Highlights
-
•
While blood CD8+ T cells control HIV using cytolytic functions, lymphoid tissue CD8+ T cells do so via non-cytolytic functions
-
•
HIV-specific CD8+ T cells in lymphoid and mucosal tissues are largely tissue-resident memory cells
-
•
The frequency of follicular CD8+ T cells negatively correlates with HIV viral load
-
•
Expression of inhibitory receptors by CD8+ T cells in lymphoid tissues do not necessarily indicate exhaustion
-
•
Lessons from autoimmune diseases suggest that a cure strategy inducing cytotoxic CD8+ T cells might cause undesired pathology
Keywords: HIV, Lymphoid tissue, CD8+ T cells
Abstract
CD8+ T cells are crucial for immunity against viral infections, including HIV. Several characteristics of CD8+ T cells, such as polyfunctionality and cytotoxicity, have been correlated with effective control of HIV. However, most of these correlates have been established in the peripheral blood. Meanwhile, HIV primarily replicates in lymphoid tissues. Therefore, it is unclear which aspects of CD8+ T cell biology are shared and which are different between blood and lymphoid tissues in the context of HIV infection. In this review, we will recapitulate the latest advancements of our knowledge on lymphoid tissue CD8+ T cells during HIV infection and discuss the insights these advancements might provide for the development of a HIV cure.
1. Introduction
Conventional wisdom dictates that cytotoxic T cell (CTL)-mediated immune surveillance of pathogen infected or otherwise aberrant cells is mediated by the selective elimination of the offending targets through cytotoxic activity. In the setting of HIV infection, CTL activity is largely attributed to control of disease progression (Hersperger et al., 2010; Jin et al., 1999; Koup et al., 1994; Matano et al., 1998; Migueles et al., 2002, 2008; Schmitz et al., 1999), but in the case of autoimmunity, it can lead to pathogenic effects within target tissues (Molodtsov and Turk, 2018). CD8+ T cells are the major T cell type that mediates cytotoxicity, leading to the general inter-usage of the terminology “CTL” with CD8+ T cells. However, cytotoxic activity is primarily mediated by a specific subset of CD8+ T cells, which we define as those CD8+ T cells that express perforin, granzyme B, and high levels of the transcription factor T-bet known to regulate the cytotoxic effector gene cassette (Hersperger et al., 2011; Sullivan et al., 2003). While much of our knowledge of CTL activity derives from studies of vascular-derived human lymphocytes or mouse spleens (an organ contiguous with the vasculature), it is becoming increasingly clear, through studies in other organs, that CD8+ T cells have a wide array of functions beyond cytotoxicity, including immune modulation via cytokine production, chemoattraction, and non-cytotoxic suppression of pathogen gene expression (Beura et al., 2018; Guidotti and Chisari, 2001; Nigam et al., 2010; Y. Yu et al., 2018). Moreover, the different CD8+ T cell populations mediating these functions likely have specialized functional roles relating to their specific environment, whether in the vasculature, at barrier sites, lymphoid tissues, or within non-lymphoid organs. Defining how different CD8+ T cell populations function within relevant target sites is paramount to advancing our knowledge in order to therapeutically modulate these cells for specific infection and autoimmune settings.
In this review, we will re-evaluate current models of CD8+ T cell-mediated immune responses in control of HIV by discussing their functions within the tissue environment, specifically lymphoid tissues where the majority of HIV replication occurs (Estes, 2013; Pantaleo et al., 1991; Vago et al., 1989). We will advance the concept that the CD8+ T cells in blood and tissues utilize different mechanisms to control viral replication. This concept is supported by observations that unlike in the blood, CD8+ T cells in HIV-infected lymphoid and mucosal tissues are poorly cytotoxic and therefore inefficient at eliminating infected cells (Kiniry et al., 2017, 2018a; Reuter et al., 2017). Instead, at these sites, tissue resident memory CD8+ T cell populations dominate the response and are likely critical in maintaining control of HIV replication, despite the absence of potent cytotoxic functions (Buggert et al., 2018; Kiniry et al., 2018b). Additionally, in spite of expressing several inhibitory receptors, it is unclear if these resident memory CD8+ T cells are functionally exhausted. We will also review the importance of follicular CD8+ T cells in combating HIV infection, especially since B cell follicles have been shown to be a major sanctuary for the viral reservoir (Banga et al., 2016; Connick et al., 2007; Folkvord et al., 2005; Fukazawa et al., 2015; Perreau et al., 2013). Together, the concepts of tissue-specific viral control mechanisms, tissue residency, exhaustion, and follicle-trafficking add complexity to the search for a cure strategy. Current cure strategies, such as “shock and kill”(Deeks, 2012), favor the elimination of the HIV reservoir, which requires the presence of cytotoxic CD8+ T cells in tissues. However, the elevation of cytotoxic CD8+ T cells in tissues has been correlated with pathology in autoimmune diseases. Therefore, is it a good idea to induce these cells in HIV-infected tissues? Should mechanisms of viral suppression rather than elimination be pursued? Additionally, is it important to invoke a large frequency of tissue-resident and follicle-homing CD8+ T cells as a part of a cure strategy? Does the expression of inhibitory receptors of tissue CD8+ T cells render them dysfunctional? We will touch upon issues related to these topics and draw parallels to CD8+ T cell responses against other infections and in autoimmune conditions, with the hope of providing new insights for the development of a HIV cure strategy.
2. CD8+ T cell function and control of HIV: how do peripheral blood CTL control HIV infection?
The primary CD8+ T cell effector mechanism attributed to control of HIV involves cytotoxic elimination of infected CD4+ T cells (Migueles et al., 2002, 2008). The exact mechanisms of how CD8+ T cells execute their cytotoxic functions have been well defined. After recognition of their cognate antigens presented on MHC-class I molecules, CD8+ T cells undergo major cytoskeletal reorganization in order to form an immunological synapse with their targets (Griffiths et al., 2010) and subsequently release lytic granules that trigger apoptosis in the target cells (Peters et al., 1991). Perforin, a pore-forming protein isolated from lytic granules of peripheral blood-derived CD8+ T cell clones in the mid 1980s (Henkart et al., 1984; Podack et al., 1985), and granzyme B, a serine protease that induces apoptosis by direct cleavage of caspase-3, caspase-8, PARP-1, BID, lamin B, ICAD, DNA-PK, and Nu-Ma (Chowdhury and Lieberman, 2008; Darmon et al., 1995), act in concert to induce the death of cells targeted by CD8+ T cell, with perforin mediating the delivery of granzyme B. Human CD8+ T cells can also express other granzyme proteins, including A, H, K, and M, each of which can induce apoptosis through a variety of mechanisms that may or may not require perforin-mediated delivery (Lieberman, 2003; Voskoboinik et al., 2015).
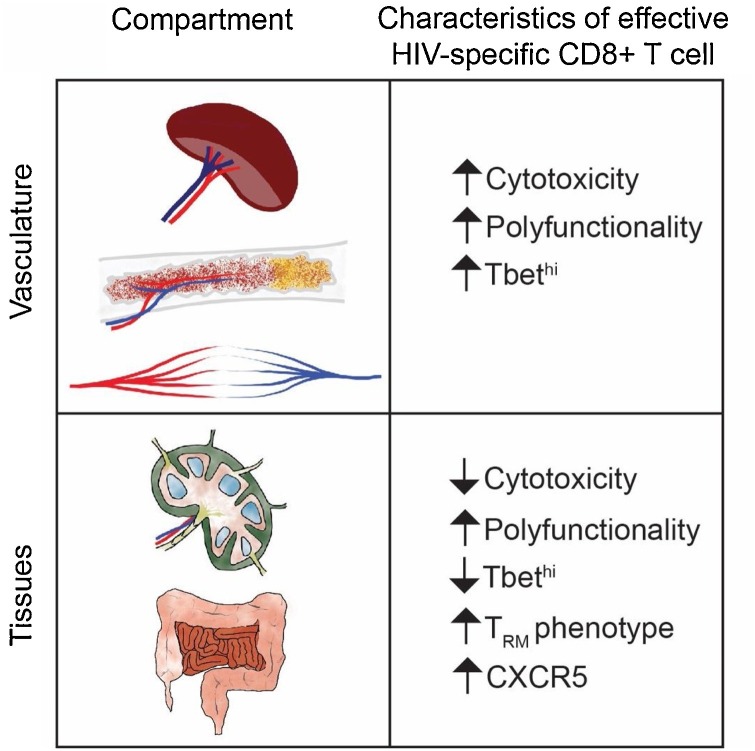
There is a clear association between the expression of perforin and granzyme family members and T cell memory differentiation. In particular, perforin and granzyme B are rarely expressed by central memory CD8+ T cells, those cells with the ability to directly traffic into lymph nodes (LNs) via CD62 L and CCR7 (Butcher and Picker, 1996; Chattopadhyay et al., 2009; Sallusto et al., 1999). The highest expression of perforin and granzyme B is found within the terminally differentiated CD8+ T cell subset defined by the absence of CCR7 and CD62 L and re-expression of CD45RA (Chattopadhyay et al., 2009), also called the CD8+ TEMRA cell. These cells, as well as specific subsets of the CCR7-CD62L-CD45RO+ (TEM) CD8+ T cell population, are largely believed to mediate viral control in HIV infection. Polyfunctional CD8+ T cells (defined by coordinate expression of IL-2, TNF, IFNg, MIP1b, and CD107a) have also been shown to correlate with control of viremia (Fig. 1 ) (Betts et al., 2006; Ferre et al., 2009). However, these polyfunctional CD8+ T cells typically are not cytotoxic, as they do not express perforin prior to or after stimulation (Makedonas et al., 2010).
Fig. 1.
Summary of HIV-specific CD8+ T cell functional properties in tissues and vasculature. In vasculature, HIV-specific CD8+ T cells associated with HIV control are highly cytolytic, which is associated with a high level of Tbet expression. Subsets of these cells are also highly polyfunctional. In tissues, CD8+ T cells linked with HIV control are largely non-cytolytic. However, they display a profile of TRM and polyfunctionality. Subsets of these cells also have potential to traffic to B cell follicles by virtue of CXCR5 expression.
There are clear correlative studies showing that peripheral blood CD8+ T cell cytotoxicity is associated with control of HIV replication; but is this a direct or indirect correlation? How, and where, would a peripheral blood cytotoxic CD8+ T cell encounter, recognize, and eliminate an infected CD4+ T cell? Given the rarity of HIV-RNA+ CD4+ T cells in the blood, as well as the volume and physical forces active within the vasculature, it would seem unlikely that an HIV-specific CD8+ T cell would encounter and eliminate an HIV-infected CD4+ T cell in the blood itself. One potential site where vascular HIV-specific CD8+ T cells could encounter HIV-infected CD4+ T cells is the spleen, which filters blood and therefore contains a similar complement of cytotoxic CD8+ T cells to the peripheral blood. Interestingly, total body quantification of viral RNA-producing CD4+ T cells in viremic SIV-infected rhesus macaques yields an extraordinarily low proportion (∼0.2%) of total body infected CD4+ T cells in the spleen compared to other lymphoid organs that are not contiguous with the vasculature (Estes et al., 2017). Thus, one could hypothesize that the correlates of control of HIV infection obtained from peripheral blood CD8+ T cells involving cytotoxicity actually reflect the efficient elimination of HIV-infected CD4+ T cells within the splenic environment. Whether parameters of peripheral blood CD8+ T cells relate to control of HIV-infected CD4+ T cells in lymphoid tissues, however, is less clear.
3. CD8+ T cell function and control of HIV: how do lymph node CD8+ T cells control HIV infection?
Based upon studies in SIV-infected rhesus macaque (RM) controller models, it is clear that CD8+ T cells play a significant role in controlling viral replication within lymphoid tissues (Fukazawa et al., 2015). In these studies, depletion of CD8+ T cells in Mamu-B*08/B*17 controller animals resulted in recrudescence of viral replication in the T cell zone of lymph nodes, whereas in the presence of CD8+ T cells viral replication was restricted to lymphoid follicles. However, it is not clear whether this viral control is mediated by CD8+ T cell cytotoxicity or other CD8+ T cell functional mechanisms.
The differential expression pattern of perforin and granzyme B within CD8+ T cell subsets is especially relevant when considering the potential role of peripheral blood CD8+ T cell cytotoxicity and control of HIV within the vascular (blood, spleen, bone marrow) versus the lymphoid tissue space. As discussed above, vascular T cell access to LNs is provided by L-selectin (CD62 L) and CCR7, allowing transmigration through high endothelial venules towards a CCL19/21 gradient (Butcher and Picker, 1996; Sallusto et al., 1999). TEM have access to non-lymphoid tissues (NLT) and can return to blood via the lymphatics (with possible stops along draining LNs). Transit to NLT is controlled by homing receptors (e.g. CLA, CCR4, CCR6, CCR9, CXCR3, CXCR6, a4b7) (for a detailed review see Griffith et al., 2014)). TEMRA are found in the blood and spleen at steady-state, but may express CXCR3 and enter NLTs under inflammatory conditions (Pirozyan et al., 2019; Uno et al., 2010). Given the absence of CD62 L expression on cytotoxic CD8+ T cell subsets in the peripheral blood, it is unlikely that CD8+ CTL (TEM and TEMRA) traffic directly into the LN across high endothelial venules (Butcher and Picker, 1996). However, as mentioned above, inflammatory signals can drive vascular CTL into tissues (Sallusto et al., 1999) and HIV is known to drive inflammation in lymphoid tissue (Biancotto et al., 2007). Until recently however, it has been unclear whether peripheral blood CD8+ CTL are recruited into lymphoid tissues or lymphoid tissue CD8+ T cells acquire CTL activity during chronic HIV infection in an effort to control viral replication.
Many studies have demonstrated the presence of CD8+ T cells, including HIV- and SIV-specific CD8+ T cells, in the lymph nodes of HIV-infected humans and SIV-infected RM (Andersson et al., 1999; Connick et al., 2014, 2007; Folkvord et al., 2003; Li et al., 2016). However, variations in the parameters measured, mis- or overinterpretation of results, and the overall interchangeable usage of the term “CTL” with CD8+ T cells have led to misconceptions regarding whether peripheral blood CD8+ CTL are found in lymphoid tissue. It is clear that both perforin and granzyme A and B expression can be found in lymphoid tissue CD8+ T cells, and that the frequency of cells expressing these proteins increases in HIV- and SIV- infection compared to uninfected controls (Andersson et al., 1999; Connick et al., 2014; Petrovas et al., 2017; Reuter et al., 2017). However, the coordinate expression of perforin and granzyme B is lower in HIV-infected lymphoid tissue CD8+ T cells compared to peripheral blood, in terms of both the frequency of co-expressing cells and the expression level of each protein on a per-cell basis (Fig. 2 B-E) (Reuter et al., 2017). Moreover, expression of perforin and granzyme in lymphoid tissue CD8+ T cells is dissociated from T-bet expression compared to peripheral blood in HIV uninfected as well as HIV/SIV-infected lymph nodes (Reuter et al., 2017; Roberts et al., 2016). As a result of these shortcomings, it is not entirely surprising that lymphoid tissue CD8+ T cells (whether from HIV + or HIV- LNs) generally have poor in vitro target killing ability compared to peripheral blood CD8+ T cells (Nguyen et al., 2019; Reuter et al., 2017).
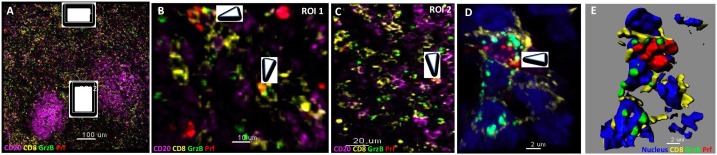
Fig. 2.
Low level of perforin and granzyme B co-expression by lymph node CD8+ T cells. (A) Multiplexed confocal image (20x) showing the expression of CD20 (magenta), CD8 (yellow), granzyme B (GrzB, green) and perforin (Prf, red) in a chronically HIV infected lymph node. The follicular areas, characterized by abundant CD20 staining, and two zoomed areas (white boxes), one in distance from the follicle (ROI 1) and one at the T – B cell border area (ROI 2), are also shown. (B, C) The presence of GrzB+ Prf+ CD8 T cells in the zoomed ROI 1 and 2 areas is show (white arrows). (D) A 63X confocal image showing the presence of a GrzB+ Prf+ CD8+ T cell (nuclear staining in blue) as well as the computationally derived surfaces showing the spatial organization of GrzB and Prf (E). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
During the acute induction phase of CD8+ T cell responses in LCMV infected mice, CD8+ T cells initially have high killing capacity in lymphoid tissues (Wolint et al., 2004). Subsequent to viral clearance, lymphoid tissue LCMV-specific CD8+ T cells transition to a memory state lacking cytotoxic ability. The same transition occurs during acute SIV infection: acute SIV-specific CD8+ T cells display high levels of perforin and granzyme B until approximately day 28 post infection, after which expression of both molecules decreases, reaching baseline levels by day 90 even though viremia is not cleared (Quigley et al., 2006; Roberts et al., 2016). In the same animals, peripheral blood SIV-specific CD8+ T cells partially retain perforin and granzyme B expression through day 90. During acute HIV infection, very high levels of perforin and granzyme B+ CD8+ T cells are found in both peripheral blood (Demers et al., 2016) and LN (Nguyen et al., 2019), but with a notable absence of T-bet expression in the LN CD8+ T cells. After establishment of chronic HIV infection, coordinate perforin and granzyme expression by LN CD8+ T cells is mostly lost, and only partially retained in peripheral blood CD8+ T cells (Demers et al., 2016; Nguyen et al., 2019; Reuter et al., 2017).
HIV elite controllers have peripheral blood HIV-specific CD8+ T cells with higher cytotoxic capacity compared to HIV viremic individuals (Fig. 1) (Hersperger et al., 2010; Migueles et al., 2008), raising the question as to whether these individuals also have superior lymphoid tissue cytolytic HIV-specific CD8+ T cells. Surprisingly, we found the opposite: HIV elite controller lymphoid tissue HIV-specific CD8+ T cells are resoundingly non-cytolytic, being nearly indistinguishable from an HIV-uninfected individual (Fig. 1) (Nguyen et al., 2019). Importantly, these cells are capable of suppressing virus replication through alternative mechanisms. As mentioned previously, CD8+ T cells produce many different types of immunomodulatory cytokines and potentially antiviral products. Non-cytolytic mechanisms of HIV inhibition were first documented in 1986, where CD8+ T cells were found to suppress HIV replication without killing infected cells in vitro (Walker et al., 1986). In addition, CD8+ T cell depletion in rhesus macaques did not increase the life-span of SIV-infected cells, indicating that direct killing was unlikely the main mechanism antagonizing viral replication (Klatt et al., 2010; Wong et al., 2010). The suppressive effect is attributed, at least in part, to a still unidentified soluble molecule known as cellular antiviral factor or CAF (Levy, 2003; Walker et al., 1986). In addition to CAF, beta-chemokines produced by CD8+ T cells such as CCL3 (MIP1-α), CCL4 (MIP1-β), and CCL5 (RANTES) have been shown to exert anti-HIV activities as these molecules interfere with viral entry by binding to CCR5, a key co-receptor of HIV (Cocchi et al., 1995). We found evidence of HIV-specific CD8+ T cells in the LNs of HIV elite controllers producing enhanced levels of non-cytolytic molecules (Nguyen et al., 2019), some of which have been shown to display antiviral activities including CCL5, TNF, RNase-1, and IL-32 (Bedoya et al., 2006; Cocchi et al., 1995; Lane et al., 1999; Rasool et al., 2008; Ribeiro-Dias et al., 2017). Importantly, these cells did not upregulate cytotoxic properties upon short (6–8 hours) or long (2–3 days) stimulation, suggesting that the absence of cytotoxic properties ex vivo was not simply due to the absence of in vivo stimulation but rather represents a stable differentiation state to a non-cytolytic subset that can control viral replication without eliminating infected CD4+ T cells (Nguyen et al., 2019).
4. CD8+ T cell function and control of HIV: the role of lymphoid tissue resident memory CD8+ T cells
Recent studies have found that the numerically most abundant memory CD8+ T cells in human tissues are considered to be “tissue resident” (TRM) (Thome et al., 2014). These cells can be identified by the expression of CD69, an inhibitor of S1PR1-mediated trafficking, and other cell adhesion molecules (Bankovich et al., 2010; Masopust et al., 2001; Shiow et al., 2006). TRM do not express tissue exit and lymphoid tissue homing cues such as S1PR1, CD62 L and CCR7. These cells have distinct transcriptional and functional signatures, and play a crucial role as sentinels within their local milieu upon antigenic re-exposure, reinfection, or reactivation in the case of chronic/episodic pathogens; for thorough reviews of TRM biology see (Gebhardt et al., 2018; Masopust and Soerens, 2019; Schenkel and Masopust, 2014). While reports of TRM cytotoxic effector function vary, TRM can secrete cytokines with potential direct effects on pathogens and chemokines to promote influx of other innate and adaptive immune cells to the site of infection (Park et al., 2018; Schenkel et al., 2014, 2013).
We and others have recently examined CD8+ TRM in HIV-infected lymphoid and gut tissues, finding that the majority of HIV-specific CD8+ T cells in these tissues bear a resident memory phenotype (Buggert et al., 2019, 2018; Kiniry et al., 2018b; Shacklett et al., 2019). In lymphoid tissues, CD8+ TRM represent the majority of cells that bear an effector memory phenotype (Farber et al., 2014; Thome et al., 2014), and in HIV infection, the proportion of lymphoid tissue HIV-specific CD8+ T cells with a TRM phenotype tends to be higher in HIV elite controllers, potentially implying a role in the control of viremia (Fig. 1) (Buggert et al., 2018). In both HIV-infected lymphoid tissues and gut mucosa, HIV-specific TRM appear to have limited immediate cytotoxic potential based on low or absent expression of perforin and or granzyme B and poor target cell killing ex vivo. Whether TRM in HIV-infected tissues can acquire cytolytic activity upon encountering an HIV infected CD4+ T cell remains unclear, though in vitro these cells do not appear to acquire cytolytic function in short term cultures. Thus, as discussed above, non-cytolytic functions by TRM may be the primary HIV-specific response invoked in lymphoid tissues and non-lymphoid tissues. Because they are primarily non-cytolytic, might their function actually suppress virus replication and therefore support establishment of latency and the latent reservoir in lymphoid tissues? Do they drive viral escape? Do they become exhausted in a similar fashion to peripheral blood CD8+ T cells? Given the potential importance of TRM as mediators of tissue-based immunity in the control of HIV, focused efforts should be made towards understanding these and other questions in future studies in both lymphoid and non-lymphoid tissues.
5. CD8+ T cell function and control of HIV: follicular CD8+ T cells
Lymph nodes, are typically organized into two regions: the medulla and cortex. The cortex is further subdivided into B cell follicles and the T cell zone (or paracortex) (von Andrian and Mempel, 2003). While most T cells are found in the T cell zone, CD4+ T cells, and to lesser extent CD8+, are capable of entering B cell follicles. Follicular-homing CD4+ T cells, or TFH, play a major role in regulating the germinal center reaction and the eventual antibody response (Vinuesa et al., 2016). Given the importance of CD4+ TFH for the formation and maintenance of the HIV/SIV reservoir (Aid et al., 2018; Banga et al., 2016; Boritz et al., 2016; Kohler et al., 2016; Lindqvist et al., 2012; Miller et al., 2017; Perreau et al., 2013; Xu et al., 2017a) the dynamics of CD8+ T cells within the follicular areas have been the focus of recent studies (Buggert et al., 2018; Ferrando-Martinez et al., 2018; Fukazawa et al., 2015; He et al., 2016; J. J. Hong et al., 2012; Li et al., 2019; Miles et al., 2016; Mylvaganam et al., 2017; Petrovas et al., 2017; Rahman et al., 2018; Reuter et al., 2017). Chronic HIV/SIV infection is characterized by an increased presence of CD8+ T cells in the lymph nodes, particularly in the follicular areas (Ferrando-Martinez et al., 2018; Leong et al., 2016; Petrovas et al., 2017; Reuter et al., 2017). Follicular CD8+ T cells upregulate the expression of CXCR5, although at significantly lower levels compared to CD4+ TFH (Ferrando-Martinez et al., 2018), and express a unique gene signature (Mylvaganam et al., 2017; Petrovas et al., 2017; Quigley et al., 2007). Advanced imaging assays have allowed the comprehensive investigation of these cells with regards to their topology and surrounding microenvironment (Estes et al., 2018). Despite the recent efforts, many questions related to follicular CD8+ T cell biology and dynamics remain to be addressed.
What is the lineage origin of follicular CD8+ T cells? Similar to CD4+ TFH, follicular CD8+ T cells express a unique phenotype and molecular profile (Mylvaganam et al., 2017; Petrovas et al., 2017; D. Yu and Ye, 2018). Furthermore, cytokines/chemokines (like TGF-b1, IL-23, IL-12) and transcription factors (including Bcl-6, Blimp1, TCF1, E2A), involved in the development of CD4+ TFH cells, also regulate follicular CD8+ T cells (Perdomo-Celis et al., 2019; Schmitt et al., 2014; D. Yu and Ye, 2018). Whether follicular CD8+ T cells represent an advanced differentiation stage of a particular CD8+ population or trafficking of bulk CD8+ T cells present in the T cell zone into the follicular areas is not known. It is also possible that non-follicular CXCR5lo CD8+ T cells give rise to the follicular CXCR5hi CD8+ T cells driven primarily by TGFb present in chronic HIV/SIV infection (Estes et al., 2007; Mylvaganam et al., 2017; Zeng et al., 2011). In support of this concept, an increased frequency of lymph node CCR7loCXCR5lo CD8+ T cells was found in early chronic SIV infection, preceding the accumulation of follicular CCR7loCXCR5hi CD8+ T cells observed in chronic SIV infection (Ferrando-Martinez et al., 2018).
What makes B cell follicles an immunologically privileged area? Historically, immune privileged areas are tissue sites that have been adapted to avoid tissue damage from local inflammation and recruitment of effector immune cells (Dai et al., 2005; S. Hong and Van Kaer, 1999). The presence of CD8+ T cells within follicles, particularly germinal centers, and the expression of cytolytic proteins are limited during homeostasis. The majority of follicular CD4+ and B cells express increased amounts of CD95/Fas (Koncz and Hueber, 2012; Marinova et al., 2006) making them highly sensitive to Fas-induced cell death. Therefore, one could hypothesize that limiting the access of CD8+ CTL into follicles protects the follicular populations from such deleterious signals. However, the molecular and cellular nature of mechanisms restricting the trafficking of CD8+ T cells into follicles are not known. The relative low expression of CXCR5 in lymph node CD8+ T cells could be a part of these mechanisms. In chronic SIV infection, a significantly higher frequency of follicular CD8+ T cells were found in both enlarged/lysed and intact mature follicles (Ferrando-Martinez et al., 2018), suggesting that trafficking of potential CTLs into follicles/germinal centers is not just a passive process. Comparing the local follicular environment in diseases characterized by irregular sequestration of CD8+ T cells in follicles could provide useful information regarding cellular and molecular pathways mediating this process.
Cognate vs non-cognate immune reactions; impact on follicular CD8+ T cell accumulation during chronic infection. HIV/SIV-specific follicular CD8+ T cells, defined by tetramer or intracellular cytokine staining, represent a small fraction of the follicular CD8+ T cell pool found in chronic HIV/SIV (Connick et al., 2007; Ferrando-Martinez et al., 2018; Li et al., 2019; Petrovas et al., 2017; Reuter et al., 2017). Although the presence of CD8+ T cell responses against other antigens cannot be excluded, it is possible that many of the follicular CD8+ T cells found in chronic infection are the result of a non-cognate differentiation process that is mediated by stimuli from the local microenvironment. No accumulation of follicular CD8+ T cells was observed in acute and early chronic SIV infection (Ferrando-Martinez et al., 2018; Li et al., 2019), while in chronic HIV/SIV infection accumulation of LN and follicular CD8+ T cells is associated with increased frequencies of circulating effector memory CD8+ T cells. Therefore, one could hypothesize that circulating CD8+ T cells can traffic back to LN possibly by a CXCR3-mediated mechanism (Alanio et al., 2018; Ferrando-Martinez et al., 2018; Khan et al., 2000; Reinhart et al., 2002), contributing to their accumulation in the T and B cell areas. The absence of CD8+ T cell accumulation in the LN areas (Ferrando-Martinez et al., 2018) in chronically SIV infected African Green Monkeys (AGMs, SIV natural host species), an infection characterized by low levels of immune activation (Silvestri et al., 2003), further supports the role of tissue inflammation and immune activation for this process.
Can follicular CD8+ T cells eliminate or control the virus? Virus elimination is a multistep process requiring either local CD8+ T cells to differentiate into CTLs, or CTLs to traffic from blood into the inflamed tissue (lymph node), navigate to follicular areas, scan/recognize infected cells, and finally secrete their killing mediators in a target-specific way through the immunological synapse. Therefore, this process is a matter of functionality and trafficking. Several reports have shown the limited presence of HIV/SIV-specific cytolytic CD8+ T cells in the follicles (Connick et al., 2014, 2007; Fukazawa et al., 2015; Kohler et al., 2016; Li et al., 2016). Even when present, these cells are usually found at the T-B border area (Fig. 2C). Furthermore, the perforin/granzyme B-mediated killing ability of LN antigen-specific CD8+ T cells, which express several inhibitory receptors (Petrovas et al., 2017), is probably compromised at least compared to their blood counterparts (Reuter et al., 2017). However, the frequency of CXCR5+ CD8+ T cells has been shown to be negatively correlated with viral load, implying that these cells can potentially control viral replication through cytolytic-independent mechanisms (Fig. 1) (Nguyen et al., 2019; Reuter et al., 2017). Therefore, the identification of these mechanisms and potential induction of them in follicular CD8+ T cells should be pursued. An alternative approach that takes advantage of the increased presence of follicular CD8+ T cells in chronic infection is the use of immunotherapies like bispecific/tri-specific (Petrovas et al., 2017; Xu et al., 2017b) antibodies that can direct bulk follicular CD8+ T cells to the infected cells in a manner that is not affected by the limited TCR repertoire, inhibitory receptors expression, and specificity of the very few antigen-specific CD8+ T cells in follicles.
6. Are lymphoid tissue CD8+ T cells dysfunctional in HIV infection?
Based upon the low frequency of cytolytic CD8+ T cells within lymphoid tissues of HIV-infected individuals, one could propose that these cells are generally dysfunctional in chronic HIV infection. However, numerous reports have demonstrated that fully differentiated cytolytic CD8+ T cells are rarely, if ever, found in lymphoid tissue or non-lymphoid tissues at steady-state in HIV negative individuals (Buggert et al., 2018; Reuter et al., 2017; Thome et al., 2014; Woon et al., 2016). Considering these observations, are CD8+ T cells in HIV-infected lymphoid tissues actually dysfunctional, or do their properties simply reflect the normal biology of lymphoid tissues?
In the context of acute viral infections, viral-specific CD8+ T cells have been shown to be important for protection against primary challenge or re-infection. In several acute respiratory infections, including severe acute respiratory syndrome coronavirus (SARS-CoV), influenza, and respiratory syncytial virus (RSV) infection, CD8+ T cells, presumably within the respiratory tract, can mediate protection upon challenge or in vaccine-challenge models (Channappanavar et al., 2014; Graham et al., 1991; Schmidt et al., 2018; Slutter et al., 2013). However, in these infections, protection is not likely mediated directly in lymphoid tissues, but rather at the respiratory epithelial barrier. In chronic human viral infections, such as Epstein-Barr virus (EBV) and cytomegalovirus (CMV), CD8+ T cells are important for long-term control and preventing disease progression, in spite of their inability to clear these viruses. Primary EBV infection causes a massive expansion of CD8+ T cells that ultimately contracts as antigen load decreases, indicating a role for CD8+ T cells in clearing virally infected cells (Bharadwaj et al., 2001; Hoshino et al., 1999; Tomkinson et al., 1987). Because EBV infects B cells, lymphoid tissue EBV-specific CD8+ T cell responses may be of particular importance for control, especially follicular CD8+ T cells. EBV-specific CD8+ T cells have been shown to establish residency within and outside of follicular areas of the human nasopharyngeal lymphoid tissues (Hislop et al., 2005; Leong et al., 2016). While these cells are attributed to the control of EBV at these sites, the mechanism by which they do so remains unclear. CMV-specific CD8+ T cells in these same tissues are present, but do not appear to establish residency (Woon et al., 2016). Whether CMV-specific CD8+ T cells in lymphoid tissues have cytolytic properties remains undefined. Of note, EBV-specific CD8+ T cells, both in the periphery and in lymphoid tissues, express a combination of inhibitory receptors, including PD-1, TIGIT, TIM-3, and KLRG1 (Chatterjee et al., 2019; Duraiswamy et al., 2011), yet are able to potently produce cytokines and undergo cell division upon stimulation (Chatterjee et al., 2019). CD8+ T cells, including HIV-specific CD8+ T cells, in LN of HIV- and HIV+ individuals typically express high levels of PD-1, TIGIT, CD160, and 2B4, yet respond upon stimulation by producing cytokines and proliferating, but not necessarily acquiring cytolytic ability (Petrovas et al., 2017; Reuter et al., 2017). These observations indicate a key disconnect between the simple presence of inhibitory receptors and dysfunction/exhaustion, and instead implies an undescribed functional regulation that may specifically be characteristic of CD8+ T cells that are present in lymphoid tissues.
7. Is inducing cytotoxicity of CD8+ T cells in HIV-infected lymph nodes a good idea?
While it may be thought that having strong cytotoxic responses in lymphoid or other tissues is beneficial by enabling the elimination of HIV-infected CD4+ T cells, simply increasing the frequency or potency of cytotoxic CD8+ T cells may have untoward consequences. CD8+ T cells can cause significant damage to tissues by secreting pro-inflammatory cytokines and/or by direct killing of target cells. Pathogenic effects of CD8+ T cells have been demonstrated in several parasitic and viral infections, including Leishmaniasis, Malaria, Chagas disease, Coxsackie virus, and Zika virus infections (Ferreira et al., 2017; Henke et al., 1995; Jurado et al., 2018; Nitcheu et al., 2003; Novais et al., 2017, 2013). In each of these diseases, the observed pathology has been attributed largely to CD8+ T cells with aberrant pathogen-specific perforin-mediated cytotoxic activity in the target tissues. Cytokine production by these T cells largely does not confer pathogenic effects in model systems of each of these infections (Gebhard et al., 1998; Haque et al., 2011; Novais et al., 2013; Silverio et al., 2012). Whether the presence of pathogenic cytotoxic CD8+ T cells reflects migration from the peripheral blood or differentiation within tissue remains unclear, but either scenario in the case of HIV infection could readily yield undesired tissue pathology in lymphoid or non-lymphoid tissues.
Pathogenic resident-memory CD8+ T cells have also been described in autoimmune settings. However, the mechanisms underlying their pathogenic effects is not restricted to cytotoxic activity. For example, in type 1 diabetes mellitus, CD8+ T cells are known to infiltrate pancreatic islets, where they are believed to mediate the destruction of beta-cells (Coppieters et al., 2012; Skowera et al., 2008; Unger et al., 2012; Willcox et al., 2009). While there is evidence of traditional perforin/granzyme B-mediated cytotoxic activity, cytokine-mediated mechanisms have also been implicated in beta-cell destruction (Coppieters and von Herrath, 2011; Knight et al., 2013; Trivedi et al., 2016). Similarly, differential pathogenic mechanisms have been implicated in aberrant self-reactivity by skin CD8+ TRM in vitiligo (cytotoxicity and cytokine production) and psoriasis (cytokine-mediated inflammation) (Cheuk et al., 2017, 2014; van den Boorn et al., 2009). Finally, multiple sclerosis (MS), a neurodegenerative disease caused by demyelination of nerve fibers in the CNS, is largely believed to be mediated by T cells with aberrant functions in the central nervous system. T cell receptor analyses of T cells isolated from the CSF, brain biopsies and peripheral blood of MS patients showed increased oligoclonality among CD8+, but not CD4+, T cells, indicating clonal expansion (Babbe et al., 2000; Jacobsen et al., 2002; Skulina et al., 2004). The mechanism responsible for neuronal loss is still not fully understood, but there is some evidence that IFNγ and IL-17A play a role (Annibali et al., 2011; Tzartos et al., 2008).
8. Conclusion
Given the potential role of CD8+ T cells in viral control and/or elimination, particularly in lymph node/follicles, several approaches aiming to favor the influx or expansion of CD8+ T cells in these areas are under development. in vivo administration of IL-15, a pleiotropic cytokine (Waldmann, 2006), was able to increase the presence of CD8+ T cells in the follicular/germinal centers and suppress the virus locally (Watson et al., 2018; Webb et al., 2018). Inhibition of T cell egress from the LN, using an S1PR1 antagonist, increased the frequency of follicular CD8+ T cells and decreased infection of follicular CD4+ T cells (Pino et al., 2019). In another approach, genetically modified CD8+ T cells overexpressing CXCR5 were able to traffic into the spleen follicular areas (Ayala et al., 2017). Combinatorial immunotherapies (for example co-administration of bispecific/trispecific antibodies and reagents promoting the trafficking of CD8+ T cells into follicles) represent another potential strategy for virus control or elimination.
However, the challenge of all of these strategies is to understand precisely what type of CD8+ T cell response is desired, and whether it will be possible to induce and durably maintain it within the lymphoid tissue environment. Studies by our group and others challenge the notion that cytotoxic CD8+ T cell responses are necessary for control of HIV replication in tissues. Hence, improving non-cytolytic CD8+ T cell mediated suppression of viral replication could be sufficient to maintain durable control of viremia. Given the accumulating data that cytotoxic function exerted by tissue CD8+ T cells is rare and likely heavily regulated through inhibitory pathways, attempting to permanently invoke cytolytic function in lymphoid tissues may be more difficult than anticipated and yield undesirable results through the induction of deleterious pathology. Further studies should aim to determine the exact non-cytolytic mechanisms involved in CD8+ T cell-mediated control of HIV. The elucidation of these mechanisms can provide targets for the development of functional cure strategies.
Acknowledgement
Funding was provided by NIH grants UC4 DK112217 and R01 AI118694 (MRB).
References
- Aid M., Dupuy F.P., Moysi E., Moir S., Haddad E.K., Estes J.D., Sekaly R.P., Petrovas C., Ribeiro S.P. Follicular CD4 t helper cells As a major HIV reservoir compartment: a molecular perspective. Front. Immunol. 2018;9:895. doi: 10.3389/fimmu.2018.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanio C., Barreira da Silva R., Michonneau D., Bousso P., Ingersoll M.A., Albert M.L. CXCR3/CXCL10 Axis shapes tissue distribution of memory phenotype CD8(+) t cells in nonimmunized mice. J. Immunol. 2018;200:139–146. doi: 10.4049/jimmunol.1700564. [DOI] [PubMed] [Google Scholar]
- Andersson J., Behbahani H., Lieberman J., Connick E., Landay A., Patterson B., Sonnerborg A., Lore K., Uccini S., Fehniger T.E. Perforin is not co-expressed with granzyme A within cytotoxic granules in CD8 T lymphocytes present in lymphoid tissue during chronic HIV infection. AIDS. 1999;13:1295–1303. doi: 10.1097/00002030-199907300-00005. [DOI] [PubMed] [Google Scholar]
- Annibali V., Ristori G., Angelini D.F., Serafini B., Mechelli R., Cannoni S., Romano S., Paolillo A., Abderrahim H., Diamantini A., Borsellino G., Aloisi F., Battistini L., Salvetti M. CD161(high)CD8+T cells bear pathogenetic potential in multiple sclerosis. Brain. 2011;134:542–554. doi: 10.1093/brain/awq354. [DOI] [PubMed] [Google Scholar]
- Ayala V.I., Deleage C., Trivett M.T., Jain S., Coren L.V., Breed M.W., Kramer J.A., Thomas J.A., Estes J.D., Lifson J.D., Ott D.E. CXCR5-dependent entry of CD8 t cells into Rhesus macaque B-Cell follicles achieved through T-Cell engineering. J. Virol. 2017:91. doi: 10.1128/JVI.02507-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbe H., Roers A., Waisman A., Lassmann H., Goebels N., Hohlfeld R., Friese M., Schroder R., Deckert M., Schmidt S., Ravid R., Rajewsky K. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga R., Procopio F.A., Noto A., Pollakis G., Cavassini M., Ohmiti K., Corpataux J.M., de Leval L., Pantaleo G., Perreau M. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat. Med. 2016;22:754–761. doi: 10.1038/nm.4113. [DOI] [PubMed] [Google Scholar]
- Bankovich A.J., Shiow L.R., Cyster J.G. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J. Biol. Chem. 2010;285:22328–22337. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoya V.I., Boasso A., Hardy A.W., Rybak S., Shearer G.M., Rugeles M.T. Ribonucleases in HIV type 1 inhibition: effect of recombinant RNases on infection of primary T cells and immune activation-induced RNase gene and protein expression. AIDS Res. Hum. Retroviruses. 2006;22:897–907. doi: 10.1089/aid.2006.22.897. [DOI] [PubMed] [Google Scholar]
- Betts M.R., Nason M.C., West S.M., De Rosa S.C., Migueles S.A., Abraham J., Lederman M.M., Benito J.M., Goepfert P.A., Connors M., Roederer M., Koup R.A. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura L.K., Mitchell J.S., Thompson E.A., Schenkel J.M., Mohammed J., Wijeyesinghe S., Fonseca R., Burbach B.J., Hickman H.D., Vezys V., Fife B.T., Masopust D. Intravital mucosal imaging of CD8(+) resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat. Immunol. 2018;19:173–182. doi: 10.1038/s41590-017-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj M., Burrows S.R., Burrows J.M., Moss D.J., Catalina M., Khanna R. Longitudinal dynamics of antigen-specific CD8+ cytotoxic T lymphocytes following primary Epstein-Barr virus infection. Blood. 2001;98:2588–2589. doi: 10.1182/blood.v98.8.2588. [DOI] [PubMed] [Google Scholar]
- Biancotto A., Grivel J.C., Iglehart S.J., Vanpouille C., Lisco A., Sieg S.F., Debernardo R., Garate K., Rodriguez B., Margolis L.B., Lederman M.M. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–4279. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boritz E.A., Darko S., Swaszek L., Wolf G., Wells D., Wu X., Henry A.R., Laboune F., Hu J., Ambrozak D., Hughes M.S., Hoh R., Casazza J.P., Vostal A., Bunis D., Nganou-Makamdop K., Lee J.S., Migueles S.A., Koup R.A., Connors M., Moir S., Schacker T., Maldarelli F., Hughes S.H., Deeks S.G., Douek D.C. Multiple origins of virus persistence during natural control of HIV infection. Cell. 2016;166:1004–1015. doi: 10.1016/j.cell.2016.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggert M., Japp A.S., Betts M.R. Everything in its right place: resident memory CD8+ T cell immunosurveillance of HIV infection. Curr. Opin. HIV AIDS. 2019;14:93–99. doi: 10.1097/COH.0000000000000523. [DOI] [PubMed] [Google Scholar]
- Buggert M., Nguyen S., Salgado-Montes de Oca G., Bengsch B., Darko S., Ransier A., Roberts E.R., Del Alcazar D., Brody I.B., Vella L.A., Beura L., Wijeyesinghe S., Herati R.S., Del Rio Estrada P.M., Ablanedo-Terrazas Y., Kuri-Cervantes L., Sada Japp A., Manne S., Vartanian S., Huffman A., Sandberg J.K., Gostick E., Nadolski G., Silvestri G., Canaday D.H., Price D.A., Petrovas C., Su L.F., Vahedi G., Dori Y., Frank I., Itkin M.G., Wherry E.J., Deeks S.G., Naji A., Reyes-Teran G., Masopust D., Douek D.C., Betts M.R. Identification and characterization of HIV-specific resident memory CD8(+) T cells in human lymphoid tissue. Sci. Immunol. 2018;3 doi: 10.1126/sciimmunol.aar4526. eaar4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E.C., Picker L.J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Channappanavar R., Fett C., Zhao J., Meyerholz D.K., Perlman S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 2014;88:11034–11044. doi: 10.1128/JVI.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee B., Deng Y., Holler A., Nunez N., Azzi T., Vanoaica L.D., Muller A., Zdimerova H., Antsiferova O., Zbinden A., Capaul R., Dreyer J.H., Nadal D., Becher B., Robinson M.D., Stauss H., Munz C. CD8+ T cells retain protective functions despite sustained inhibitory receptor expression during Epstein-Barr virus infection in vivo. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay P.K., Betts M.R., Price D.A., Gostick E., Horton H., Roederer M., De Rosa S.C. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J. Leukoc. Biol. 2009;85:88–97. doi: 10.1189/jlb.0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuk S., Schlums H., Gallais Serezal I., Martini E., Chiang S.C., Marquardt N., Gibbs A., Detlofsson E., Introini A., Forkel M., Hoog C., Tjernlund A., Michaelsson J., Folkersen L., Mjosberg J., Blomqvist L., Ehrstrom M., Stahle M., Bryceson Y.T., Eidsmo L. CD49a expression defines tissue-resident CD8(+) t cells poised for cytotoxic function in human skin. Immunity. 2017;46:287–300. doi: 10.1016/j.immuni.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuk S., Wiken M., Blomqvist L., Nylen S., Talme T., Stahle M., Eidsmo L. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J. Immunol. 2014;192:3111–3120. doi: 10.4049/jimmunol.1302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury D., Lieberman J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu. Rev. Immunol. 2008;26:389–420. doi: 10.1146/annurev.immunol.26.021607.090404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F., DeVico A.L., Garzino-Demo A., Arya S.K., Gallo R.C., Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Connick E., Folkvord J.M., Lind K.T., Rakasz E.G., Miles B., Wilson N.A., Santiago M.L., Schmitt K., Stephens E.B., Kim H.O., Wagstaff R., Li S., Abdelaal H.M., Kemp N., Watkins D.I., MaWhinney S., Skinner P.J. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J. Immunol. 2014;193:5613–5625. doi: 10.4049/jimmunol.1401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connick E., Mattila T., Folkvord J.M., Schlichtemeier R., Meditz A.L., Ray M.G., McCarter M.D., Mawhinney S., Hage A., White C., Skinner P.J. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J. Immunol. 2007;178:6975–6983. doi: 10.4049/jimmunol.178.11.6975. [DOI] [PubMed] [Google Scholar]
- Coppieters K.T., Dotta F., Amirian N., Campbell P.D., Kay T.W., Atkinson M.A., Roep B.O., von Herrath M.G. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters K.T., von Herrath M.G. Viruses and cytotoxic T lymphocytes in type 1 diabetes. Clin. Rev. Allergy Immunol. 2011;41:169–178. doi: 10.1007/s12016-010-8220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z., Nasr I.W., Reel M., Deng S., Diggs L., Larsen C.P., Rothstein D.M., Lakkis F.G. Impaired recall of CD8 memory T cells in immunologically privileged tissue. J. Immunol. 2005;174:1165–1170. doi: 10.4049/jimmunol.174.3.1165. [DOI] [PubMed] [Google Scholar]
- Darmon A.J., Nicholson D.W., Bleackley R.C. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature. 1995;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- Deeks S.G. HIV: shock and kill. Nature. 2012;487:439–440. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- Demers K.R., Makedonas G., Buggert M., Eller M.A., Ratcliffe S.J., Goonetilleke N., Li C.K., Eller L.A., Rono K., Maganga L., Nitayaphan S., Kibuuka H., Routy J.P., Slifka M.K., Haynes B.F., McMichael A.J., Bernard N.F., Robb M.L., Betts M.R. Temporal dynamics of CD8+ t cell effector responses during primary HIV infection. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraiswamy J., Ibegbu C.C., Masopust D., Miller J.D., Araki K., Doho G.H., Tata P., Gupta S., Zilliox M.J., Nakaya H.I., Pulendran B., Haining W.N., Freeman G.J., Ahmed R. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J. Immunol. 2011;186:4200–4212. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes J.D. Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol. Rev. 2013;254:65–77. doi: 10.1111/imr.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes J.D., Kityo C., Ssali F., Swainson L., Makamdop K.N., Del Prete G.Q., Deeks S.G., Luciw P.A., Chipman J.G., Beilman G.J., Hoskuldsson T., Khoruts A., Anderson J., Deleage C., Jasurda J., Schmidt T.E., Hafertepe M., Callisto S.P., Pearson H., Reimann T., Schuster J., Schoephoerster J., Southern P., Perkey K., Shang L., Wietgrefe S.W., Fletcher C.V., Lifson J.D., Douek D.C., McCune J.M., Haase A.T., Schacker T.W. Defining total-body AIDS-virus burden with implications for curative strategies. Nat. Med. 2017;23:1271–1276. doi: 10.1038/nm.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes J.D., LeGrand R., Petrovas C. Visualizing the immune system: providing key insights into HIV/SIV infections. Front. Immunol. 2018;9:423. doi: 10.3389/fimmu.2018.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes J.D., Wietgrefe S., Schacker T., Southern P., Beilman G., Reilly C., Milush J.M., Lifson J.D., Sodora D.L., Carlis J.V., Haase A.T. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J. Infect. Dis. 2007;195:551–561. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- Farber D.L., Yudanin N.A., Restifo N.P. Human memory T cells: generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando-Martinez S., Moysi E., Pegu A., Andrews S., Nganou Makamdop K., Ambrozak D., McDermott A.B., Palesch D., Paiardini M., Pavlakis G.N., Brenchley J.M., Douek D., Mascola J.R., Petrovas C., Koup R.A. Accumulation of follicular CD8+ T cells in pathogenic SIV infection. J. Clin. Invest. 2018;128:2089–2103. doi: 10.1172/JCI96207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre A.L., Hunt P.W., Critchfield J.W., Young D.H., Morris M.M., Garcia J.C., Pollard R.B., Yee H.F., Jr., Martin J.N., Deeks S.G., Shacklett B.L. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood. 2009;113:3978–3989. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira L.R., Ferreira F.M., Nakaya H.I., Deng X., Candido D.D., de Oliveira L.C., Billaud J.N., Lanteri M.C., Rigaud V.O., Seielstad M., Kalil J., Fernandes F., Ribeiro A.L., Sabino E.C., Cunha-Neto E. Blood gene signatures of chagas cardiomyopathy with or without ventricular dysfunction. J. Infect. Dis. 2017;215:387–395. doi: 10.1093/infdis/jiw540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkvord J.M., Anderson D.M., Arya J., MaWhinney S., Connick E. Microanatomic relationships between CD8+ cells and HIV-1-producing cells in human lymphoid tissue in vivo. J. Acquir. Immune Defic. Syndr. 2003;32:469–476. doi: 10.1097/00126334-200304150-00001. [DOI] [PubMed] [Google Scholar]
- Folkvord J.M., Armon C., Connick E. Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Res. Hum. Retroviruses. 2005;21:363–370. doi: 10.1089/aid.2005.21.363. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y., Lum R., Okoye A.A., Park H., Matsuda K., Bae J.Y., Hagen S.I., Shoemaker R., Deleage C., Lucero C., Morcock D., Swanson T., Legasse A.W., Axthelm M.K., Hesselgesser J., Geleziunas R., Hirsch V.M., Edlefsen P.T., Piatak M., Jr., Estes J.D., Lifson J.D., Picker L.J. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med. 2015;21:132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard J.R., Perry C.M., Harkins S., Lane T., Mena I., Asensio V.C., Campbell I.L., Whitton J.L. Coxsackievirus B3-induced myocarditis: perforin exacerbates disease, but plays no detectable role in virus clearance. Am. J. Pathol. 1998;153:417–428. doi: 10.1016/S0002-9440(10)65585-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T., Palendira U., Tscharke D.C., Bedoui S. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol. Rev. 2018;283:54–76. doi: 10.1111/imr.12650. [DOI] [PubMed] [Google Scholar]
- Graham B.S., Bunton L.A., Wright P.F., Karzon D.T. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J.W., Sokol C.L., Luster A.D. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- Griffiths G.M., Tsun A., Stinchcombe J.C. The immunological synapse: a focal point for endocytosis and exocytosis. J. Cell Biol. 2010;189:399–406. doi: 10.1083/jcb.201002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti L.G., Chisari F.V. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- Haque A., Best S.E., Unosson K., Amante F.H., de Labastida F., Anstey N.M., Karupiah G., Smyth M.J., Heath W.R., Engwerda C.R. Granzyme B expression by CD8+ T cells is required for the development of experimental cerebral malaria. J. Immunol. 2011;186:6148–6156. doi: 10.4049/jimmunol.1003955. [DOI] [PubMed] [Google Scholar]
- He R., Hou S., Liu C., Zhang A., Bai Q., Han M., Yang Y., Wei G., Shen T., Yang X., Xu L., Chen X., Hao Y., Wang P., Zhu C., Ou J., Liang H., Ni T., Zhang X., Zhou X., Deng K., Chen Y., Luo Y., Xu J., Qi H., Wu Y., Ye L. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537:412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- Henkart P.A., Millard P.J., Reynolds C.W., Henkart M.P. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J. Exp. Med. 1984;160:75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke A., Huber S., Stelzner A., Whitton J.L. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J. Virol. 1995;69:6720–6728. doi: 10.1128/jvi.69.11.6720-6728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersperger A.R., Martin J.N., Shin L.Y., Sheth P.M., Kovacs C.M., Cosma G.L., Makedonas G., Pereyra F., Walker B.D., Kaul R., Deeks S.G., Betts M.R. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood. 2011;117:3799–3808. doi: 10.1182/blood-2010-12-322727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersperger A.R., Pereyra F., Nason M., Demers K., Sheth P., Shin L.Y., Kovacs C.M., Rodriguez B., Sieg S.F., Teixeira-Johnson L., Gudonis D., Goepfert P.A., Lederman M.M., Frank I., Makedonas G., Kaul R., Walker B.D., Betts M.R. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop A.D., Kuo M., Drake-Lee A.B., Akbar A.N., Bergler W., Hammerschmitt N., Khan N., Palendira U., Leese A.M., Timms J.M., Bell A.I., Buckley C.D., Rickinson A.B. Tonsillar homing of Epstein-Barr virus-specific CD8+ T cells and the virus-host balance. J. Clin. Invest. 2005;115:2546–2555. doi: 10.1172/JCI24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J.J., Amancha P.K., Rogers K., Ansari A.A., Villinger F. Spatial alterations between CD4(+) T follicular helper, B, and CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J. Immunol. 2012;188:3247–3256. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Van Kaer L. Immune privilege: keeping an eye on natural killer T cells. J. Exp. Med. 1999;190:1197–1200. doi: 10.1084/jem.190.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Morishima T., Kimura H., Nishikawa K., Tsurumi T., Kuzushima K. Antigen-driven expansion and contraction of CD8+-activated T cells in primary EBV infection. J. Immunol. 1999;163:5735–5740. [PubMed] [Google Scholar]
- Jacobsen M., Cepok S., Quak E., Happel M., Gaber R., Ziegler A., Schock S., Oertel W.H., Sommer N., Hemmer B. Oligoclonal expansion of memory CD8+ T cells in cerebrospinal fluid from multiple sclerosis patients. Brain. 2002;125:538–550. doi: 10.1093/brain/awf059. [DOI] [PubMed] [Google Scholar]
- Jin X., Bauer D.E., Tuttleton S.E., Lewin S., Gettie A., Blanchard J., Irwin C.E., Safrit J.T., Mittler J., Weinberger L., Kostrikis L.G., Zhang L., Perelson A.S., Ho D.D. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado K.A., Yockey L.J., Wong P.W., Lee S., Huttner A.J., Iwasaki A. Antiviral CD8 T cells induce Zika-virus-associated paralysis in mice. Nat. Microbiol. 2018;3:141–147. doi: 10.1038/s41564-017-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I.A., MacLean J.A., Lee F.S., Casciotti L., DeHaan E., Schwartzman J.D., Luster A.D. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity. 2000;12:483–494. doi: 10.1016/s1074-7613(00)80200-9. [DOI] [PubMed] [Google Scholar]
- Kiniry B.E., Ganesh A., Critchfield J.W., Hunt P.W., Hecht F.M., Somsouk M., Deeks S.G., Shacklett B.L. Predominance of weakly cytotoxic, T-bet(Low)Eomes(Neg) CD8(+) T-cells in human gastrointestinal mucosa: implications for HIV infection. Mucosal Immunol. 2017;10:1008–1020. doi: 10.1038/mi.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiniry B.E., Hunt P.W., Hecht F.M., Somsouk M., Deeks S.G., Shacklett B.L. Differential expression of CD8(+) t cell cytotoxic effector molecules in blood and gastrointestinal mucosa in HIV-1 infection. J. Immunol. 2018;200:1876–1888. doi: 10.4049/jimmunol.1701532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiniry B.E., Li S., Ganesh A., Hunt P.W., Somsouk M., Skinner P.J., Deeks S.G., Shacklett B.L. Detection of HIV-1-specific gastrointestinal tissue resident CD8(+) T-cells in chronic infection. Mucosal Immunol. 2018;11:909–920. doi: 10.1038/mi.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt N.R., Shudo E., Ortiz A.M., Engram J.C., Paiardini M., Lawson B., Miller M.D., Else J., Pandrea I., Estes J.D., Apetrei C., Schmitz J.E., Ribeiro R.M., Perelson A.S., Silvestri G. CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R.R., Kronenberg D., Zhao M., Huang G.C., Eichmann M., Bulek A., Wooldridge L., Cole D.K., Sewell A.K., Peakman M., Skowera A. Human beta-cell killing by autoreactive preproinsulin-specific CD8 T cells is predominantly granule-mediated with the potency dependent upon T-cell receptor avidity. Diabetes. 2013;62:205–213. doi: 10.2337/db12-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S.L., Pham M.N., Folkvord J.M., Arends T., Miller S.M., Miles B., Meditz A.L., McCarter M., Levy D.N., Connick E. Germinal center t follicular helper cells are highly permissive to HIV-1 and alter their phenotype during virus replication. J. Immunol. 2016;196:2711–2722. doi: 10.4049/jimmunol.1502174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz G., Hueber A.O. The Fas/CD95 receptor regulates the death of autoreactive B cells and the selection of antigen-specific B cells. Front. Immunol. 2012;3:207. doi: 10.3389/fimmu.2012.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup R.A., Safrit J.T., Cao Y., Andrews C.A., McLeod G., Borkowsky W., Farthing C., Ho D.D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane B.R., Markovitz D.M., Woodford N.L., Rochford R., Strieter R.M., Coffey M.J. TNF-alpha inhibits HIV-1 replication in peripheral blood monocytes and alveolar macrophages by inducing the production of RANTES and decreasing C-C chemokine receptor 5 (CCR5) expression. J. Immunol. 1999;163:3653–3661. [PubMed] [Google Scholar]
- Leong Y.A., Chen Y., Ong H.S., Wu D., Man K., Deleage C., Minnich M., Meckiff B.J., Wei Y., Hou Z., Zotos D., Fenix K.A., Atnerkar A., Preston S., Chipman J.G., Beilman G.J., Allison C.C., Sun L., Wang P., Xu J., Toe J.G., Lu H.K., Tao Y., Palendira U., Dent A.L., Landay A.L., Pellegrini M., Comerford I., McColl S.R., Schacker T.W., Long H.M., Estes J.D., Busslinger M., Belz G.T., Lewin S.R., Kallies A., Yu D. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat. Immunol. 2016;17:1187–1196. doi: 10.1038/ni.3543. [DOI] [PubMed] [Google Scholar]
- Levy J.A. The search for the CD8+ cell anti-HIV factor (CAF) Trends Immunol. 2003;24:628–632. doi: 10.1016/j.it.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Li S., Folkvord J.M., Kovacs K.J., Wagstaff R.K., Mwakalundwa G., Rendahl A.K., Rakasz E.G., Connick E., Skinner P.J. Low levels of SIV-specific CD8+ T cells in germinal centers characterizes acute SIV infection. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Folkvord J.M., Rakasz E.G., Abdelaal H.M., Wagstaff R.K., Kovacs K.J., Kim H.O., Sawahata R., MaWhinney S., Masopust D., Connick E., Skinner P.J. Simian immunodeficiency virus-producing cells in follicles are partially suppressed by CD8+ cells in vivo. J. Virol. 2016;90:11168–11180. doi: 10.1128/JVI.01332-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat. Rev. Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- Lindqvist M., van Lunzen J., Soghoian D.Z., Kuhl B.D., Ranasinghe S., Kranias G., Flanders M.D., Cutler S., Yudanin N., Muller M.I., Davis I., Farber D., Hartjen P., Haag F., Alter G., Schulze zur Wiesch J., Streeck H. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J. Clin. Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makedonas G., Hutnick N., Haney D., Amick A.C., Gardner J., Cosma G., Hersperger A.R., Dolfi D., Wherry E.J., Ferrari G., Betts M.R. Perforin and IL-2 upregulation define qualitative differences among highly functional virus-specific human CD8 T cells. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinova E., Han S., Zheng B. Human germinal center T cells are unique Th cells with high propensity for apoptosis induction. Int. Immunol. 2006;18:1337–1345. doi: 10.1093/intimm/dxl066. [DOI] [PubMed] [Google Scholar]
- Masopust D., Soerens A.G. Tissue-resident t cells and other resident leukocytes. Annu. Rev. Immunol. 2019;37:521–546. doi: 10.1146/annurev-immunol-042617-053214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D., Vezys V., Marzo A.L., Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Matano T., Shibata R., Siemon C., Connors M., Lane H.C., Martin M.A. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migueles S.A., Laborico A.C., Shupert W.L., Sabbaghian M.S., Rabin R., Hallahan C.W., Van Baarle D., Kostense S., Miedema F., McLaughlin M., Ehler L., Metcalf J., Liu S., Connors M. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 2002;3:1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- Migueles S.A., Osborne C.M., Royce C., Compton A.A., Joshi R.P., Weeks K.A., Rood J.E., Berkley A.M., Sacha J.B., Cogliano-Shutta N.A., Lloyd M., Roby G., Kwan R., McLaughlin M., Stallings S., Rehm C., O’Shea M.A., Mican J., Packard B.Z., Komoriya A., Palmer S., Wiegand A.P., Maldarelli F., Coffin J.M., Mellors J.W., Hallahan C.W., Follman D.A., Connors M. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles B., Miller S.M., Folkvord J.M., Levy D.N., Rakasz E.G., Skinner P.J., Connick E. Follicular regulatory CD8 t cells impair the germinal center response in SIV and ex vivo HIV infection. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.M., Miles B., Guo K., Folkvord J., Meditz A.L., McCarter M.D., Levy D.N., MaWhinney S., Santiago M.L., Connick E. Follicular regulatory t cells are highly permissive to R5-Tropic HIV-1. J. Virol. 2017;91:430. doi: 10.1128/JVI.00430-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molodtsov A., Turk M.J. Tissue resident CD8 memory t cell responses in Cancer and autoimmunity. Front. Immunol. 2018;9:2810. doi: 10.3389/fimmu.2018.02810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylvaganam G.H., Rios D., Abdelaal H.M., Iyer S., Tharp G., Mavigner M., Hicks S., Chahroudi A., Ahmed R., Bosinger S.E., Williams I.R., Skinner P.J., Velu V., Amara R.R. Dynamics of SIV-specific CXCR5+ CD8 t cells during chronic SIV infection. Proc. Natl. Acad. Sci. U. S. A. 2017;114:1976–1981. doi: 10.1073/pnas.1621418114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen S., Deleage C., Darko S., Ransier A., Truong D.P., Agarwal D., Japp A.S., Wu V.H., Kuri-Cervantes L., Abdel-Mohsen M., Del Rio Estrada P.M., Ablanedo-Terrazas Y., Gostick E., Hoxie J.A., Zhang N.R., Naji A., Reyes-Teran G., Estes J.D., Price D.A., Douek D.C., Deeks S.G., Buggert M., Betts M.R. Elite control of HIV is associated with distinct functional and transcriptional signatures in lymphoid tissue CD8(+) T cells. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aax4077. eaax4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam P., Velu V., Kannanganat S., Chennareddi L., Kwa S., Siddiqui M., Amara R.R. Expansion of FOXP3+ CD8 T cells with suppressive potential in colorectal mucosa following a pathogenic simian immunodeficiency virus infection correlates with diminished antiviral T cell response and viral control. J. Immunol. 2010;184:1690–1701. doi: 10.4049/jimmunol.0902955. [DOI] [PubMed] [Google Scholar]
- Nitcheu J., Bonduelle O., Combadiere C., Tefit M., Seilhean D., Mazier D., Combadiere B. Perforin-dependent brain-infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. J. Immunol. 2003;170:2221–2228. doi: 10.4049/jimmunol.170.4.2221. [DOI] [PubMed] [Google Scholar]
- Novais F.O., Carvalho A.M., Clark M.L., Carvalho L.P., Beiting D.P., Brodsky I.E., Carvalho E.M., Scott P. CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1beta production. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novais F.O., Carvalho L.P., Graff J.W., Beiting D.P., Ruthel G., Roos D.S., Betts M.R., Goldschmidt M.H., Wilson M.E., de Oliveira C.I., Scott P. Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Butini L., Pizzo P.A., Schnittman S.M., Kotler D.P., Fauci A.S. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991;88:9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.L., Zaid A., Hor J.L., Christo S.N., Prier J.E., Davies B., Alexandre Y.O., Gregory J.L., Russell T.A., Gebhardt T., Carbone F.R., Tscharke D.C., Heath W.R., Mueller S.N., Mackay L.K. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 2018;19:183–191. doi: 10.1038/s41590-017-0027-5. [DOI] [PubMed] [Google Scholar]
- Perdomo-Celis F., Feria M.G., Taborda N.A., Rugeles M.T. Induction of follicular-like CXCR5+ CD8+ t cells by TGF-β1/IL-23 is limited during HIV infection. Viral Immunol. 2019 doi: 10.1089/vim.2019.0029. vim.2019.0029. [DOI] [PubMed] [Google Scholar]
- Perreau M., Savoye A.L., De Crignis E., Corpataux J.M., Cubas R., Haddad E.K., De Leval L., Graziosi C., Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J. Exp. Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters P.J., Borst J., Oorschot V., Fukuda M., Krahenbuhl O., Tschopp J., Slot J.W., Geuze H.J. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med. 1991;173:1099–1109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C., Ferrando-Martinez S., Gerner M.Y., Casazza J.P., Pegu A., Deleage C., Cooper A., Hataye J., Andrews S., Ambrozak D., Del Rio Estrada P.M., Boritz E., Paris R., Moysi E., Boswell K.L., Ruiz-Mateos E., Vagios I., Leal M., Ablanedo-Terrazas Y., Rivero A., Gonzalez-Hernandez L.A., McDermott A.B., Moir S., Reyes-Teran G., Docobo F., Pantaleo G., Douek D.C., Betts M.R., Estes J.D., Germain R.N., Mascola J.R., Koup R.A. Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aag2285. eaag2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino M., Paganini S., Deleage C., Padhan K., Harper J.L., King C.T., Micci L., Cervasi B., Mudd J.C., Gill K.P., Jean S.M., Easley K., Silvestri G., Estes J.D., Petrovas C., Lederman M.M., Paiardini M. Fingolimod retains cytolytic T cells and limits T follicular helper cell infection in lymphoid sites of SIV persistence. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirozyan M.R., Nguyen N., Cameron B., Luciani F., Bull R.A., Zekry A., Lloyd A.R. Chemokine-regulated recruitment of antigen-specific T-Cell subpopulations to the liver in acute and chronic hepatitis C infection. J. Infect. Dis. 2019;219:1430–1438. doi: 10.1093/infdis/jiy679. [DOI] [PubMed] [Google Scholar]
- Podack E.R., Young J.D., Cohn Z.A. Isolation and biochemical and functional characterization of perforin 1 from cytolytic T-cell granules. Proc Natl Acad Sci U S A. 1985;82:8629–8633. doi: 10.1073/pnas.82.24.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M.F., Abel K., Zuber B., Miller C.J., Sandberg J.K., Shacklett B.L. Perforin expression in the gastrointestinal mucosa is limited to acute simian immunodeficiency virus infection. J. Virol. 2006;80:3083–3087. doi: 10.1128/JVI.80.6.3083-3087.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M.F., Gonzalez V.D., Granath A., Andersson J., Sandberg J.K. CXCR5+ CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur. J. Immunol. 2007;37:3352–3362. doi: 10.1002/eji.200636746. [DOI] [PubMed] [Google Scholar]
- Rahman M.A., McKinnon K.M., Karpova T.S., Ball D.A., Venzon D.J., Fan W., Kang G., Li Q., Robert-Guroff M. Associations of simian immunodeficiency virus (SIV)-Specific follicular CD8(+) t cells with other follicular t cells suggest complex contributions to SIV viremia control. J. Immunol. 2018;200:2714–2726. doi: 10.4049/jimmunol.1701403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasool S.T., Tang H., Wu J., Li W., Mukhtar M.M., Zhang J., Mu Y., Xing H.X., Wu J., Zhu Y. Increased level of IL-32 during human immunodeficiency virus infection suppresses HIV replication. Immunol. Lett. 2008;117:161–167. doi: 10.1016/j.imlet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Reinhart T.A., Fallert B.A., Pfeifer M.E., Sanghavi S., Capuano S., Rajakumar P., 3rd, Murphey-Corb M., Day R., Fuller C.L., Schaefer T.M. Increased expression of the inflammatory chemokine CXC chemokine ligand 9/monokine induced by interferon-gamma in lymphoid tissues of rhesus macaques during simian immunodeficiency virus infection and acquired immunodeficiency syndrome. Blood. 2002;99:3119–3128. doi: 10.1182/blood.v99.9.3119. [DOI] [PubMed] [Google Scholar]
- Reuter M.A., Del Rio Estrada P.M., Buggert M., Petrovas C., Ferrando-Martinez S., Nguyen S., Sada Japp A., Ablanedo-Terrazas Y., Rivero-Arrieta A., Kuri-Cervantes L., Gunzelman H.M., Gostick E., Price D.A., Koup R.A., Naji A., Canaday D.H., Reyes-Teran G., Betts M.R. HIV-specific CD8(+) t cells exhibit reduced and differentially regulated cytolytic activity in lymphoid tissue. Cell Rep. 2017;21:3458–3470. doi: 10.1016/j.celrep.2017.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Dias F., Saar Gomes R., de Lima Silva L.L., Dos Santos J.C., Joosten L.A. Interleukin 32: a novel player in the control of infectious diseases. J. Leukoc. Biol. 2017;101:39–52. doi: 10.1189/jlb.4RU0416-175RR. [DOI] [PubMed] [Google Scholar]
- Roberts E.R., Carnathan D.G., Li H., Shaw G.M., Silvestri G., Betts M.R. Collapse of cytolytic potential in SIV-Specific CD8+ t cells following acute SIV infection in Rhesus macaques. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Schenkel J.M., Fraser K.A., Beura L.K., Pauken K.E., Vezys V., Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel J.M., Fraser K.A., Vezys V., Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat. Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel J.M., Masopust D. Tissue-resident memory t cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.E., Knudson C.J., Hartwig S.M., Pewe L.L., Meyerholz D.K., Langlois R.A., Harty J.T., Varga S.M. Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N., Liu Y., Bentebibel S.E., Munagala I., Bourdery L., Venuprasad K., Banchereau J., Ueno H. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat. Immunol. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J.E., Kuroda M.J., Santra S., Sasseville V.G., Simon M.A., Lifton M.A., Racz P., Tenner-Racz K., Dalesandro M., Scallon B.J., Ghrayeb J., Forman M.A., Montefiori D.C., Rieber E.P., Letvin N.L., Reimann K.A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Shacklett B.L., Ferre A.L., Kiniry B.E. Tissue issues: mucosal T-cell responses in HIV-1 infection. Curr. Opin. HIV AIDS. 2019;14:100–107. doi: 10.1097/COH.0000000000000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow L.R., Rosen D.B., Brdickova N., Xu Y., An J., Lanier L.L., Cyster J.G., Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Silverio J.C., Pereira I.R., Cipitelli Mda C., Vinagre N.F., Rodrigues M.M., Gazzinelli R.T., Lannes-Vieira J. CD8+ T-cells expressing interferon gamma or perforin play antagonistic roles in heart injury in experimental Trypanosoma cruzi-elicited cardiomyopathy. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri G., Sodora D.L., Koup R.A., Paiardini M., O’Neil S.P., McClure H.M., Staprans S.I., Feinberg M.B. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- Skowera A., Ellis R.J., Varela-Calvino R., Arif S., Huang G.C., Van-Krinks C., Zaremba A., Rackham C., Allen J.S., Tree T.I., Zhao M., Dayan C.M., Sewell A.K., Unger W.W., Drijfhout J.W., Ossendorp F., Roep B.O., Peakman M. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J. Clin. Invest. 2008;118:3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulina C., Schmidt S., Dornmair K., Babbe H., Roers A., Rajewsky K., Wekerle H., Hohlfeld R., Goebels N. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci U S A. 2004;101:2428–2433. doi: 10.1073/pnas.0308689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutter B., Pewe L.L., Kaech S.M., Harty J.T. Lung airway-surveilling CXCR3(hi) memory CD8(+) T cells are critical for protection against influenza A virus. Immunity. 2013;39:939–948. doi: 10.1016/j.immuni.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan B.M., Juedes A., Szabo S.J., von Herrath M., Glimcher L.H. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J.J., Yudanin N., Ohmura Y., Kubota M., Grinshpun B., Sathaliyawala T., Kato T., Lerner H., Shen Y., Farber D.L. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson B.E., Wagner D.K., Nelson D.L., Sullivan J.L. Activated lymphocytes during acute Epstein-Barr virus infection. J. Immunol. 1987;139:3802–3807. [PubMed] [Google Scholar]
- Trivedi P., Graham K.L., Krishnamurthy B., Fynch S., Slattery R.M., Kay T.W., Thomas H.E. Perforin facilitates beta cell killing and regulates autoreactive CD8+ T-cell responses to antigen in mouse models of type 1 diabetes. Immunol. Cell Biol. 2016;94:334–341. doi: 10.1038/icb.2015.89. [DOI] [PubMed] [Google Scholar]
- Tzartos J.S., Friese M.A., Craner M.J., Palace J., Newcombe J., Esiri M.M., Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger W.W., Pearson T., Abreu J.R., Laban S., van der Slik A.R., der Kracht S.M., Kester M.G., Serreze D.V., Shultz L.D., Griffioen M., Drijfhout J.W., Greiner D.L., Roep B.O. Islet-specific CTL cloned from a type 1 diabetes patient cause beta-cell destruction after engraftment into HLA-A2 transgenic NOD/scid/IL2RG null mice. PLoS One. 2012;7:e49213. doi: 10.1371/journal.pone.0049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno S., Imagawa A., Saisho K., Okita K., Iwahashi H., Hanafusa T., Shimomura I. Expression of chemokines, CXC chemokine ligand 10 (CXCL10) and CXCR3 in the inflamed islets of patients with recent-onset autoimmune type 1 diabetes. Endocr. J. 2010;57:991–996. doi: 10.1507/endocrj.k10e-076. [DOI] [PubMed] [Google Scholar]
- Vago L., Antonacci M.C., Cristina S., Parravicini C., Lazzarin A., Moroni M., Negri C., Uberti-Foppa C., Musicco M., Costanzi G. Morphogenesis, evolution and prognostic significance of lymphatic tissue lesions in HIV infection. Appl. Pathol. 1989;7:298–309. [PubMed] [Google Scholar]
- van den Boorn J.G., Konijnenberg D., Dellemijn T.A., van der Veen J.P., Bos J.D., Melief C.J., Vyth-Dreese F.A., Luiten R.M. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J. Invest. Dermatol. 2009;129:2220–2232. doi: 10.1038/jid.2009.32. [DOI] [PubMed] [Google Scholar]
- Vinuesa C.G., Linterman M.A., Yu D., MacLennan I.C. Follicular helper t cells. Annu. Rev. Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- von Andrian U.H., Mempel T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Voskoboinik I., Whisstock J.C., Trapani J.A. Perforin and granzymes: function, dysfunction and human pathology. Nat. Rev. Immunol. 2015;15:388–400. doi: 10.1038/nri3839. [DOI] [PubMed] [Google Scholar]
- Waldmann T.A. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- Walker C.M., Moody D.J., Stites D.P., Levy J.A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Watson D.C., Moysi E., Valentin A., Bergamaschi C., Devasundaram S., Fortis S.P., Bear J., Chertova E., Bess J., Sowder R., Jr., Venzon D.J., Deleage C., Estes J.D., Lifson J.D., Petrovas C., Felber B.K., Pavlakis G.N. Treatment with native heterodimeric IL-15 increases cytotoxic lymphocytes and reduces SHIV RNA in lymph nodes. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb G.M., Li S., Mwakalundwa G., Folkvord J.M., Greene J.M., Reed J.S., Stanton J.J., Legasse A.W., Hobbs T., Martin L.D., Park B.S., Whitney J.B., Jeng E.K., Wong H.C., Nixon D.F., Jones R.B., Connick E., Skinner P.J., Sacha J.B. The human IL-15 superagonist ALT-803 directs SIV-specific CD8(+) t cells into B-cell follicles. Blood Adv. 2018;2:76–84. doi: 10.1182/bloodadvances.2017012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox A., Richardson S.J., Bone A.J., Foulis A.K., Morgan N.G. Analysis of islet inflammation in human type 1 diabetes. Clin. Exp. Immunol. 2009;155:173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolint P., Betts M.R., Koup R.A., Oxenius A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J. Exp. Med. 2004;199:925–936. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J.K., Strain M.C., Porrata R., Reay E., Sankaran-Walters S., Ignacio C.C., Russell T., Pillai S.K., Looney D.J., Dandekar S. In vivo CD8+ T-cell suppression of siv viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon H.G., Braun A., Li J., Smith C., Edwards J., Sierro F., Feng C.G., Khanna R., Elliot M., Bell A., Hislop A.D., Tangye S.G., Rickinson A.B., Gebhardt T., Britton W.J., Palendira U. Compartmentalization of total and virus-specific tissue-resident memory CD8+ t cells in human lymphoid organs. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Pegu A., Rao E., Doria-Rose N., Beninga J., McKee K., Lord D.M., Wei R.R., Deng G., Louder M., Schmidt S.D., Mankoff Z., Wu L., Asokan M., Beil C., Lange C., Leuschner W.D., Kruip J., Sendak R., Kwon Y.D., Zhou T., Chen X., Bailer R.T., Wang K., Choe M., Tartaglia L.J., Barouch D.H., O’Dell S., Todd J.P., Burton D.R., Roederer M., Connors M., Koup R.A., Kwong P.D., Yang Z.Y., Mascola J.R., Nabel G.J. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science. 2017;358:85–90. doi: 10.1126/science.aan8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Phetsouphanh C., Suzuki K., Aggrawal A., Graff-Dubois S., Roche M., Bailey M., Alcantara S., Cashin K., Sivasubramaniam R., Koelsch K.K., Autran B., Harvey R., Gorry P.R., Moris A., Cooper D.A., Turville S., Kent S.J., Kelleher A.D., Zaunders J. HIV-1 and SIV predominantly use CCR5 expressed on a precursor population to establish infection in t follicular helper cells. Front. Immunol. 2017;8:376. doi: 10.3389/fimmu.2017.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Ye L. A portrait of CXCR5(+) follicular cytotoxic CD8(+) t cells. Trends Immunol. 2018;39:965–979. doi: 10.1016/j.it.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Yu Y., Ma X., Gong R., Zhu J., Wei L., Yao J. Recent advances in CD8(+) regulatory T cell research. Oncol. Lett. 2018;15:8187–8194. doi: 10.3892/ol.2018.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M., Smith A.J., Wietgrefe S.W., Southern P.J., Schacker T.W., Reilly C.S., Estes J.D., Burton G.F., Silvestri G., Lifson J.D., Carlis J.V., Haase A.T. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J. Clin. Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]