Abstract
Background: The efficacy of paclitaxel and bevacizumab (PB) compared with other chemotherapies in patients with human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer is unclear. Patients and Methods: We retrospectively investigated 301 patients with HER2− ABC who received first-line chemotherapy from January 2011 to December 2016. Results: We included 114 patients who received PB and 187 patients who received other chemotherapies. After propensity score matching, the PB group showed a significantly superior overall response rate (77.8% vs. 38.9%, p<0.0001) and median time to treatment failure (7.3 vs. 5.9 months, p=0.035). In subgroup analyses, PB improved the median overall survival of patients with pleural lesions or pulmonary lymphangiopathy (not reached vs. 18.9 months, p=0.037), and of patients with three or more metastatic sites without liver metastases, (48.0 vs. 27.3 months, p=0.015). Conclusion: Compared with conventional chemotherapy, PB improved the overall response rate and time to treatment failure in patients with HER2− advanced breast cancer and improved overall survival in some patient subgroups.
Keywords: Advanced breast cancer, bevacizumab, HER2-negative, propensity score matching, real world
Anthracyclines, taxanes, and capecitabine are used in cytotoxic regimens against human epidermal growth factor receptor-2 negative (HER2−) advanced breast cancer (ABC); bevacizumab combined with a cytotoxic agent is only considered for selected patients (1). In some clinical trials, paclitaxel with bevacizumab (PB) improved progression-free survival (PFS) and the objective response rate (ORR) of patients with HER2− ABC; however, overall survival (OS) was not improved (2-8). Recently, significant improvements in OS with PB as first-line chemotherapy (CTx) for HER2− ABC have been reported based on the Epidemiological Strategy and Medical Economics (ESME) database, showing the efficacy of first-line PB in prolonging OS in a real-world setting (9).
However, no trials have compared PB with CTx (e.g. docetaxel, capecitabine, anthracyclines) other than paclitaxel alone as first-line treatment for HER2− ABC. Furthermore, subgroups of patients whose OS might be improved by PB have not been identified. Therefore, we retrospectively investigated the effectiveness of PB as first-line CTx, as well as the characteristics of patients with HER2− ABC whose OS was improved by PB in a multicentre, real-world setting.
Patients and Methods
Patients. We retrospectively analyzed patients with HER2− ABC who received first-line CTx at a registered site (Fukuyama City Hospital, Hiroshima City Hiroshima Citizens Hospital, or Shizuoka Cancer Centre). Medical information of patients who were treated at these three institutions between January 2011 and December 2016 were extracted from medical records and their characteristics and outcomes analyzed. Tumour subtypes were classified according to the American Society of Clinical Oncology/College of American Pathologists guidelines (10). Pathological reports of surgical specimens or initial biopsy specimens were used. We also preferentially used biopsies from metastases and recurrent tumours when available. Treatment responses were assessed by the Response Evaluation Criteria in Solid Tumours (RECIST) (11). Surveillance intervals were determined by each physician’s based on the patient’s clinical need. This retrospective study was approved by the Institutional Review Boards of each participating institution (approval number: Fukuyama City Hospital: 359; Hiroshima City Hiroshima Citizens Hospital: 2019-73; and Shizuoka Cancer Center: T30-25). All procedures performed were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was waived because this study used retrospective clinical data that were analysed anonymously.
Treatments. CTx regimens, including dose modifications, interruptions, or discontinuations, were determined based on the physician's judgment and patient preferences.
Patients were divided into two groups, PB and non-PB, according to the first-line therapy each patient received. The non-PB group was further classified by their treatment regimens into anthracycline (such as epirubicin with cyclophosphamide), taxane (such as docetaxel), eribulin, 5-fluorouracil [5-FU; such as capecitabine, S-1 (combination of tegafur, gimeracil, and oteracil potassium)], and ‘other’ (such as vinorelbine or gemcitabine) groups. We were able to use eribulin as first-line CTx because eribulin is reimbursed as any-line CTx in Japan. Because of the retrospective nature of the study, some patients in the non-PB group eventually received PB as later-line therapy.
We defined OS as the time from the initiation of first-line CTx until death from any cause, disease-free survival (DFS) as the time from the initiation of primary therapy for primary disease to the diagnosis of recurrence, and time to treatment failure (TTF) as the time from the initiation of CTx to discontinuation of CTx for any reason (e.g. disease progression, intolerable toxicity, withdraw of consent or death from any cause). ORR was defined as the proportion of patients who achieved complete response (CR) or partial response (PR) according to the RECIST criteria. The clinical benefit rate (CBR) was defined as the proportion of patients who achieved CR, PR, or maintained stable disease for more than 6 months.
Statistical analysis. Student’s t-test was used to compare averages of age and Fisher’s exact tests to compare proportions of categorical variables (such as metastatic sites) between the groups. Survival analyses were estimated using the Kaplan-Meier method. Comparisons between the groups were performed using the log-rank test or generalized Wilcoxon test. For multivariate analyses, Cox regression models were used. Data were subjected to propensity score matching (PSM). The adjustment factors used in the PSM were selected according to the ESME (9) and TURANDOT studies (12) and included age, diagnosis (advanced or recurrent), subtype [oestrogen receptor (ER)+/ER−], liver metastases (yes/no), visceral disease (yes/no), number of metastatic sites (<3/≥3), disease-free survival (DFS; <24 months/≥24 months), and prior (neo)adjuvant anthracycline-based regimen and/or taxane-based regimen (yes/no). Values of p<0.05 were considered statistically significant. Statistical analyses were performed using JMP 14.0 Japanese version (SAS Institute Inc., Cary, NC, USA).
Results
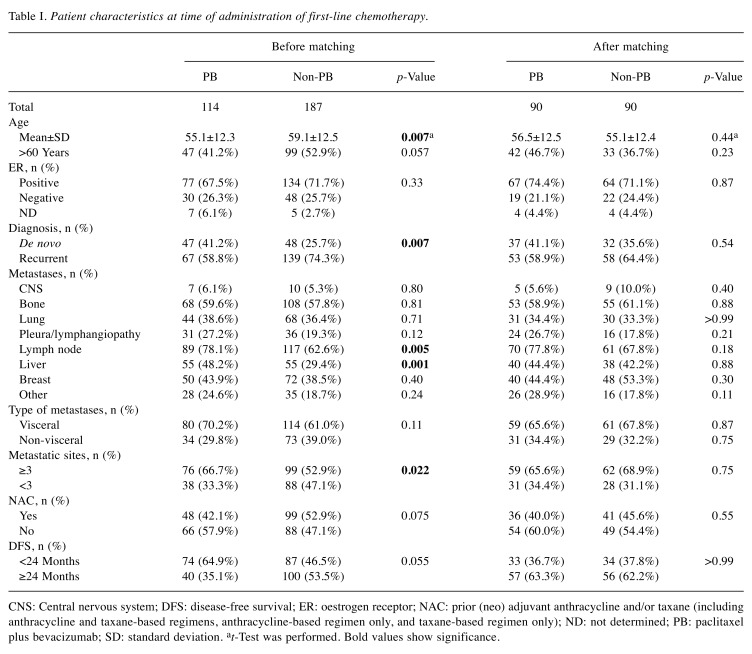
Patient characteristics. We identified 301 patients with HER2− ABC among three institutions, with 114 patients in the PB group and 187 patients in the non-PB group. Regimens in the non-PB group were anthracycline in 24, taxane in 28, eribulin in 23, 5-FU in 99, and other in 13. The median follow-up duration was 28.8 months (95% confidence interval (CI)=25.2-32.7 months). Characteristics at baseline (at the initiation of first-line CTx) are shown in Table I. The mean age in the PB group (55.1 years) was significantly younger than that in the non-PB group (59.1 years; p=0.0073). The PB group was also diagnosed less often with recurrent disease (58.8% vs. 74.3%, p=0.0071), but had a higher prevalence of lymph node (78.1% vs. 62.6%, p=0.005) and liver (48.2% vs. 29.4%, p=0.0013) metastases. Patients in the PB group also had more sites of metastases (≥3; 66.7% vs. 52.9%, p=0.022), and tended to have shorter DFS (<24 months; 64.9% vs. 53.5%, p=0.055). After PSM, these characteristics were generally well-balanced between the two groups (Table I). Of the 301 patients, 44 (14.6%) discontinued first-line CTx due to toxicity prior to PSM (PB: 21/114 patients, 18.4%; non-PB: 23/187 patients, 12.3%).
Table I. Patient characteristics at time of administration of first-line chemotherapy.
CNS: Central nervous system; DFS: disease-free survival; ER: oestrogen receptor; NAC: prior (neo) adjuvant anthracycline and/or taxane (including anthracycline and taxane-based regimens, anthracycline-based regimen only, and taxane-based regimen only); ND: not determined; PB: paclitaxel plus bevacizumab; SD: standard deviation. at-Test was performed. Bold values show significance
Response rates. ORR and CBR were higher in the PB group than in the non-PB group (ORR: 75.4% vs. 34. 2%, p<0.0001, CBR: 81.6% vs. 56. 7%, p<0.0001). ORR and CBR by CTx regimen were 62.5% and 66.7% for those treated with anthracycline, 39.3% and 57.1% for those treated with taxane, 17.4% and 43.5% for the group treated with eribulin, 29.3% and 56.6% for treatment with 5-FU, and 38.5% and 61.5% for the group treated with other agents, respectively.
After PSM, ORR and CBR were still higher in the PB group than in the non-PB group (ORR: 77.8% vs. 38.9%, p<0.0001; CBR: 84.4% vs. 62.2%, p=0.0012). ORR and CBR by CTx group were 70.6% and 76.5%, 42.9% and 64.3%, 27.3% and 54.5%, 27.1% and 52.1%, and 14.3% and 42.9%, respectively.
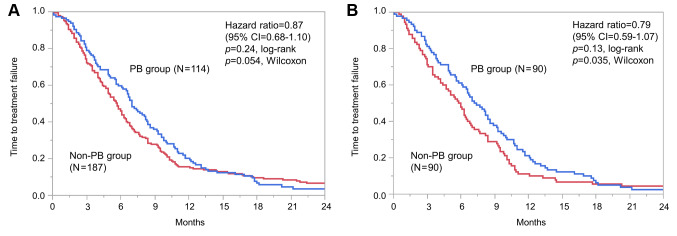
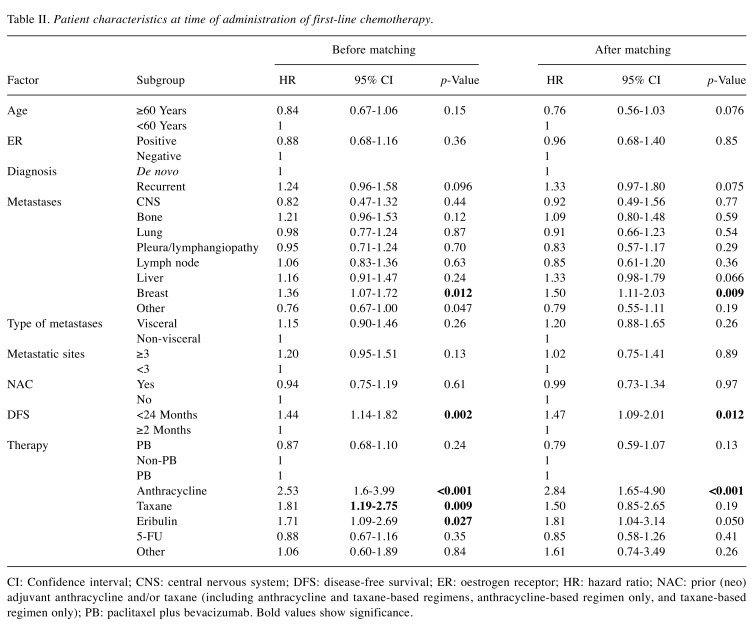
Time to treatment failure. Median TTF did not significantly differ between the PB and non-PB groups [7.0 vs. 5.7 months; hazard ratio (HR)=0.87; 95% CI=0.68-1.10, log-rank p=0.24; Wilcoxon p=0.054; Figure 1A]. After PSM, significant differences were observed between the two groups based on Wilcoxon tests (7.3 vs. 5.9 months; HR=0.79; 95% CI=0.59-1.07, log-rank p=0.13, Wilcoxon p=0.035; Figure 1B). We used univariate analysis to determine the effect of PB on PFS (Table II). After PSM, DFS (<24 vs. ≥24 months; HR=1.47; 95% CI=1.09-2.01) was associated with TTF. When compared by CTx administered, the PB group showed a trend for superior TTF to the other groups.
Figure 1. Time to treatment failure before (A) and after (B) propensity score matching. CI: Confidence interval; PB: paclitaxel and bevacizumab therapy.
Table II. Patient characteristics at time of administration of first-line chemotherapy.
CI: Confidence interval; CNS: central nervous system; DFS: disease-free survival; ER: oestrogen receptor; HR: hazard ratio; NAC: prior (neo) adjuvant anthracycline and/or taxane (including anthracycline and taxane-based regimens, anthracycline-based regimen only, and taxane-based regimen only); PB: paclitaxel plus bevacizumab. Bold values show significance
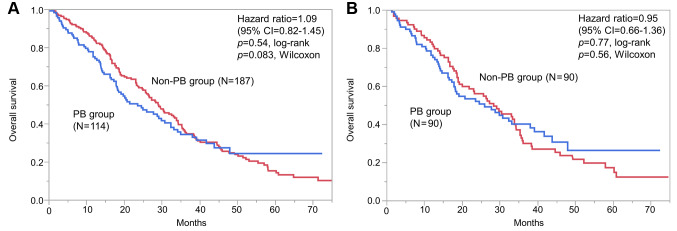
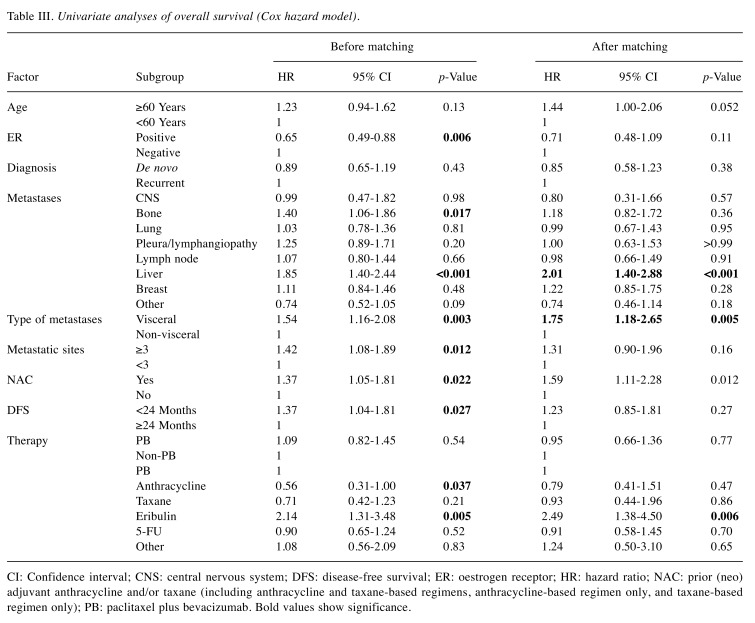
Overall survival. Median OS did not significantly differ between the two groups before PSM (23.6 vs. 29.1 months; HR=1.09; 95% CI=0.82-1.45, log-rank p=0.54; Wilcoxon p=0.083; Figure 2A), nor after PSM (26.0 vs. 28.4 months; HR=0.95; 95% CI=0.66-1.36, log-rank p=0.77; Wilcoxon p=0.56; Figure 2B). We used univariate analysis to determine the effect of PB on OS (Table III). After PSM, liver metastases (HR=2.01, 95% CI=1.40-2.88), visceral disease (HR=1.75, 95% CI=1.18-2.65), and prior (neo)adjuvant anthracyclines- and/or taxane-based regimen (HR=1.59, 95% CI=1.11-2.28) were significantly associated with OS. When compared by CTx administered, the PB group did not appear to experience an obvious OS benefit.
Figure 2. Overall survival before (A) and after (B) propensity score matching. CI: Confidence interval; PB: paclitaxel and bevacizumab therapy.
Table III. Univariate analyses of overall survival (Cox hazard model).
CI: Confidence interval; CNS: central nervous system; DFS: disease-free survival; ER: oestrogen receptor; HR: hazard ratio; NAC: prior (neo) adjuvant anthracycline and/or taxane (including anthracycline and taxane-based regimens, anthracycline-based regimen only, and taxane-based regimen only); PB: paclitaxel plus bevacizumab. Bold values show significance
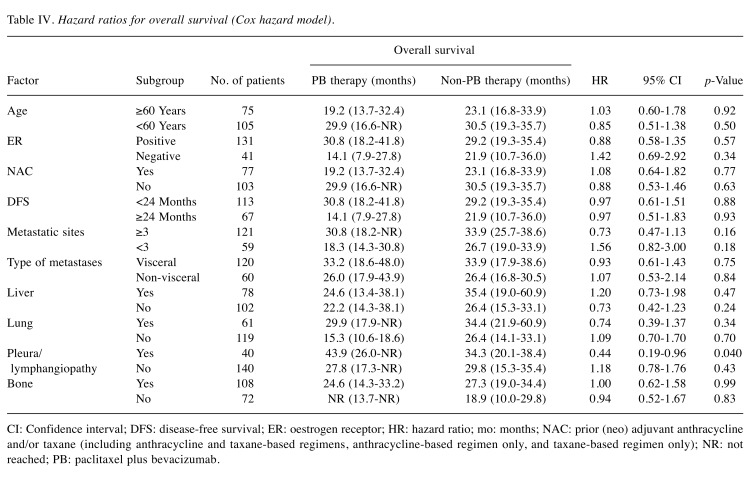
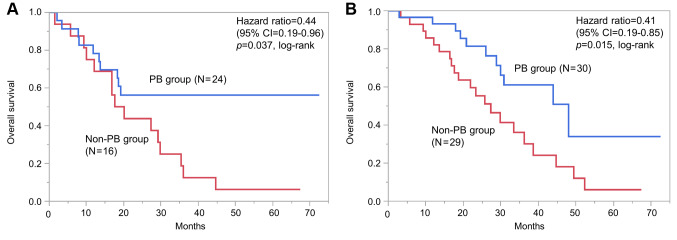
Subgroup analyses. To identify patients who experienced an OS benefit from PB, subgroup analyses were performed in groups of patients after PSM (Table IV). In the group with pleural lesion, pulmonary carcinomatous lymphangitis or both in 40, median OS was significantly better in the PB group than in the non-PB group [not reached (NR) (95% CI=13.7 months-NR) vs. 18.9 months (95% CI=10.0-29.8); HR=0.44 (95% CI=0.19-0.96) log-rank p=0.037; Figure 3A].
Table IV. Hazard ratios for overall survival (Cox hazard model).
CI: Confidence interval; DFS: disease-free survival; ER: oestrogen receptor; HR: hazard ratio; mo: months; NAC: prior (neo) adjuvant anthracycline and/or taxane (including anthracycline and taxane-based regimens, anthracycline-based regimen only, and taxane-based regimen only); NR: not reached; PB: paclitaxel plus bevacizumab
Figure 3. Subgroup analyses for overall survival after propensity score matching among patients treated with and without paclitaxel plus bevacizumab (PB). A: Patients with pleural lesion, pulmonary carcinomatous lymphangitis or both. B: Patients with three or more metastatic sites but without liver metastases. CI: Confidence interval; PB: paclitaxel and bevacizumab therapy.
In addition, patients who benefited from PB were investigated based on combinations of risk factors. When limited to patients with three or more metastatic sites but without liver metastases in 59, significantly better median OS was observed in the PB group compared with the non-PB group [48.0 months (95% CI=28.8-NR) vs. 27.3 months (95% CI=17.6-36.2); HR=0.41 (95% CI=0.19-0.85); log-rank p=0.015; Figure 3B]. Furthermore, two subgroups which obtained significantly superior median TTF by PB therapy were identified; patients with pleural lesion, pulmonary carcinomatous lymphangitis or both [8.0 vs. 5.5 months; HR=0.49 (95% CI=0.24-0.98), log-rank p=0.037], and patients with three or more metastatic sites excluding liver ([10.2 vs. 6.0 months; HR=0.42 (95% CI=0.24-0.72), log-rank p=0.0012].
Discussion
Bevacizumab with CTx is reportedly efficacious as first-line CTx for HER2− ABC (2,12,13). In the E2100 study that compared paclitaxel monotherapy with PB, significant improvements in median PFS (11.8 vs. 5.9 months; HR=0.60, p<0.001) and ORR (36.9% vs. 21.2%, p<0.001) were observed in the PB arm (2). Furthermore, the AVADO study, which compared docetaxel monotherapy with docetaxel plus bevacizumab, showed significantly better PFS (10.1 vs. 8.2 months; HR=0.77, p=0.006) and ORR (64.1% vs. 46.4%, p<0.001) in the docetaxel plus bevacizumab arm (13). Additionally, the RIBBON-1 study, which compared conventional CTx (based on taxane, anthracycline, or capecitabine) with CTx with bevacizumab, found similar favourable results for both PFS and ORR with the combination therapy (14). However, none of these three prospective, randomized trials found OS to have improved; a meta-analysis that included these three studies showed bevacizumab provided significant PFS and ORR benefit [HR=0.70 (95% CI=0.57-0.86) and HR=1.81 (95% CI=1.53-2.14) respectively] but no significant gain in OS (HR=0.95, 95% CI=0.85-1.06) (15), and a recent report from Miyashita et al. showed similar results based on a meta-analysis of eight prospective clinical trials (16). Bevacizumab was approved for HER2− ABC by the US Food and Drug Administration in 2008 based on the results of the E2100 trial (2), however, this approval was withdrawn in 2011 due to the lack of OS benefit and concerns about toxicities raised by subsequent studies (13,14). Nevertheless, bevacizumab has been approved in combination with paclitaxel in Japan, based on the results of the Japanese clinical trial (4). Under these circumstances, the ESME study found an OS benefit with PB over paclitaxel monotherapy as first-line CTx using real-world data (9). Furthermore, recent findings of a cost-effectiveness analysis using the ESME database showed that in France, the addition of bevacizumab to paclitaxel compared with paclitaxel alone was likely to represent a cost-effective treatment for patients with HER2− ABC (17).
The ESME study aimed to describe outcomes after use of first-line paclitaxel with or without bevacizumab in France, with OS as the primary endpoint. PB prolonged median OS compared with paclitaxel monotherapy [27.7 vs. 19.8 months; HR=0.739 (95% CI=0.672-0.813)]. The results for OS and PFS with PB were consistent with those in clinical trials, and the ESME study showed the superior efficacy of the combination therapy compared with paclitaxel monotherapy (9). The efficacy of PB in the present study after PSM was comparable with that in the ESME and other clinical trials (present study vs. ESME study vs. clinical trials (ORR: 77.8% vs. no data vs. 36.9-74.0%; TTF: 7.3 vs. 8.1 vs. 8.1-12.9 months; OS: 26.0 vs. 27.7 vs. 26.7-35.8 months, respectively) (2-9,12). In addition, results with CTx alone (the non-PB group in the present study) were comparable with those of the control groups (paclitaxel-, docetaxel-, capecitabine-, or anthracycline-based) in previous studies (ORR: 38.9% vs. 21.2-46.4%; TTF: 5.9 vs. 5.7-8.8 months; OS: 28.4 vs. 21.2-31.9 months, respectively) (2-8,13,14). As previously mentioned, the effects of PB on TTF and ORR in the present study were very similar to those in clinical trials (2-8,12-14) and the ESME study (9), however, unlike the ESME study, the present study and the clinical trials showed no OS benefit with PB. In prospective, randomized clinical trials, possible reasons for these discrepancies between PFS and OS included the facts that some patients in the control group eventually crossed over to receive PB, post-treatment was heterogeneous, and there may have been an influence of post-progression survival (2,14,18). Several reasons may account for why OS did not significantly differ in this study after PSM. Firstly, in our non-PB group, 50.3% (94/187) of patients before PSM and 54.4% (49/90) after PSM underwent PB in second- or later-line therapies. Secondly, our non-PB group had a better median OS (28.4 months) than did the control groups (paclitaxel only) in the clinical trials (19.8-25.8 months) (2,3,7,9). Therefore, in order to identify patients who experienced OS benefit from PB in this study, we performed subgroup analyses in groups of patients after PSM (Table IV).
Some clinical trials have attempted to identify factors associated with the effect of bevacizumab, including vascular endothelial growth factor A (VEGF), which has been implicated in tumour progression and metastasis (19,20) and has therefore been studied as a predictive biomarker for the efficacy of bevacizumab in various carcinomas. However, the predictive value of plasma VEGFA levels is unclear (7,21-25), and it is not easily measured in ordinary clinical practice. We therefore investigated predictors of PB efficacy that we would be able to access conveniently, such as patient clinicopathological characteristics. We performed subgroup analyses after PSM to identify patients whose OS improved after PB, and found that, among those with pleural lesions and/or pulmonary carcinomatous lymphangitis, OS was significantly better in the PB group than in the non-PB group. Bevacizumab has been shown to be effective against malignant pleural effusion in lung cancer (26-28), and our results may demonstrate its utility in HER2− ABC. We also found that PB improved OS among patients with three or more metastatic sites, but without liver metastases. Liver metastases are a poor prognostic factor for patients with ABC (29-31); Llombart-Cussac et al. reported that the presence of liver metastases was a risk factor for patients with HER2− ABC treated with first-line CTx with bevacizumab (32). Our results also demonstrate that liver metastasis is a risk factor in the real world, and the clinical utility of PB therapy in HER2− ABC will be limited when patients have liver metastases. The above subgroup analyses should be interpreted with caution because of the multiplicity issue due to the repetition of statistical tests.
This study has some limitations because of its retrospective design. Therefore, in order to minimize selection biases from the results, PSM was used to adjust for differences in patient characteristics between the groups. In this study, the effectiveness of PB may have been obscured because many patients in the non-PB group eventually used PB after their cancer progressed. However, a strength of our study was its use of real-world data, which allowed us to compare PB with anthracycline- or 5-FU-based regimens, which are not used as comparators in clinical trials; thus, our findings might assist in managing HER2− ABC. Furthermore, real-world setting evidence was important for describing the efficacy and safety of investigated treatments and for bridging the gap between clinical trials and clinical practice (16). Thus, we should note both clinical trials results and real-world setting evidence. In addition, we did not assess adverse events. Prospective studies are needed to verify the benefit/harm profile of PB as first-line CTx for patients with HER2− ABC.
By using PSM, we have shown PB to significantly improve TTF and ORR as a first-line CTx option for HER2− ABC in a real-world setting and have identified a subgroup of patients whose OS can benefit from PB.
Conflicts of Interest
Dr. Nakamoto reports personal fees from Chugai Pharmaceuticals, Eisai, and Taiho Pharmaceuticals outside the submitted work; Dr. Watanabe reports personal fees from AstraZeneca, Chugai Pharmaceuticals, Daiichi-Sankyo, Eisai, Eli-Lilly, Novartis Pharma, Pfizer, and Taiho Pharmaceuticals outside the submitted work; Dr. Ohtani reports personal fees from AstraZeneca, Chugai Pharmaceuticals, Eli-Lilly, and Pfizer outside the submitted work; Dr. Morita reports personal fees from Chugai Pharmaceuticals outside the submitted work; Dr. Ikeda reports personal fees from AstraZeneca, Chugai Pharmaceuticals, Daiichi-Sankyo, Eisai, Eli-Lilly, Kyowa Kirin, Pfizer, Nippon Kayaku, and Sawai Pharmaceuticals outside the submitted work.
Authors’ Contributions
All Authors contributed to the study conception and design. Material preparation and data collection were performed by Shogo Nakamoto and Shoichiro Ohtani. Analysis was performed by Shogo Nakamoto. The first draft of the article was written by Shogo Nakamoto and all Authors commented on previous versions of the article. All Authors read and approved the final article.
Acknowledgements
The Authors thank H. Nikki March, Ph.D., and Marla Brunker, M.Sc., from Edanz Group (www.edanzediting.com/ac), for editing a draft of this article.
References
- 1.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, Harbeck N, Aguilar Lopez B, Barrios CH, Bergh J, Biganzoli L, Boers-Doets CB, Cardoso MJ, Carey LA, Cortés J, Curigliano G, Diéras V, El Saghir NS, Eniu A, Fallowfield L, Francis PA, Gelmon K, Johnston SRD, Kaufman B, Koppikar S, Krop IE, Mayer M, Nakigudde G, Offersen BV, Ohno S, Pagani O, Paluch-Shimon S, Penault-Llorca F, Prat A, Rugo HS, Sledge GW, Spence D, Thomssen C, Vorobiof DA, Xu B, Norton L, Winer EP. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4) Ann Oncol. 2018;29(8):1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 3.Gray R, Bhattacharya S, Bowden C, Miller K, Comis RL. Independent review of E2100: A phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2009;27(30):4966–4972. doi: 10.1200/JCO.2008.21.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aogi K, Masuda N, Ohno S, Oda T, Iwata H, Kashiwaba M, Fujiwara Y, Kamigaki S, Ito Y, Ueno T, Takashima S. First-line bevacizumab in combination with weekly paclitaxel for metastatic breast cancer: efficacy and safety results from a large, open-label, single-arm Japanese study. Breast Cancer Res Treat. 2011;129(3):829–838. doi: 10.1007/s10549-011-1685-x. [DOI] [PubMed] [Google Scholar]
- 5.Lang I, Brodowicz T, Ryvo L, Kahan Z, Greil R, Beslija S, Stemmer SM, Kaufman B, Zvirbule Z, Steger GG, Melichar B, Pienkowski T, Sirbu D, Messinger D, Zielinski C, Central European Cooperative Oncology Group Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer: Interim efficacy results of the randomised, open-label, non-inferiority, phase 3 TURANDOT trial. Lancet Oncol. 2013;14(2):125–133. doi: 10.1016/S1470-2045(12)70566-1. [DOI] [PubMed] [Google Scholar]
- 6.Zielinski C, Láng I, Inbar M, Kahán Z, Greil R, Beslija S, Stemmer SM, Zvirbule Z, Steger GG, Melichar B, Pienkowski T, Sirbu D, Petruzelka L, Eniu A, Nisenbaum B, Dank M, Anghel R, Messinger D, Brodowicz T, TURANDOT investigators Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer (TURANDOT): Primary endpoint results of a randomised, open-label, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(9):1230–1239. doi: 10.1016/S1470-2045(16)30154-1. [DOI] [PubMed] [Google Scholar]
- 7.Miles D, Cameron D, Bondarenko I, Manzyuk L, Alcedo JC, Lopez RI, Im SA, Canon JL, Shparyk Y, Yardley DA, Masuda N, Ro J, Denduluri N, Hubeaux S, Quah C, Bais C, O'Shaughnessy J. Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): A double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. Eur J Cancer. 2017;70:146–155. doi: 10.1016/j.ejca.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Miles D, Cameron D, Hilton M, Garcia J, O'Shaughnessy J. Overall survival in MERiDiAN, a double-blind placebo-controlled randomised phase III trial evaluating first-line bevacizumab plus paclitaxel for HER2-negative metastatic breast cancer. Eur J Cancer. 2018;90:153–155. doi: 10.1016/j.ejca.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Delaloge S, Pérol D, Courtinard C, Brain E, Asselain B, Bachelot T, Debled M, Dieras V, Campone M, Levy C, Jacot W, Lorgis V, Veyret C, Dalenc F, Ferrero JM, Uwer L, Kerbrat P, Goncalves A, Mouret-Reynier MA, Petit T, Jouannaud C, Vanlemmens L, Chenuc G, Guesmia T, Robain M, Cailliot C. Paclitaxel plus bevacizumab or paclitaxel as first-line treatment for HER2-negative metastatic breast cancer in a multicenter national observational study. Ann Oncol. 2016;27(9):1725–1732. doi: 10.1093/annonc/mdw260. [DOI] [PubMed] [Google Scholar]
- 10.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Brodowicz T, Lang I, Kahan Z, Greil R, Beslija S, Stemmer SM, Kaufman B, Petruzelka L, Eniu A, Anghel R, Koynov K, Vrbanec D, Pienkowski T, Melichar B, Spanik S, Ahlers S, Messinger D, Inbar MJ, Zielinski C. Selecting first-line bevacizumab-containing therapy for advanced breast cancer: TURANDOT risk factor analyses. Br J Cancer. 2014;111(11):2051–2057. doi: 10.1038/bjc.2014.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miles DW, Chan A, Dirix LY, Cortés J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28(20):3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 14.Robert NJ, Diéras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, Phan SC, O'Shaughnessy J. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29(10):1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 15.Rossari JR, Metzger-Filho O, Paesmans M, Saini KS, Gennari A, de Azambuja E, Piccart-Gebhart M. Bevacizumab and breast cancer: A meta-analysis of first-line phase III studies and a critical reappraisal of available evidence. J Oncol. 2012;2012:417673. doi: 10.1155/2012/417673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyashita M, Hattori M, Takano T, Toyama T, Iwata H. Risks and benefits of bevacizumab combined with chemotherapy for advanced or metastatic breast cancer: A meta-analysis of randomized controlled trials. Breast Cancer. 2020 doi: 10.1007/s12282-020-01052-9. [DOI] [PubMed] [Google Scholar]
- 17.Petitjean A, Smith-Palmer J, Valentine W, Tehard B, Roze S. Cost-effectiveness of bevacizumab plus paclitaxel versus paclitaxel for the first-line treatment of HER2-negative metastatic breast cancer in specialist oncology centers in France. BMC Cancer. 2019;19(1):140. doi: 10.1186/s12885-019-5335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101(23):1642–1649. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis LM. Mechanisms of action of bevacizumab as a component of therapy for metastatic colorectal cancer. Semin Oncol. 2006;33(5 Suppl 10):S1–S7. doi: 10.1053/j.seminoncol.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31(9):1219–1230. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- 21.Lambrechts D, Claes B, Delmar P, Reumers J, Mazzone M, Yesilyurt BT, Devlieger R, Verslype C, Tejpar S, Wildiers H, de Haas S, Carmeliet P, Scherer SJ, Van Cutsem E. VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: An analysis of data from the AViTA and AVOREN randomised trials. Lancet Oncol. 2012;13(7):724–733. doi: 10.1016/S1470-2045(12)70231-0. [DOI] [PubMed] [Google Scholar]
- 22.Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, Peng Yong W, Langer B, Delmar P, Scherer SJ, Shah MA. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012;30(17):2119–2127. doi: 10.1200/JCO.2011.39.9824. [DOI] [PubMed] [Google Scholar]
- 23.Miles DW, de Haas SL, Dirix LY, Romieu G, Chan A, Pivot X, Tomczak P, Provencher L, Cortés J, Delmar PR, Scherer SJ. Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer. 2013;108(5):1052–1060. doi: 10.1038/bjc.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegde PS, Jubb AM, Chen D, Li NF, Meng YG, Bernaards C, Elliott R, Scherer SJ, Chen DS. Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin Cancer Res. 2013;19(4):929–937. doi: 10.1158/1078-0432.CCR-12-2535. [DOI] [PubMed] [Google Scholar]
- 25.Maru D, Venook AP, Ellis LM. Predictive biomarkers for bevacizumab: Are we there yet. Clin Cancer Res. 2013;19(11):2824–2827. doi: 10.1158/1078-0432.CCR-12-3409. [DOI] [PubMed] [Google Scholar]
- 26.Tamiya M, Tamiya A, Yamadori T, Nakao K, Asami K, Yasue T, Otsuka T, Shiroyama T, Morishita N, Suzuki H, Okamoto N, Okishio K, Kawaguchi T, Atagi S, Kawase I, Hirashima T. Phase 2 study of bevacizumab with carboplatin-paclitaxel for non-small cell lung cancer with malignant pleural effusion. Med Oncol. 2013;30(3):676. doi: 10.1007/s12032-013-0676-7. [DOI] [PubMed] [Google Scholar]
- 27.Du N, Li X, Li F, Zhao H, Fan Z, Ma J, Fu Y, Kang H. Intrapleural combination therapy with bevacizumab and cisplatin for non-small cell lung cancer-mediated malignant pleural effusion. Oncol Rep. 2013;29(6):2332–2340. doi: 10.3892/or.2013.2349. [DOI] [PubMed] [Google Scholar]
- 28.Qi N, Li F, Li X, Kang H, Zhao H, Du N. Combination use of paclitaxel and avastin enhances treatment effect for the NSCLC patients with malignant pleural effusion. Medicine. 2016;95(47):e5392. doi: 10.1097/MD.0000000000005392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leone BA, Vallejo CTx, Romero AO, Machiavelli MR, Pérez JE, Leone J, Leone JP. Prognostic impact of metastatic pattern in stage IV breast cancer at initial diagnosis. Breast Cancer Res Treat. 2017;161(3):537–548. doi: 10.1007/s10549-016-4066-7. [DOI] [PubMed] [Google Scholar]
- 30.Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, Courdi A, Hannoun-Levi JM, Ettore F, Birtwisle-Peyrottes I, Balu-Maestro C, Marcy PY, Raoust I, Lallement M, Chamorey E. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19(12):2012–2019. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto N, Watanabe T, Katsumata N, Omuro Y, Ando M, Fukuda H, Takue Y, Narabayashi M, Adachi I, Takashima S. Construction and validation of a practical prognostic index for patients with metastatic breast cancer. J Clin Oncol. 1998;16(7):2401–2408. doi: 10.1200/JCO.1998.16.7.2401. [DOI] [PubMed] [Google Scholar]
- 32.Llombart-Cussac A, Pivot X, Biganzoli L, Cortes-Funes H, Pritchard K, Pierga JY, Smith I, Thomssen C, Srock S, Sampayo M, Cortes J. A prognostic factor index for overall survival in patients receiving first-line chemotherapy for HER2-negative advanced breast cancer: an analysis of the ATHENA trial. Breast. 2014;23(5):656–662. doi: 10.1016/j.breast.2014.06.017. [DOI] [PubMed] [Google Scholar]