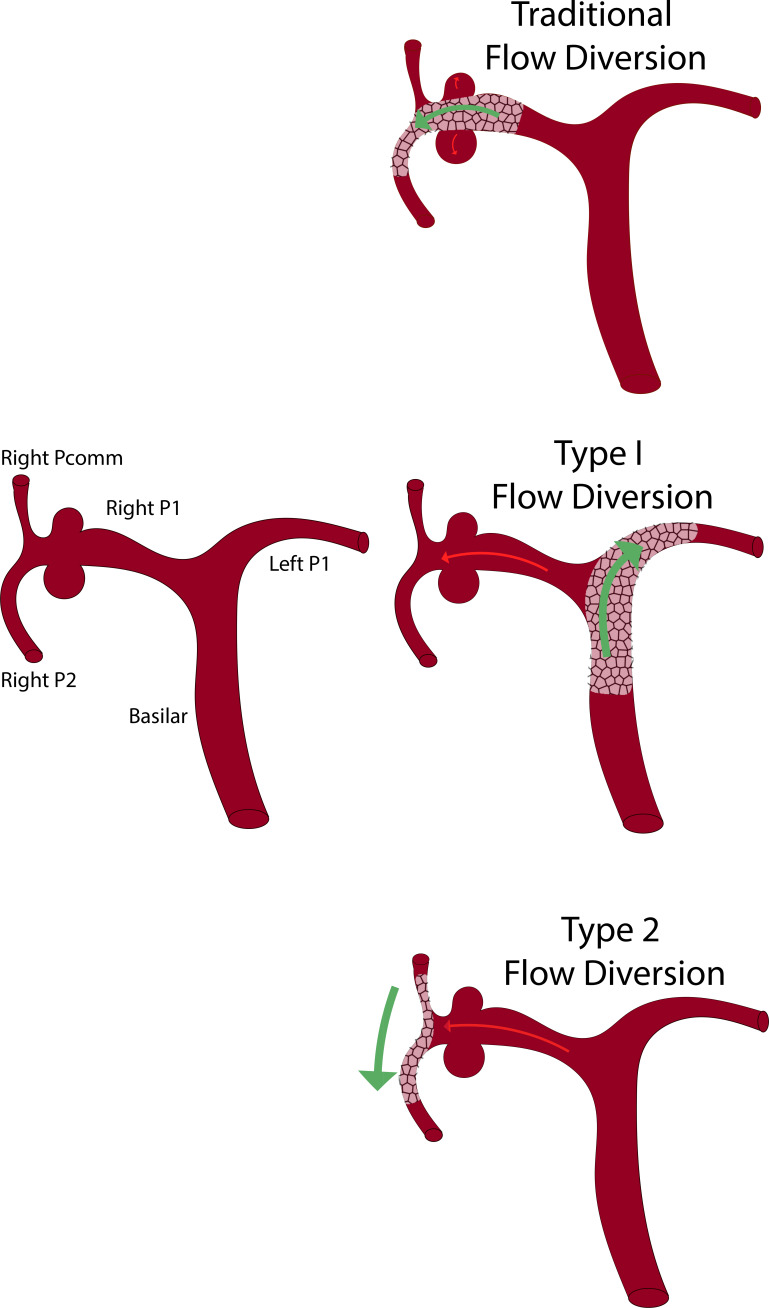
Proposed classification scheme for competitive flow diversion.
Figure 6.
Digital subtraction angiography of right carotid and vertebral arteries performed prior to flow-diverting stent deployment (left panels) and at 9-month follow-up (right panels). A progressive increase in the right posterior communicating artery (Pcomm, green arrows) and a decrease in size of the right posterior cerebral artery (PCA)-P1 caliber (red arrow) are observed. Decreased P1 associated flow contribution to the right occipital-parietal lobe (orange arrows) is evidenced by a paucity of contrast on the vertebral artery injection due to increased competitive flow from the right PComm after stenting and PCA-P1 segment flow remodeling.
Discussion
FDS across the neck of an intracranial aneurysm is an established treatment that allows for parent vessel remodeling and obliteration of intracranial aneurysms.1 2 Originally, competitive flow diversion was described as FDS into a normal vessel, 'jailing' the origin of the parent artery proximal to an aneurysm. This allows for progressive aneurysm deconstruction by redirecting flow into the normal artery, reducing flow in the diseased aneurysmal parent artery and enabling the development of leptomeningeal collaterals.3 4 We classify this as type I competitive flow diversion (figure 5).
To our knowledge, we report the first use of competitive flow diversion by stenting distal to an aneurysm within the circle of Willis. We classify this as type II competitive flow diversion (figure 5). This technique mitigates concerns regarding insufficient collateral circulation. It does not preclude future endovascular access by jailing the parent artery proximal to the aneurysm. We hypothesized that redirection of the primary vector of blood flow to the distal PCA territory would diminish flow across the P1 segment, reducing aneurysm inflow and wall shear stress. We proposed that this redistribution of flow would ultimately lead to obliteration of the aneurysms. At 12-month follow-up this proved to be the case. Augmentation of the stented Pcomm-P2 segment, attenuation of the P1 segment, and complete regression of the aneurysms was noted (figures 4 and 6). Stenting from the Pcomm-P2 segment with a non-flow-diverting stent with subsequent coiling of the P1 aneurysms represents another treatment option but was not chosen given the risk of distal P1 perforator compromise.
The patient had a small thalamic infarct that resulted in temporary hemiparesis. This infarct most likely occurred during the numerous attempts to navigate microwires and microcatheters into the P2, resulting in thromboemboli or injury to a thalamoperferator. It is less likely that the infarct occurred as a result of diminished flow to a thalamoperforator after stenting.
This treatment resulted in delayed occlusion of two aneurysms that were most likely unruptured. In cases of unequivocally ruptured aneurysms, competitive flow diversion may not be feasible due to the need for antiplatelet medication and the ongoing risk of SAH. However, traditional FDS across the aneurysm neck has been used successfully with acutely ruptured aneurysms, suggesting that it may be sufficient to diminish flow into an aneurysm to reduce the risk of re-rupture.5 Further investigation with computational flow modeling may yield predictive models for successful competitive flow diversion in the future.
In this case we accomplished delayed aneurysmal occlusion with competitive flow diversion across the parent artery distal to the aneurysms. The classification described here provides a structured framework for interventionalists to select and describe competitive flow diversion treatment options. Further systematic study of competitive flow diversion is warranted to outline outcomes and risks. The patient had a complete neurological recovery. Type II competitive flow diversion could be considered in aneurysms around the circle of Willis when traditional methods of securing aneurysms are not feasible.
Learning points.
Flow diversion stenting can be used to divert blood flow away from aneurysmal parent arteries rather than stenting across the aneurysm neck.
We classify competitive flow diversion into two distinct types. Type I competitive flow diversion is accomplished when the parent artery harboring the aneurysm is 'jailed' proximally. Type II competitive flow diversion occurs when flow is diverted from the parent artery distal to the aneurysm origin.
We describe the first case of aneurysm occlusion in the circle of Willis with type II competitive flow diversion and the first use of competitive flow diversion for the treatment of multiple adjacent aneurysms.
Footnotes
Contributors: Conception and design: AW and VMP. Acquisition of data: MAM and TJH. Analysis and interpretation of data: all authors. Drafting the article: AW, MAM. Critically revising the article: AW, MHM, MHS, TJH and VMP. Review of the final article prior to submission: all authors. Approval of the final version of the manuscript on behalf of all the authors: AW. Administrative/technical/material support: AW. Drawing of proposed classification scheme (Figure 5): AW. Study supervision: AW.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Walcott BP, Stapleton CJ, Choudhri O, et al. Flow diversion for the treatment of intracranial aneurysms. JAMA Neurol 2016;73:1002–8. 10.1001/jamaneurol.2016.0609 [DOI] [PubMed] [Google Scholar]
- 2.Walcott BP, Koch MJ, Stapleton CJ, et al. Blood flow diversion as a primary treatment method for ruptured brain aneurysms - concerns, controversy, and future directions. Neurocrit Care 2017;26:465–73. 10.1007/s12028-016-0318-y [DOI] [PubMed] [Google Scholar]
- 3.Wajnberg E, Silva TS, Johnson AK, et al. Progressive deconstruction: a novel aneurysm treatment using the pipeline embolization device for competitive flow diversion: case report. Neurosurgery 2014;10 Suppl 1:E161–6. 10.1227/NEU.0000000000000029 [DOI] [PubMed] [Google Scholar]
- 4.Johnson AK, Tan LA, Lopes DK, et al. Progressive deconstruction of a distal posterior cerebral artery aneurysm using competitive flow diversion. Neurointervention 2016;11:46–9. 10.5469/neuroint.2016.11.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madaelil TP, Moran CJ, Cross DT, et al. Flow diversion in ruptured intracranial aneurysms: a meta-analysis. AJNR Am J Neuroradiol 2017;38:590–5. 10.3174/ajnr.A5030 [DOI] [PMC free article] [PubMed] [Google Scholar]