Abstract
Objective:
To describe an unusual immune-related adverse event (irAE), acquired generalized lipodystrophy (AGL), from checkpoint inhibitor therapy in a patient treated with pembrolizumab.
Methods:
This is a case report of a 67-year-old male with metastatic melanoma who was treated with pembrolizumab. Prior to pembrolizumab, the patient was treated with another immune-checkpoint inhibitor and developed autoimmune hemolytic anemia. After starting pembrolizumab, he developed a scrotal mass consistent with panniculitis and after several subsequent cycles, he developed AGL.
Results:
Loss of subcutaneous fat, unexplained weight loss in combination with worsening insulin resistance and worsening hypertriglyceridemia after initiation of pembrolizumab were consistent with AGL. Autoimmune disorders and other etiologies were ruled out. Despite this irAE, the patient continued to receive pembrolizumab given stabilization of melanoma with treatment.
Conclusion:
We report the second case of a patient who developed AGL secondary to pembrolizumab, and the fourth case to report such complication secondary to antiprogrammed cell death receptor-1 inhibitors. As use of checkpoint inhibitors becomes more common to treat several types of cancer, it is vital for clinicians to recognize these rare irreversible complications that are not frequently reported in clinical trials.
INTRODUCTION
Pembrolizumab is a fully humanized antiprogrammed cell death receptor-1 (PD-1) monoclonal antibody that has shown significant antitumor activity in a variety of cancers with improvement of survival outcomes in patients with advanced melanoma (1). However, enhancement of the antitumor response is associated with several well-characterized endocrine side effects including thyroiditis, hypophysitis, and type 1 diabetes (2–5).
Acquired lipodystrophy is a rare but potential immune-related adverse event (irAE) that has been described in 3 case reports so far (6,7,8). We report the second case of acquired generalized lipodystrophy (AGL) in a patient treated with pembrolizumab for advanced melanoma. This syndrome is characterized by the selective loss of fat from the face, arms, and legs, with higher risk to develop insulin resistance and dyslipidemia. It has previously been associated with autoimmune diseases, infections causing panniculitis, and certain medications such as protease inhibitors (9). The pathogenic mechanism of fat destruction in AGL remains unknown but evidence suggests an autoimmune origin (10–12). With the increased use of PD-1 therapies for several cancer subtypes, clinicians need to be aware of this rare irAE which may present similarly to checkpoint inhibitor-associated diabetes but with significant phenotypic changes consistent with lipodystrophy, and without evidence of ketoacidosis (8).
CASE REPORT
A 67-year-old man with a history of type 2 diabetes mellitus (T2DM) and hypertriglyceridemia was evaluated at our institution in 2015 after he was found to have a right inguinal mass and multiple pulmonary nodules which were consistent with metastatic malignant melanoma. Prior to initiating any treatment for melanoma, the patient had a history of well controlled T2DM (hemoglobin A1c 5.9% [41 mmol/mol]) and was on metformin and long acting insulin therapy (0.1 units/kg/day). He was obese with a body mass index (BMI) of 38.5 kg/m2. His hypertriglyceridemia was managed with statins and omega 3 fatty acids, with persistent hypertriglyceridemia (400 mg/dL) despite therapy. He also had a history of primary hypothyroidism and was on thyroid hormone replacement therapy. He did not have any history of being infected with the human immunodeficiency virus, autoimmune diseases, or use of medications associated with lipodystrophy.
For treatment of metastatic melanoma, patient was initiated on nivolumab in combination with ipilimumab; however, ipilimumab was discontinued after 2 doses due to the development of immune-mediated hemolytic anemia and thrombocytopenia, which were treated with high dose steroids. The patient continued to receive nivolumab as a single agent therapy for a total of 20 doses, which was completed in 2016 during which his melanoma remained stable.
In 2018, he had a recurrence of melanoma and was reinitiated on therapy with pembrolizumab. After 2 doses of pembrolizumab, he developed an enlarging left scrotal mass which was evaluated further with ultrasound. A scrotal ultrasound revealed a 4.9 × 10 × 3 cm lobulated echogenic mass with increased vascularity in the left scrotal sac. A biopsy of this mass showed adipose tissue with reactive changes, and extensive macrophage infiltration which was consistent with fat necrosis and panniculitis. Prior to starting pembrolizumab, the patient was still on metformin and long acting insulin therapy (0.1 units/kg/day) for T2DM management with hemoglobin A1c of 7.0% (53 mmol/mol).
After cycle 5 of pembrolizumab, the patient presented to the endocrine clinic with a rapid unexplained weight loss of 30 pounds over the course of the following 4 months. Physical examination revealed facial lipoatrophy, with loss of buccal fat pads, prominence of the zygomatic arch, and loss of subcutaneous fat in the neck and chest. The patient had a prominence of the superficial veins in the upper arms and abdomen with an increase in truncal fat. The patient was noted to have fat loss affecting his lower extremities and buttocks with increased muscularity of the upper arms and lower legs (Fig. 1). Despite weight loss and a reduction of his BMI to 27 kg/m2, he was noted to have worsening hyperglycemia with glucose values ranging from 160 to 330 mg/dL despite increasing basal and bolus insulin therapy (1.3 units/kg/day). Since his presentation was concerning for lipodystrophy, insulin doses were increased further, and pioglitazone was added to decrease insulin resistance. He denied any family history of lipodystrophy.
Fig. 1.
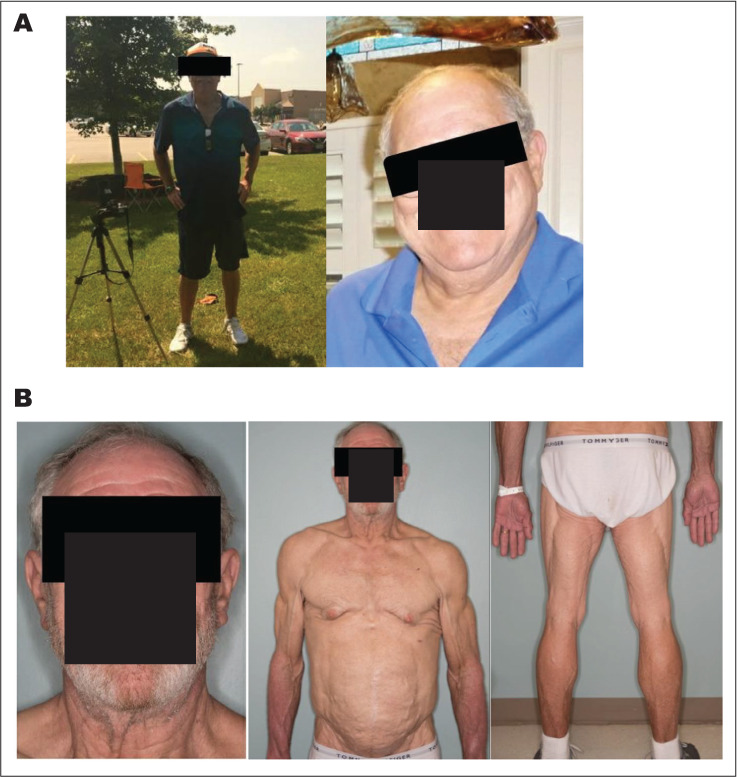
Clinical features of pembrolizumab-induced lipodystrophy. Clinical photographs that demonstrate the change of fat distribution before and after treatment with pembrolizumab. A, Before treatment. B, After initiation of immunotherapy. There is central obesity and decreased adipose tissue in the extremities.
Laboratory evaluation included c-peptide 6.2 ng/mL (normal, 1.1 to 4.4 ng/mL) with serum glucose of 141 mg/dL. Leptin level was less than 0.6 ng/mL (normal leptin levels for patient's BMI of 27, 0.7 to 5.3 ng/mL). Adiponectin was evaluated and it was undetectable. Thyroid function tests were within normal limits. There was no elevation of liver enzymes and the hepatitis profile was negative. Evaluation for autoimmune diseases including systemic lupus erythematosus, myositis, and other systemic diseases such as sarcoidosis were ruled out clinically and serologically. A lipid profile revealed extreme elevation of triglycerides level (1,708 mg/dL) and low high-density lipoprotein (HDL; 18 mg/dL). As the patient was unable to tolerate higher doses of statin therapy, he was advised to initiate fibrate therapy, and continue omega-3 fatty acids and statin treatment. Patient did not have any evidence of pancreatitis and pancreatic enzymes remained within normal limits throughout his course. There was no evidence of liver steatosis on recent imaging. Changes in weight and in metabolic profile during treatment are shown in Figure 2.
Fig. 2.
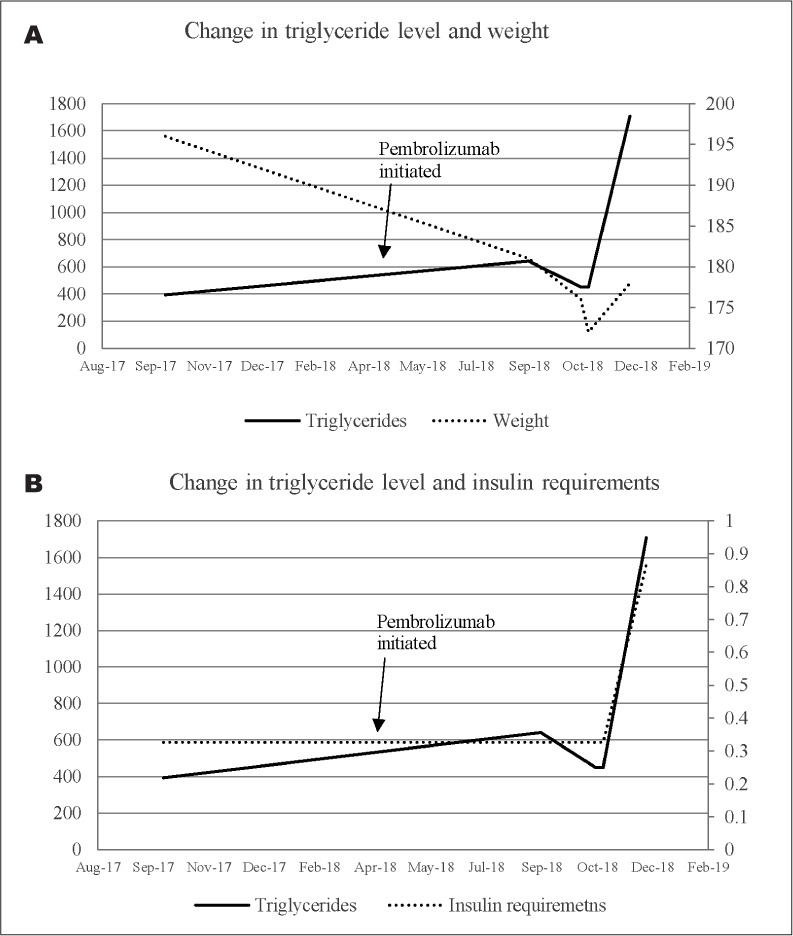
Changes in weight, triglyceride levels, and insulin requirements over time. A, Changes in triglyceride level and weight. B, Changes in triglyceride level and insulin requirements.
The overall clinical picture characterized by loss of subcutaneous fat, unexplained weight loss, marked insulin resistance and significant worsening of hypertriglyceridemia was suggestive of lipodystrophy. Of interest, the patient continued pembrolizumab despite this complication as his melanoma responded well to therapy.
DISCUSSION
Lipodystrophy syndromes are rare heterogeneous disorders characterized by loss of adipose tissue without evidence of nutritional deprivation resulting in multiple metabolic complications such as insulin resistance, diabetes, hypertriglyceridemia, polycystic ovary syndrome, and nonalcoholic fatty liver disease (13). Because of its rarity and heterogeneity, lipodystrophy may frequently be unrecognized or misdiagnosed, which is concerning because it is a progressive disease with potentially life-threatening complications.
Lipodystrophy is classified in 4 major subtypes: AGL, congenital generalized lipodystrophy, familial partial lipodystrophy, and acquired partial lipodystrophy (14). AGL is a syndrome that has been associated with autoimmune diseases, infections causing panniculitis, and certain medications such as protease inhibitors (9), interferon beta-1a (15), and more recently associated with checkpoint inhibitors in 3 other published cases (6,7,8).
Checkpoint inhibitors are agents that target PD-1 receptors, such as nivolumab and pembrolizumab, programmed death-ligand 1, such as atezolizumab and durvalumab, or cytotoxic T-lymphocyte antigen-4 such as ipilimumab. Immunotherapy has provided great clinical benefit and is currently considered part of standard treatment of multiple malignancies. Despite the clinical benefits provided by this group of medications (1), important endocrine-related adverse effects have been described with these drugs such as primary and secondary hypothyroidism, thyroiditis, hypophysitis, primary and secondary adrenal insufficiency, and type 1 diabetes (2–5). While thyroiditis and hypophysitis can resolve without sequelae, most endocrine irAE from immune checkpoint inhibitor therapy result in long term need for hormone replacement therapy and are irreversible. While this is burdensome to patients, unlike other endocrine irAEs, AGL is a unique adverse event that is associated with physical findings that are more evident and can present additional psychologic burden to the patient due to social stigma.
While the patient had many findings of metabolic syndrome at baseline prior to starting immune checkpoint inhibitor therapy, it is unknown if these factors contributed to the development of AGL. Our patient developed autoimmune hemolytic anemia while on ipilimumab which may have heralded his predisposition to develop autoimmune complications from immunotherapy. In addition, after 2 doses of pembrolizumab, the patient developed a scrotal mass consistent with panniculitis prior to getting the physical changes consistent with AGL. It is possible that localized panniculitis may predict the risk of developing generalized lipodystrophy, and if this is found in a patient on immunotherapy, one might consider the risk of AGL with continued therapy.
There are 4 case reports to date on AGL development in patients treated with immune checkpoint inhibitor therapy. Falcao et al (6) reported the first case of AGL as an adverse reaction to a PD-1 inhibitor (nivolumab) in a patient with advanced renal cell carcinoma. The second case was published by Haddad et al (7), who described the case of a patient with malignant melanoma treated with pembrolizumab who developed AGL. A recently published case described a patient with malignant melanoma who developed AGL after treatment with nivolumab (8). We report the second case of acquired lipodystrophy associated with pembrolizumab. Table 1 provides a comparison between our case and previous reported cases. We have also included a graphical representation of the steps we followed to complete the diagnostic workup of our patient (Fig. 3).
Table 1.
Description of Current and Previous Cases of AGL Related to Check-Point Inhibitors
| Case and reference | ||||
|---|---|---|---|---|
| 1a | 2 (6)b | 3 (7)b | 4 (8)b | |
| Age | 67 | 50 | 47 | 62 |
| Gender | Male | Female | Female | Female |
| Type of cancer | Melanoma | Renal cell carcinoma | Melanoma | Melanoma |
| Medication | Pembrolizumab | Nivolumab | Pembrolizumab | Pembrolizumab |
| Onset of presentation (after initiation of immunotherapy) | 6 weeks | 2 months | 2 months | 18 months |
| Hemoglobin A1c (%) at diagnosis of AGL, (mmol/mol) | 7.2% (55 mmol/mol) | 10.5% (91 mmol/mol) | 6.1% (43 mmol/mol) | 11.4% (101 mmol/mol) |
| Worsening dislipidemia | Yes | Yes | Yes | Yes |
| Leptin (ng/mL) | 0.6 ng/mL (0.7–5.3) | 0.08 ng/mL (0.08–0.38) | 27.2 ng/mL | Undetectable |
| Immunotherapy discontinued? | No | No | Yes (completed) | Yes |
Abbreviation: AGL = acquired generalized lipodystrophy.
aCase presented in this manuscript.
bThese cases are presented in the respective references, listed at the end of the manuscript.
Fig. 3.
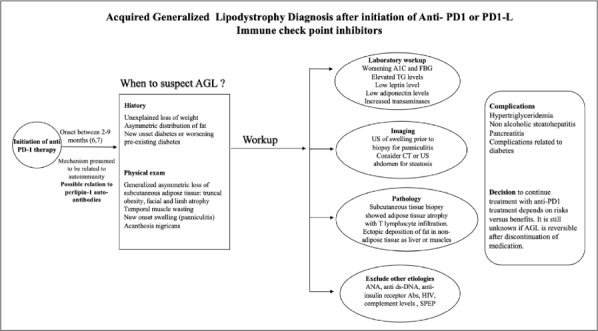
Graphic representation of the diagnostic workup of acute generalized lipodystrophy in patients treated with PD-1 therapy. Abs = antibodies; AGL = acquired generalized lipodystrophy; ANA = antinuclear antibody; anti-dsDNA = anti-double stranded deoxyribonucleic acid antibody; CT = computed tomography; FBG = fast blood glucose; HIV = human immunodeficiency virus; PD-1 = antiprogrammed cell death receptor-1; SPEP = serum protein electrophoresis; TG = triglyceride; US = ultrasound.
While the mechanism of PD-1 inhibitors-associated lipodystrophy remains unclear, there is evidence of an autoimmune process of fat destruction in lipodystrophy. Anti-adipocyte antibodies have been previously reported in patients with AGL, although their involvement in the pathogenesis has not been studied and the target antigens remain to be identified (10–12). Antiperilipin-1 IgG auto-antibodies have been recently reported in the serum of patients with autoimmune variety-AGL (10). These auto-antibodies alter the ability of perilipin-1 to regulate lipolysis in cultured preadipocytes resulting in an elevation of lipolysis (10).
Immune checkpoint inhibitors are associated with a variety of autoimmune endocrine irAEs. Given that patients often experience more than one endocrine irAE with the use of combination immunotherapies, it is possible that a single irAE may trigger metabolic changes that predispose to other irAEs. This may explain the findings reported in other case reports of immune checkpoint inhibitor-associated AGL where patients also develop other autoimmune complications.
Classic AGL usually is more common in women and is typically preceded by an infection and may be seen in association with other autoimmune conditions such as Hashimoto thyroiditis, hemolytic anemia, or rheumatoid arthritis. With pembrolizumab-associated AGL, it is unclear if there is an infectious etiology in addition to the exposure of the drug; however, it is also associated with other autoimmune complications from immune checkpoint inhibitor treatment. Of the 3 other cases of checkpoint inhibitor therapy-associated AGL, ours is unique as it occurred in a male patient who had autoimmune hemolytic anemia prior to developing AGL several years later. Regardless of the etiology, both pembrolizumab-associated AGL and classic AGL present with the same physical changes and metabolic abnormalities of insulin resistance, hypertriglyceridemia, and low HDL.
Finally, the treatment of AGL includes management of each metabolic condition. Treatment of insulin resistance requires use of insulin sensitizers such as metformin. Peroxisome proliferator-activated receptor gamma is important for adipose differentiation and thus thiazolinediones can also be used to improve insulin resistance by improving the capacity of adipose tissue to store fat, which has been described in other reports (16). Patients usually require high doses of insulin to control hyperglycemia. Hyperlipidemia can be treated with a combination therapy of statins, fibrates, omega-3 fatty acids, ezetimibe and/or proprotein convertase subtilisin/kexin type 9 inhibitors. The use of metreleptin, a human leptin analog, has shown significant improvement in metabolic profiles of patients with AGL in previous studies (17,18); however, those cases of AGL were not related to immunotherapy. As irAEs are considered to be irreversible, it is unclear if these patients would benefit from this therapy and further studies are needed to determine the effectiveness of this drug in this patient population.
CONCLUSION
Sudden unexplained insulin resistance, severe hypertriglyceridemia and loss of subcutaneous fat in a patient receiving a PD-1 inhibitor should raise suspicion of AGL as a rare irAE. This is a unique case in a male patient with metabolic syndrome and T2DM who developed localized panniculitis in the form of a scrotal mass and then presented with AGL several months later while on treatment with pembrolizumab. This complication is associated with several metabolic abnormalities and increased social stigma due to a change in the physical appearance of the patient and it is important for clinicians to familiarize themselves with the diagnosis and management of this rare side effect.
Abbreviations
- AGL
acquired generalized lipodystrophy
- BMI
body mass index
- IrAE
immune-related adverse event
- PD-1
antiprogrammed cell death receptor-1
- T2DM
type 2 diabetes mellitus
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Sahni S, Valecha G, Sahni A. Role of anti-PD-1 antibodies in advanced melanoma: the era of immunotherapy. Cureus. 2018;10 doi: 10.7759/cureus.3700. e3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baraibar I, Melero I, Ponz-Sarvise M, Castanon E. Safety and tolerability of immune checkpoint inhibitors (PD-1 and PD-L1) in cancer. Drug Saf. 2019;42:281–294. doi: 10.1007/s40264-018-0774-8. [DOI] [PubMed] [Google Scholar]
- 3.Sznol M, Postow MA, Davies MJ et al. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev. 2017;58:70–76. doi: 10.1016/j.ctrv.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Okahata S, Sakamoto K, Mitsumatsu T et al. Fulminant type 1 diabetes associated with Isolated ACTH deficiency induced by anti-programmed cell death 1 antibody-insight into the pathogenesis of autoimmune endocrinopathy. Endocr J. 2019;66:295–300. doi: 10.1507/endocrj.EJ18-0328. [DOI] [PubMed] [Google Scholar]
- 5.Chae YK, Chiec L, Mohindra N, Gentzler R, Patel J, Giles F. A case of pembrolizumab-induced type-1 diabetes mellitus and discussion of immune checkpoint inhibitor-induced type 1 diabetes. Cancer Immunol Immunother. 2017;66:25–32. doi: 10.1007/s00262-016-1913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falcao CK, Cabral MCS, Mota JM et al. Acquired lipodystrophy associated with nivolumab in a patient with advanced renal cell carcinoma. J Clin Endocrinol Metab. 2019;104:3245–3248. doi: 10.1210/jc.2018-02221. [DOI] [PubMed] [Google Scholar]
- 7.Haddad N, Vidal-Trecan T, Baroudjian B et al. Acquired generalized lipodystrophy under immune checkpoint inhibition. Br J Dermatol. 2019 doi: 10.1111/bjd.18124. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Jehl A, Cugnet-Anceau C, Vigouroux C et al. Acquired generalized lipodystrophy: a new cause of anti-PD-1 immune-related diabetes. Diabetes Care. 2019;42:2008–2010. doi: 10.2337/dc18-2535. [DOI] [PubMed] [Google Scholar]
- 9.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 10.Corvillo F, Aparicio V, López-Lera A et al. Autoantibodies against perilipin 1 as a cause of acquired generalized lipodystrophy. Front Immunol. 2018;9:2142. doi: 10.3389/fimmu.2018.02142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy Y, George J, Yona E, Shoenfeld Y. Partial lipodystrophy, mesangiocapillary glomerulonephritis, and complement dysregulation an autoimmune phenomenon. Immunol Res. 1998;18:55–60. doi: 10.1007/BF02786513. [DOI] [PubMed] [Google Scholar]
- 12.Billings JK, Milgraum SS, Gupta AK, Headington JT, Rasmussen JE. Lipoatrophic panniculitis: a possible autoimmune inflammatory disease of fat. Report of three cases. Arch Dermatol. 1987;123:1662–1666. [PubMed] [Google Scholar]
- 13.Brown RJ, Araujo-Vilar D, Cheung PT et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101:4500–4511. doi: 10.1210/jc.2016-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araujo-Vilar D, Santini F. Diagnosis and treatment of lipodystrophy: a step-by-step approach. J Endocrinol Invest. 2019;42:61–73. doi: 10.1007/s40618-018-0887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beiske AG, Myhr KM. Lipoatrophy: a non-reversible complication of subcutaneous interferon-beta 1a treatment of multiple sclerosis. J Neurol. 2006;253:377–378. doi: 10.1007/s00415-006-0898-0. [DOI] [PubMed] [Google Scholar]
- 16.Sutinen J. The effects of thiazolidinediones on metabolic complications and lipodystrophy in HIV-infected patients. PPAR Res. 2009;2009 doi: 10.1155/2009/373524. 373524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown RJ, Oral EA, Cochran E et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine. 2018;60:479–489. doi: 10.1007/s12020-018-1589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan JL, Lutz K, Cochran E et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17:922–932. doi: 10.4158/EP11229.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]


