Abstract
A 34-year-old man was admitted with acute lung injury and COVID-19 pneumonia. In the intensive care unit, he experienced episodes of prolonged asystole accompanied by hypotension without loss of consciousness. Once reversible causes were excluded, symptoms were related to dysfunction of the sinus node, and the patient underwent implantation of a pacemaker. (Level of Difficulty: Beginner.)
Key Words: bradycardia, bradycardia-tachycardia syndrome, COVID-19, electrophysiology, sick sinus dysfunction, sinus node dysfunction
Abbreviations and Acronyms: COVID-19, coronavirus-2019; CoV, coronavirus; PM, pacemaker; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; SSD, sick sinus dysfunction
Graphical abstract
A 34-year-old man was admitted with acute lung injury and COVID-19 pneumonia. In the intensive care unit, he experienced episodes of prolonged asystole…
Presentation
A 34-year-old white male arrived at the emergency department with dyspnea. Physical examination was notable for diffuse rhonchi. Initial blood pressure was 125/65 mm Hg, heart rate of 88 beats/min, oxygen saturation of 77%, breathing respiratory rate was 32/min, and temperature was 36.3°C.
Learning Objectives
-
•
To recognize cardiovascular complications among COVID-19 patients.
-
•
To demonstrate arrhythmic risk related to COVID-19 disease.
-
•
To manage sick sinus dysfunction in COVID-19 disease.
A 12-lead electrocardiogram showed sinus rhythm without abnormalities. Chest radiography showed bilateral ground glass opacities (Figure 1) and arterial blood gas tests showed respiratory alkalosis with severe hypoxemia. Noninvasive ventilation was immediately performed, bringing rapid clinical improvement. Blood test results (Table 1) revealed leukopenia, normocytic anemia, thrombocytopenia, and significant increase in C- reactive protein levels. A nasopharyngeal swab sample tested positive for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) using real time-reverse transcription-polymerase chain reaction.
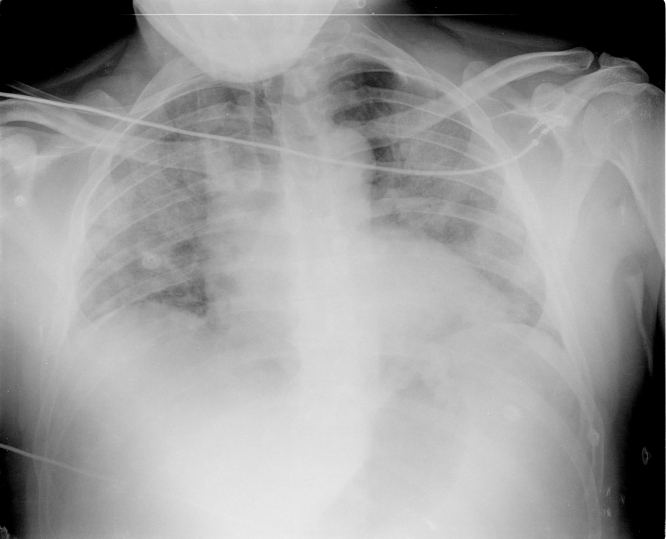
Figure 1.
Chest Radiography at Presentation
Note bilateral ground glass opacities.
Table 1.
Blood Tests During Hospitalization
| Blood Test | Day 1 | Day 2 | Day 5 | Day 10 | Day 15 | Day 25 | Day 35 |
|---|---|---|---|---|---|---|---|
| Hemoglobin, g/dl (normal values: 14.0 to 18.0 g/dl) | 12.1∗ | 10.9∗ | 9.8∗ | 9.9∗ | 11.8∗ | 10.5∗ | 12.3∗ |
| White blood cell count, ×103/μl (normal values: 4.0 to 10.8 ×103/μl) | 2.2∗ | 2.2∗ | 4.1 | 8.7 | 4.7 | 3.5∗ | 4.9 |
| Lymphocyte count (absolute), ×103/μl (normal values: 0.9 to 4.0 ×103/μl) | 0.22∗ | 0.22∗ | 0.30∗ | 0.20∗ | 0.9 | NA | NA |
| Platelet count, ×103/μl (normal values: 130 to 400 ×103/μl) | 91∗ | 94∗ | 141 | 110∗ | 186 | 170 | 136 |
| Creatinine, mg/dl (normal values: 0.7 to 1.2 mg/dl) | 1.10 | 0.80 | 0.70 | 0.60 | 0.50 | 0.50 | 0.60 |
| Sodium, mmol/l (normal values: 136 to 145 mmol/l) | 140 | 147† | 149† | 138 | 137 | 144 | 135∗ |
| Potassium, mmol/l (normal values: 3.4 to 4.5 mmol/l) | 3.5 | 3.6 | 3.8 | 5.1† | 4.8 | 3.2∗ | 3.7 |
| Calcium, mmol/l (normal values: 8.6 to 10.2 mmol/l) | NA | 8.0∗ | 8.66 | NA | 8.96 | 9.4 | 10 |
| Fibrinogen, mg/dl (normal values: 170 to 410 mg/dl) | NA | 448† | 222 | 587† | 941† | 168∗ | NA |
| D-dimer, ng/ml (normal values: <232 ng/ml) | NA | NA | 2,904† | 3,212† | 2,398† | 693† | NA |
| C-reactive protein, mg/l (normal values: <5.0 mg/l) | 116.9† | NA | 6.2† | 157.3† | 20.4† | 0.7 | NA |
| Lactate dehydrogenase, U/l (normal values: 135 to 225 U/l) | 917† | NA | NA | NA | 289† | 231† | NA |
| Ferritin, ng/ml (normal values: 30 to 400 ng/ml) | NA | NA | 915† | 759† | 676† | 447† | NA |
| Troponin, ng/l (normal values: <14/ml) | NA | 6 | 3 | NA | 7 | 13 | NA |
| Creatine kinase-MB, μg/l (normal values: <6.2 μg/l) | NA | 1.3 | NA | NA | NA | NA | NA |
NA = not available.
The value is less than normal.
The value is greater than normal.
Medical History
The patient had a medical history of bipolar disorder and hypothyroidism. He reported having fever for 1 week.
Differential Diagnosis
Given the patient’s presentation of prolonged asystole, differential diagnosis included metabolic disorders, pharmacologic agents and extracardiac diseases with special attention to thyroid hormone levels.
Investigations
On admission, there were no electrocardiographic abnormalities. During hospitalization, the patient experienced 3 episodes of short-lived asystole. Transthoracic echocardiography revealed normal left ventricle (LV) dimensions, without hypokinesis, with preserved LV ejection fraction. Electrocardiogram continuous monitoring showed other episodes of prolonged asystole accompanied by hypotension without loss of consciousness. There were no increases in myocardial necrosis enzyme values and no electrolyte abnormalities. Thyroid hormone values and lithium levels were normal.
Management
The patient was admitted to the department of internal medicine after acute lung injury and coronavirus-2019 (COVID-19) pneumonia were diagnosed.
Only home therapy with levothyroxine was administered. Lithium, olanzapine, and aripiprazole were temporarily suspended to avoid interference with antiviral drugs. Darunavir (800 mg once a day), ritonavir (100 mg once a day), hydroxychloroquine (200 mg twice a day), and enoxaparin sodium (100 IU/kg, twice a day) were administered.
Despite noninvasive ventilation, the patient suddenly deteriorated. Endotracheal intubation was performed (Figure 2), and he was transferred to the intensive care unit. Due to severe hypoxemia and poor respiratory exchanges, several patient pronation cycles were performed, and eventually, tracheostomy was carried out. This led to a progressive improvement until weaning from mechanical ventilation, with the use of low-dose oxygen therapy.
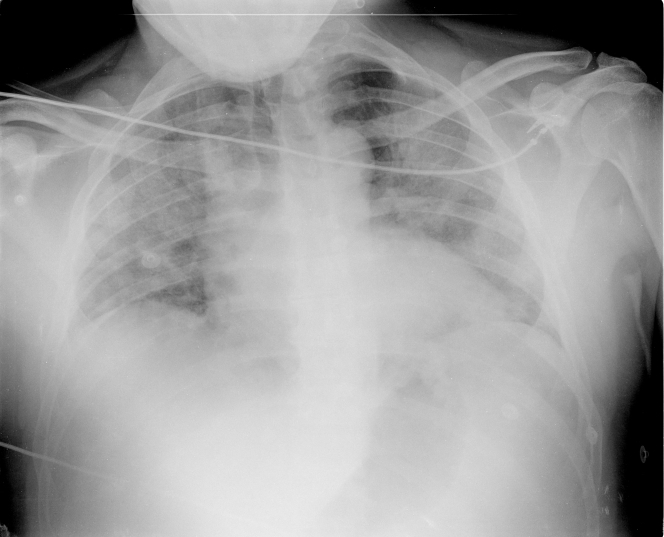
Figure 2.
Chest Radiography
Note bilateral ground glass opacities, endotracheal cannula, right central venous catheter, and the nasogastric tube.
Subsequently, the patient experienced 3 episodes of short-lived asystole, which required administration of isoproterenol and positioning of plates for external pacing. For this reason, he was transferred to the cardiology unit.
During the following days, the patient had episodes of asystole associated with hypotension but without loss of cardiac output (Figure 3). Moreover, alternating episodes of bradycardia and tachycardia and a single case of paroxysmal supraventricular tachycardia were reported. There were no increases in myocardial necrosis enzyme values and no electrolyte alterations. Thyroid hormone values and lithium levels were normal.
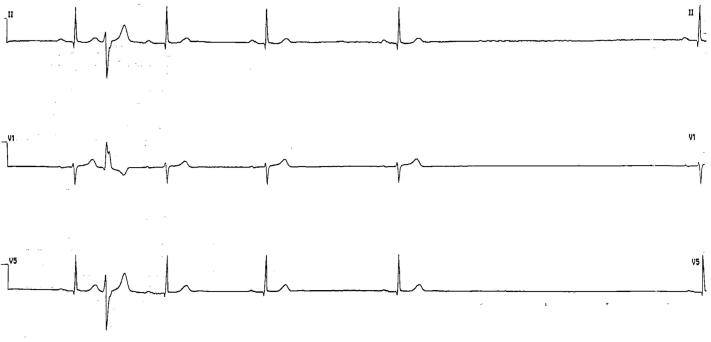
Figure 3.
Electrocardiography Continuous Monitoring Showing Asystole
Sinus activity suddenly interrupts with a 4.8-s pause.
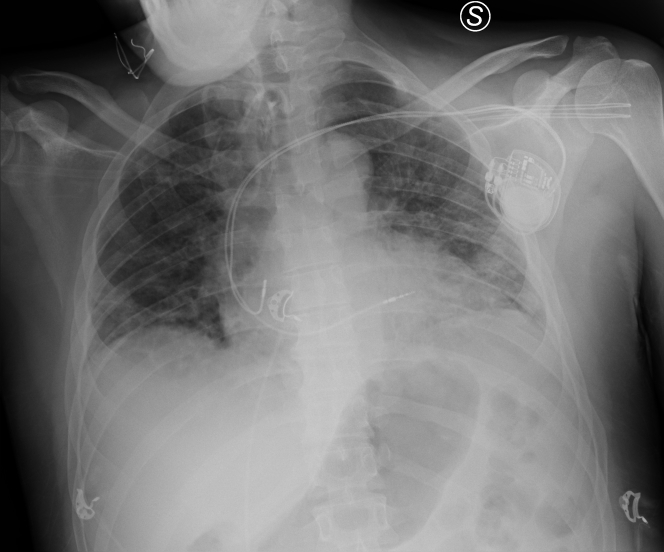
Once reversible causes were excluded, symptoms were related to dysfunction of the intrinsic sinus node, and the patient underwent dual-chamber rate-modulated implantation of a pacemaker (PM) (Figure 4).
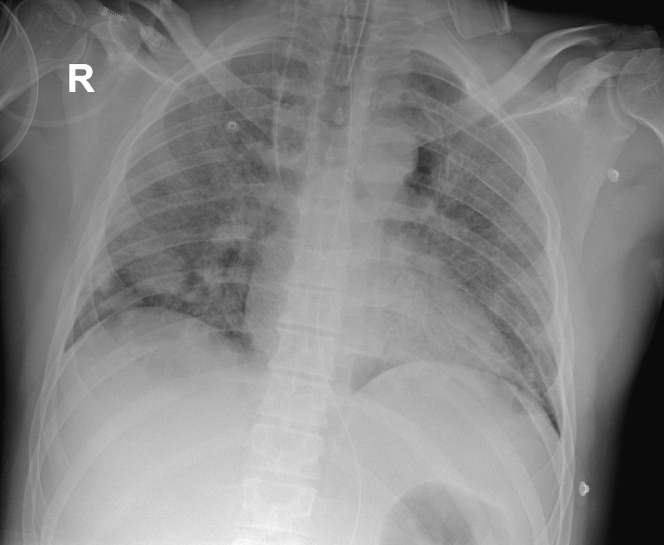
Figure 4.
Chest Radiography After Dual-Chamber Rate-Modulated Pacemaker Implantation
Follow-Up
Because of evidence of sinus tachycardia, bisoprolol was administrated. Further and later PM control showed only this episode. Transthoracic echocardiography was repeated before the patient was discharged and showed no differences from the previous one.
In consideration of his progressive clinical improvement, the endotracheal tube was removed, and the patient began to breath spontaneously. On psychiatric indication, therapy for bipolar disorder was reintroduced.
Discussion
COVID-19 patients present with a broad spectrum of pathology. (1). Moreover, there have been reports of patients experiencing late myocardial dysfunction, as well as ventricular fibrillation or cardiopulmonary arrest with pulseless electrical activity during the recovery phase of their pulmonary disease (2).
This could be due to hypoxia and electrolyte abnormalities, which could lead to episodes of cardiac arrhythmia, or due to central nervous system alterations caused by SARS-CoV-2 disease.
Increasing evidence shows that CoVs are not always confined to the respiratory tract but may also invade the central nervous system, inducing neurological diseases (1,3), and some CoVs have been shown to be able to spread to the medullary cardiorespiratory center through chemoreceptors and mechanoreceptors in the lung and lower respiratory airways through a synapse-connected route (4).
Considering that most CoVs share a similar viral structure and infection pathway (5), the infection mechanisms previously found for other CoVs may also be applicable to SARS-CoV-2.
Furthermore, the transsynaptic transfer also has been reported for avian bronchitis virus (6), in which viral antigens have been detected in the brain stem, where the infected regions included the nucleus ambiguus and the nucleus of the solitary tract. The nucleus of the solitary tract receives sensory information from chemoreceptors and mechanoreceptors in the lung respiratory tracts (7,8), whereas the efferent fibers from the nucleus ambiguus and the nucleus of the solitary tract provide innervation to airway smooth muscle, blood vessels, and glands.
Such neuroanatomical interconnections indicate that the death of infected patients may be a result of a dysfunction of the cardiorespiratory center in the brain stem (5) or a result of autonomic dysfunction through alteration of the intrinsic cardiac nervous system, with subsequent sick sinus dysfunction (SSD).
In fact, the intrinsic cardiac nervous system has regional control over different cardiac functions, such as sinus node electrical activation and propagation, as well as atrioventricular nodal conduction, and consists of ganglia composed of afferent, efferent, and interconnecting neurons to other cardiac ganglia. These ganglia coordinate the sympathetic and parasympathetic inputs received from the rest of the cardiac autonomic nervous system.
Generally, autonomic dysfunction refers to a disorder of an autonomic nervous system that may arise from intrinsic or extrinsic mechanisms. Intrinsic autonomic dysfunction arises from diseases that directly affect the autonomic nerves, such as diabetes mellitus and the various syndromes of primary autonomic failure. Extrinsic autonomic dysfunction often is secondarily induced by cardiac or other disease (9).
Patients with autonomic dysfunction commonly have poor long-term prognosis, and death can occur from pneumonia, acute respiratory failure, sudden cardiopulmonary arrest, or fatal arrhythmias related, for example, to SSD.
Specifically, SSD includes a spectrum of heart rhythm disturbances related to abnormal sinus impulse formation or propagation (10) and has different presentations, such as bradycardia, alternating episodes of bradycardia and tachycardia and sinoatrial block. In some cases, SSD presents with sinus node arrest and prolonged asystole, such as in the present patient. Symptoms related to SSD are generally fatigue and syncope or presyncope, but patients can be asymptomatic in the early phase of the disease. When symptoms are related to dysfunction of sinus node, PM implantation is required.
Conclusions
Currently, data regarding the neuroinvasive potential of SARS-CoV-2 with subsequent autonomic dysfunction are less described. Furthermore, to these authors’ knowledge, this is the first case in medical literature of SSD related to COVID-19 infection.
An improved understanding is crucial primarily for guiding the need for additional arrhythmia monitoring during hospitalization and after discharge (2). The present authors believe that recognition by the scientific community of these risks related to COVID-19 disease may be helpful for strict monitoring of affected patients and also for furthering knowledge of such complications for global public health.
Footnotes
Dr. Metra has received personal fees from Abbott Vascular, Amgen, Bayer, Edwards Therapeutics, and Vifor Pharma. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130:1787. doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakkireddy D.R., Chung M.K., Gopinathannair R. Guidance for cardiac electrophysiology during the coronavirus (COVID-19) pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology, and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology. Circulation. 2020;141:e823–e831. doi: 10.1161/CIRCULATIONAHA.120.047063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y.C., Bai W.Z., Hirano N., Hayashida T., Hashikawa T. Coronavirus infection of rat dorsal root ganglia: ultrastructural characterization of viral replication, transfer, and the early response of satellite cells. Virus Res. 2012;163:628–635. doi: 10.1016/j.virusres.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y.C., Bai W.Z., Hirano N. Neurotropic virus tracing suggests a membranous-coating-mediated mechanism for transsynaptic communication. J Comp Neurol. 2013;521:203–212. doi: 10.1002/cne.23171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan Y., Cao D., Zhang Y. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat Commun. 2017;8:15092. doi: 10.1038/ncomms15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chasey D., Alexander D.J. Morphogenesis of avian infectious bronchitis virus in primary chick kidney cells. Arch Virol. 1976;52:101–111. doi: 10.1007/BF01317869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalia M., Mesulam M.M. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- 8.Hadziefendic S., Haxhiu M.A. CNS innervation of vagal preganglionic neurons controlling peripheral airways: a transneuronal labeling study using pseudorabies virus. J Auton Nerv Syst. 1999;76:135–145. doi: 10.1016/s0165-1838(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 9.Goldberger J.J., Arora R., Buckley U., Shivkumar K. Autonomic nervous system dysfunction: JACC focus seminar. J Am Coll Cardiol. 2019;73:1189–1206. doi: 10.1016/j.jacc.2018.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Ponti R., Marazzato J., Bagliani G., Leonelli F.M., Padeletti L. Sick sinus syndrome. Card Electrophysiol Clin. 2018;10:183–195. doi: 10.1016/j.ccep.2018.02.002. [DOI] [PubMed] [Google Scholar]