Abstract
Background/Aim: We evaluated the dosimetric profiles of manually generated volumetric-modulated arc therapy (VMAT) plans and performance of a commercial knowledge-based planning system (KBP) in treating breast cancer. Materials and Methods: We defined the manually generated VMAT plan as the manual plan (MP). Twenty MPs were generated for left-sided breast cancer patients who underwent breast-conserving surgery and used to develop a KBP training set. The other five patients were used for validation. The dosimetric parameters among MPs, tangential irradiation plans (TPs), and KBP-VMAT plans (KBP-Ps) were compared. Results: D95 and homogeneity of the planning target volume (PTV) were significantly higher and greater in MPs and KBP-Ps than in TPs. Lung V20, V40. The Dmean for the left anterior descending artery was lower in MPs and KBP-Ps than in TPs. KBP could save time in generating VMAT plans. Conclusion: MPs and KBP-Ps could ensure higher dose uniformity of PTV than TPs. KBP could faster generate comparable MPs for breast cancer.
Keywords: Volumetric-modulated arc therapy, intensity-modulated radiation therapy, knowledge-based planning, breast cancer
Breast cancer is one of the most common malignancies in women worldwide (1). Partial mastectomy followed by postoperative radiotherapy is a well-established treatment for early-stage breast cancer (2,3). The tangential irradiation technique has been used in postoperative radiotherapy for breast cancer (4).
However, tangential irradiation for breast cancer presents several issues. Anatomical features such as a pigeon breast might facilitate the delivery of higher doses to the normal lungs (5). It is challenging to generate a treatment plan that facilitates both good conformity of radiation doses administered to the target and reduction in radiation doses administered to the normal organs. Therefore, intensity-modulated radiation therapy (IMRT) has been discussed to have a potential benefit in postoperative radiotherapy for breast cancer (6-12). However, IMRT modalities including volumetric-modulated arc therapy (VMAT) have been less commonly used in clinical practice (9). One of the reasons for this may be that generating IMRT or VMAT plans is more complex and time-consuming than planning for tangential irradiation.
A new commercial knowledge-based planning (KBP) optimization engine, RapidPlan (VarianMedical Systems, Palo Alto, CA, USA), was developed and released for clinical use. RapidPlan predicts achievable dose-volume histograms (DVHs) and automatically generates optimization objectives to realize the prediction. Although the benefits of RapidPlan are still being investigated, there have been many reports of improvements in sparing organs at risk (OARs) using KBP (13-18). The mechanical performance and dosimetric accuracy of KBP have also been verified, showing that KBP could be safely used in clinical practice (17-19). However, to date there are only a few reports on the application of KBP in breast cancer. Thus, this study sought to evaluate the target coverage and normal tissue-sparing profile of conventional tangential plans, manually generated VMAT plans, and VMAT plans generated by KBP in breast cancer.
Materials and Methods
This study was approved by our institutional review board (approval no. 29-133).
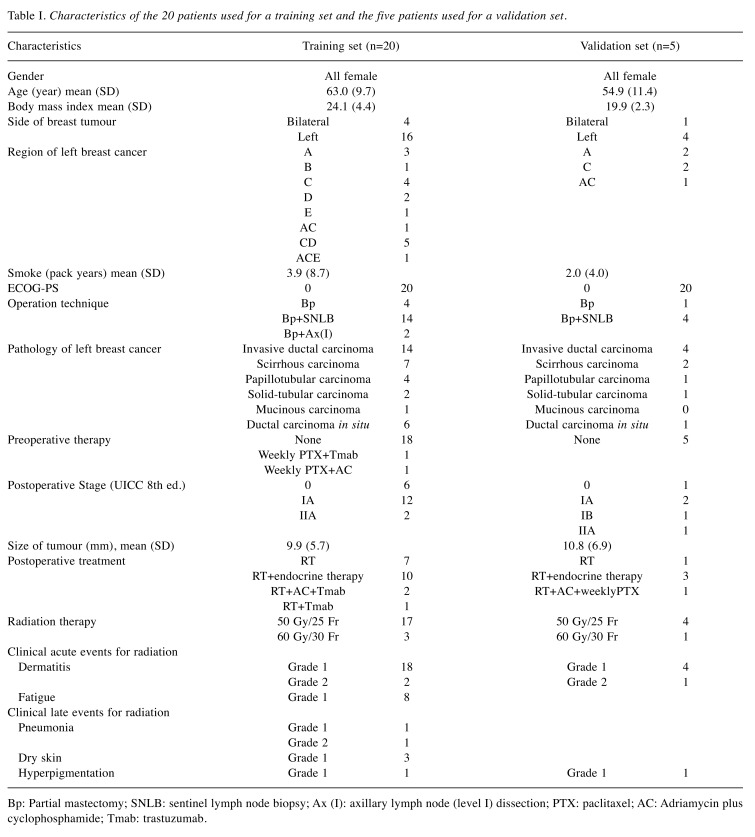
Manually generated VMAT planning and training of KBP. First, 20 VMAT plans were generated in 20 breast cancer patients as a training set for generating KBP. These 20 patients were those who received radiotherapy after breast-conserving surgery for left breast cancer or bilateral breast cancer in 2018 at our hospital. They were treated by tangential irradiation technique and prescribed 50 Gy in 25 fractions and 10 Gy in five fractions administered to the tumour bed if positive margins were suspected. Regional lymph nodes were not included in the target area. The characteristics of the included patients are presented in Table I.
Table I. Characteristics of the 20 patients used for a training set and the five patients used for a validation set.
Bp: Partial mastectomy; SNLB: sentinel lymph node biopsy; Ax (I): axillary lymph node (level I) dissection; PTX: paclitaxel; AC: Adriamycin plus cyclophosphamide; Tmab: trastuzumab.
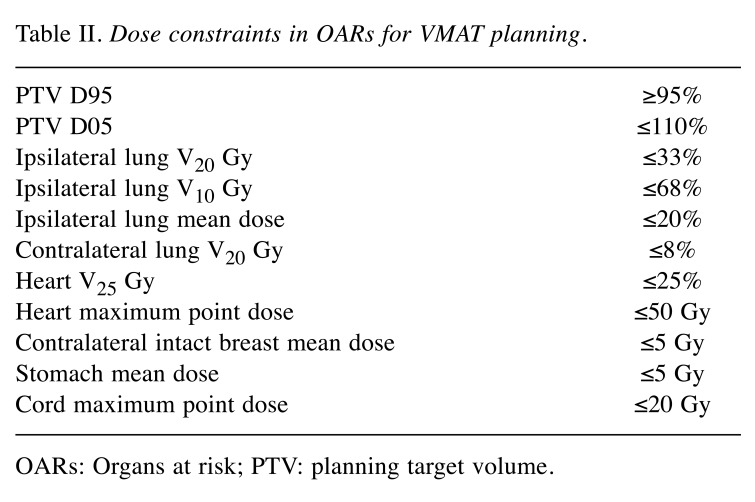
Clinical tumour volume (CTV) was defined as the whole breast. For this study, we re-contoured CTV and OARs according to the Radiation Therapy Oncology Group (RTOG) contouring atlas (20). A margin of 5 mm was added to the CTV to generate the planning target volume (PTV). For optimization, PTV was limited to within 2 mm of the body structure. The prescription dose for KBP was a total of 50 Gy in 25 fractions for each patient. Each VMAT plan was designed to cover 95% of PTV by at least 95% of the prescription dose (21). The dose constraints for OAR are shown in Table II. We defined the manually generated VMAT plan as the manual plan (MP). MPs included dose constraints to the PTV, contralateral and ipsilateral lungs, heart, right breast, and stomach. Then, the KBP was trained using the 20 MPs.
Table II. Dose constraints in OARs for VMAT planning.
OARs: Organs at risk; PTV: planning target volume.
A commercial treatment planning system (TPS) (Eclipse version 15.6; Varian Medical Systems, Palo Alto, CA, USA), 6-MV photon Flattening Filter Free beams, and four full arcs of VMAT were applied (Gantry angle: 181˚-179˚ clockwise, and 179˚-181˚ counterclockwise) for treatment using Halcyon 1.0 (Varian Medical Systems, Palo Alto, CA, USA). All VMAT plans were optimized with the photon optimizer and calculated with the Varian Analytic Anisotropic Algorithm. In this model, the geometric or dosimetric outliers were not excluded since the removal of statistical outliers had no significant impact on establishing the model (22).
Validation of KBP. The other five patients who received postoperative radiotherapy for left breast cancer were selected for validation sets. The characteristics of these patients are also summarized in Table I. These five patients were also re-contoured according to the RTOG atlas and then five MPs were generated. Five KBP-VMAT plans (KBP-Ps) were generated using KBP. In the optimization process by KBP, optimization objectives termed “line objectives” were placed along the inferior DVH prediction boundary for OARs and priority values were generated by the KBP automatically. For the PTV, upper and lower objectives were used at 50 Gy and 49.5 Gy, respectively.
Tangential irradiation plans (TPs) were re-planned for re-contoured PTV. TPs were planned to be treated by TrueBeam (Varian Medical Systems, Palo Alto, CA, USA) with 6-MV X-rays. The optimal dose distribution was achieved by using a wedge or field-in-field technique.
We compared the dose-volume parameters of each plan using single optimized KBP-Ps, MPs, and TPs. The analysed dosimetric parameters in OARs included the volume receiving 5 Gy or greater (V5), V20, and V40 for lungs; the mean dose (Dmean) for the heart; and the Dmean for the left anterior descending artery (LAD). The dose received by 95% of the PTV (D95) and homogeneity index (HI), defined as (D2 − D98)/D50, were also evaluated.
Statistical analysis. Study data are expressed as means with standard deviations in parentheses unless otherwise indicated (Table III). The data were analysed using a matched-paired t-test. All analyses were performed using GraphPad Prism version 8.2.1 (GraphPad Software, Inc., San Diego, CA, USA) and differences were considered statistically significant at a p-value of less than 0.05.
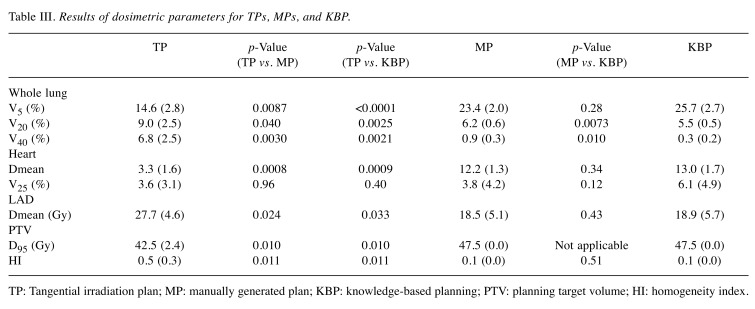
Table III. Results of dosimetric parameters for TPs, MPs, and KBP.
TP: Tangential irradiation plan; MP: manually generated plan; KBP: knowledge-based planning; PTV: planning target volume; HI: homogeneity index.
Results
Table III and Figure 1 show the dosimetric results of the TPs, MPs, and KBP-Ps. Figure 2 shows the dose distribution of the TPs, MPs, and KBP-Ps and the DVHs of the MPs and KBP-Ps.
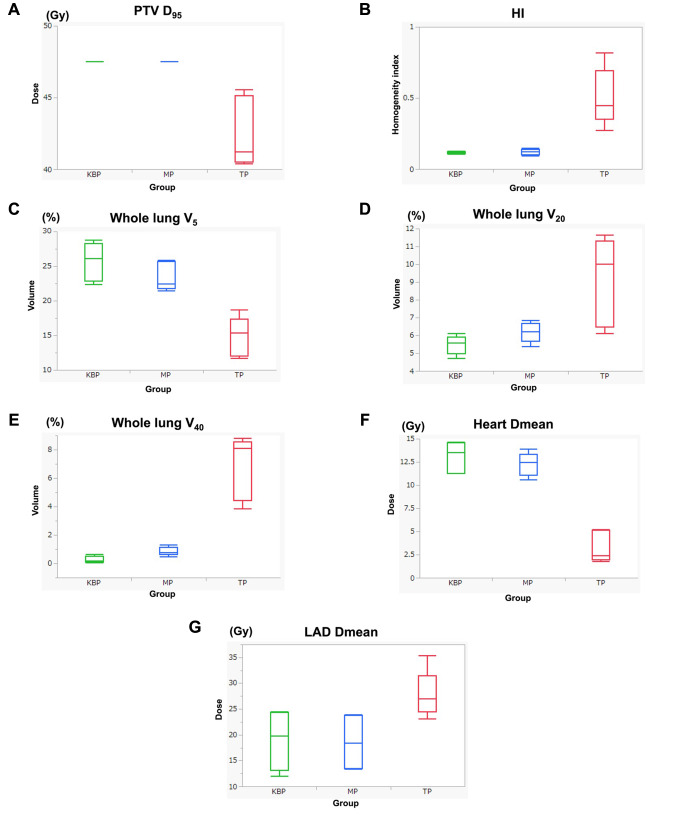
Figure 1. Comparison of dosimetric parameters in each model. (A) Dose irradiated to 95% volume (D95) for PTV, (B) HI defined as (D2 − D98)/D50×100, (C) The whole lung volume percentage receiving 5 Gy (V5), (D) V20 for the whole lung, (E) V40 for the whole lung, (F) Dmean for the heart, and (G) Dmean for the LAD. TP: Tangent irradiation plan; MP: manually generated plan; KBP: knowledge-based planning PTV: planning target volume; HI: homogeneity index.
Figure 2. Dose distribution and DVH of a patient with left breast cancer after breast-conserving surgery. (A) Treatment plans with corresponding examples of a tangent irradiation plan (TP), manually generated VMAT plan (MP), and VMAT plan using KBP (KBP). (B) The DVH of VMAT planning. The solid lines are the average of MPs and the dotted lines are the average of KBP-Ps. (red: PTV, orange: LAD, yellow: heart, blue: whole lungs). VMAT: Volumetric-modulated arc therapy; KBP: knowledge-based planning; PTV: planning target volume; LAD: left anterior descending artery.
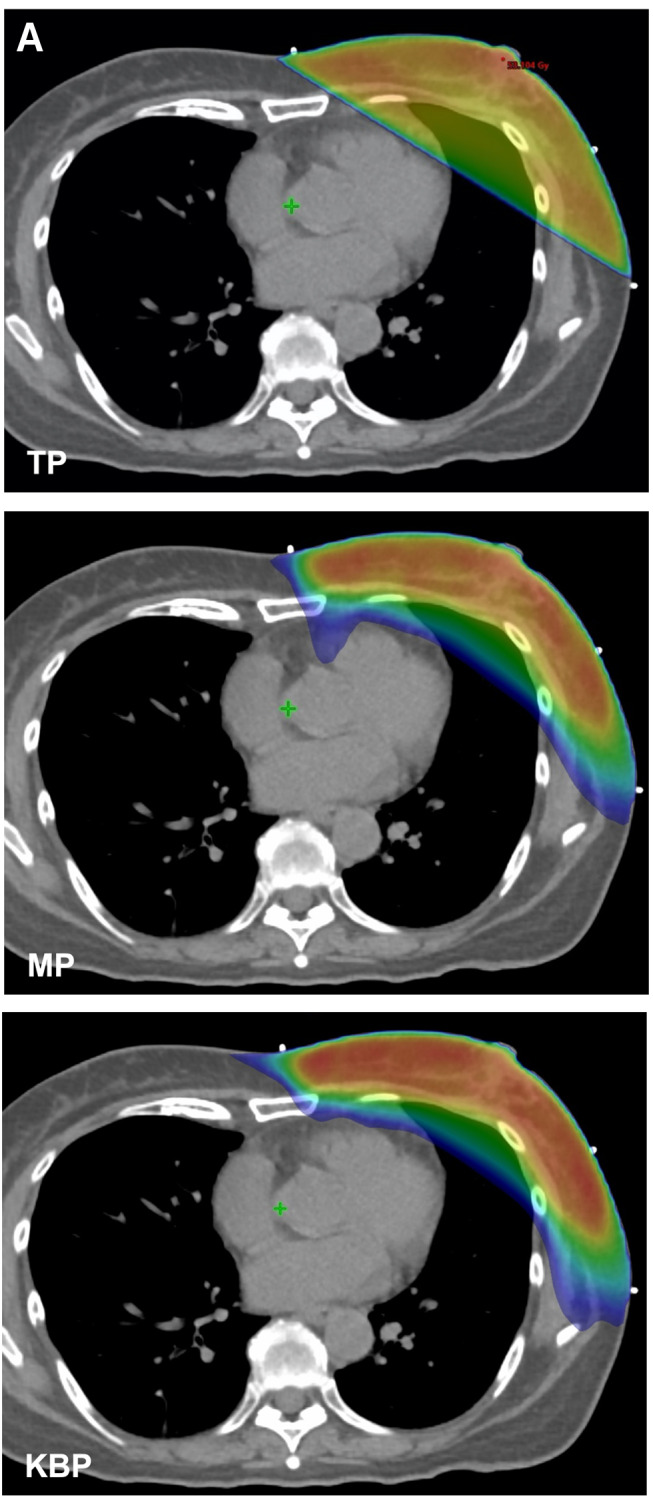
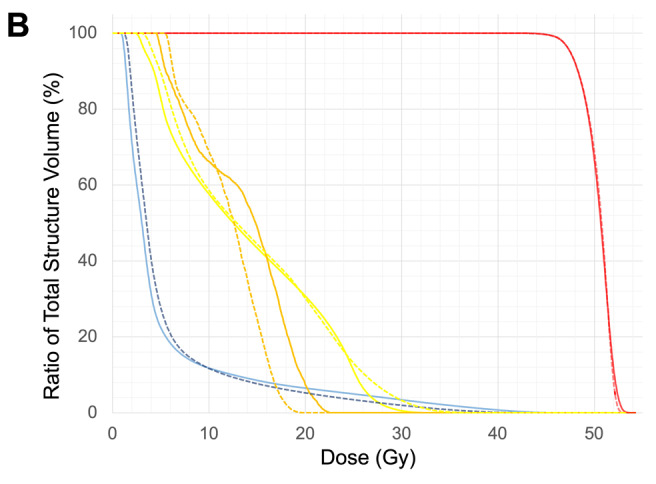
Comparison between MP and TP. The mean D95 values for PTV were 47.5 Gy and 42.6 Gy for the MPs and TPs, respectively; they were significantly different (p=0.01) (Figure 1A). The HI value of MPs was significantly lower than that of TPs (p=0.011) (Figure 1B). Lung V5 values were 23.4% and 14.6% for MPs and TPs, respectively; they were significantly different (p=0.008) (Figure 1C). Conversely, the lung V20 of MPs was significantly lower than that of TPs (6.2% and 9.0% for MPs and TPs, respectively; p=0.04) (Figure 1D). Similarly, the lung V40 values were 0.9% and 6.8% for MPs and TPs, respectively; they were significantly different (p=0.003) (Figure 1E). Separately, the heart Dmean value of MPs was 12.2 Gy, while that of TPs was 3.3 Gy (p=0.0008) (Figure 1F).The Dmean for the LAD was significantly lower in MPs than in TPs (27.7 Gy vs. 18.5 Gy; p=0.024) (Figure 1G).
Comparison between KBP-P and MP. No significant differences in lung V5, heart Dmean, and PTV D95 were observed between KBP-Ps and MPs. However, KBP-P achieved slightly but significantly lower lung V20 and V40 in comparison with MPs (p=0.0073 and 0.010, respectively) (Figure 1D and E). In terms of planning time, it took roughly two hours to generate one MP, while one KBP-P was generated almost within 15 min.
Discussion
Here, dosimetric parameters of TPs, MPs, and KBP-Ps were compared. MPs and KBP-Ps improved the homogeneity for PTV relative to TPs. However, lung V5 and heart Dmean were increased in VMAT plans, although lung V20 and V40 and LAD Dmean of VMAT plans were significantly lower than those of TPs. These results were similar to those of previous reports comparing dose parameters between TPs and IMRT/VMAT plans (10-12). Increased lung V5 and heart Dmean may constitute disadvantages of VMAT plans in comparison with TPs.
In terms of lung V5, a large-sized clinical trial for locally advanced non-small-cell lung cancer has recently shown that lung V5 was not associated with grade 3 radiation pneumonitis (23). In the RTOG0617 trial, lung V5 was 61.6%. Even though lung V5 was increased relative with TPs in this study, its values were only 23.4% and 25.7% in MPs and KBP-Ps, respectively. Lung V20 and V40 were significantly lower in VMAT plans than in TPs. These results indicate that VMAT plans were safer for radiation pneumonitis than tangential irradiation.
Radiation delivery to the heart in this context is unavoidable. In the MPs and KBP-Ps, heart Dmean was approximately four times larger than that in TPs. The heart dose is increased in this way because of the optimization method. Here, we constrained the contralateral and ipsilateral lungs’ doses strongly, and the beam angles were designed to avoid both lungs. However, those constraints could increase the radiation doses to the mediastinum area including the heart (Figure 2A). Darby et al. have shown that the rate of major coronary events for breast cancer increased linearly by 7.4% per Gy of mean heart dose (24). However, Abouegylah et al. have revealed that the dose to the LAD was a significant parameter to use to predict the risk of radiation-induced cardiotoxicity (25). In the case of left-sided tangential irradiation, The LAD is close to the PTV and tended to receive a higher dose. Here, the LAD Dmean was significantly lower in VMAT plans than in TPs. The heart Dmean is calculated from the heterogeneous dose distribution including both high- and low-dose radiation areas. Therefore, we considered the LAD Dmean as more important than heart Dmean for discerning late complications of cardiac events, so the MPs and KBP-Ps were acceptable.
Both KBP-P and MP generated similar VMAT plans with no significant differences in HI, lung V5, heart and LAD. Therefore, it can be argued that we could create VMAT plans with almost the same quality using KBP. KBP tended to suppress lung V20 and V40 doses more significantly than those seen in MPs. Only few studies have described the use of KBP in breast cancer. Some reports have suggested that KBP was useful even if the planner is a beginner (14,26,27). Overall, this study suggests that MPs and KBP-Ps were acceptable developments following breast-conserving surgery, and KBP could generate almost the same VMAT plans as MPs within a short time. Further validation studies should focus on the feasibility of KBPs for breast cancer.
Conclusion
After breast-conserving surgery, a single optimized KBP could generate VMAT plans comparable to MPs while also saving time.
Conflicts of Interest
The Authors declare that no conflicts of interest exist regarding this study.
Authors’ Contributions
Concept and design: EI, HD, HM, MT. Treatment planning: EI, HD, MI, KI, KN. Clinical evaluation: EI, HD. Data analysis: EI, HD, HM, MT. Manuscript preparation: EI, HD, HM, MT, YN. All Authors read and approved the final manuscript.
Acknowledgements
Authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
The study was partially supported by the National Cancer Center Research and Development Funds (29-A-3), and a Grant JSPS KAKENHI Grant Number JP17K16493, JP19K08135.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355:1757–1770. doi: 10.1016/S0140-6736(00)02263-7. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald SM, Napolitano B. Early breast cancer. In: Target volume delineation and field setup. Lee NY, Lu JJ (eds.). Berlin Heidelberg. Springer-Verlag. 2013. Available at: https://documents.pub/document/target-volume-delineation-and-field-setup-early-breastcancer.html. [DOI]
- 5.Das IJ, Cheng EC, Freedman G, Fowble B. Lung and heart dose volume analyses with CT simulator in radiation treatment of breast cancer. Int J Radiat Oncol Biol Phys. 1998;42(1):11–19. doi: 10.1016/s0360-3016(98)00200-4. [DOI] [PubMed] [Google Scholar]
- 6.Pignol JP, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckham W, Vu TT, Truong P, Ackerman I, Paszat L. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26(13):2085–2092. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 7.Ho AY, Ballangrud A, Li G, Gupta GP, McCormick B, Gewanter R, Gelblum D, Zinovoy M, Mueller B, Mychalczak B, Dutta P, Borofsky K, Parhar P, Reyngold M, Braunstein LZ, Chawla M, Krause K, Freeman N, Siu CT, Cost Z, Arnold BB, Zhang Z, Powell SN. Long-term pulmonary outcomes of a feasibility study of inverse-planned, multibeam intensity modulated radiation therapy in node-positive breast cancer patients receiving regional nodal irradiation. Int J Radiat Oncol Biol Phys. 2019;103(5):1100–1108. doi: 10.1016/j.ijrobp.2018.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol. 2011;84(1007):967–996. doi: 10.1259/bjr/22373346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balaji K, Subramanian B, Yadav P, Radha CA, Ramasubramanian V. Radiation therapy for breast cancer: literature review. Med Dosim. 2016;41(3):253–257. doi: 10.1016/j.meddos.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Lohr F, El-Haddad M, Dobler B, Grau R, Wertz HJ, Kraus-Tiefenbacher U, Steil V, Madyan YA, Wenz F. Potential effect of robust and simple IMRT approach for left-sided breast cancer on cardiac mortality. Int J Radiat Oncol Biol Phys. 2009;74(1):73–80. doi: 10.1016/j.ijrobp.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Aras S, İkizceli T, Aktan M. Dosimetric comparison of three-dimensional conformal radiotherapy (3D-CRT) and intensity modulated radiotherapy techniques (IMRT) with radiotherapy dose simulations for left-sided mastectomy patients. Eur J Breast Health. 2019;15(2):85–89. doi: 10.5152/ejbh.2019.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haciislamoglu E, Colak F, Canyilmaz E, Dirican B, Gurdalli S, Yilmaz AH, Yoney A, Bahat Z. Dosimetric comparison of left-sided whole-breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and volumetric arc therapy. Phys Med. 2015;31(4):360–367. doi: 10.1016/j.ejmp.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Fogliata A, Nicolini G, Bourgier C, Clivio A, De Rose F, Fenoglietto P, Lobefalo F, Mancosu P, Tomatis S, Vanetti E, Scorsetti M, Cozzi L. Performance of a knowledge-based model for optimization of volumetric modulated arc therapy plans for single and bilateral breast irradiation. PLoS One. 2015;10(12):e0145137. doi: 10.1371/journal.pone.0145137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Hu W, Yang Z, Chen X, Wu Z, Yu X, Guo X, Lu S, Li K, Yu G. Is it possible for knowledge-based planning to improve intensity modulated radiation therapy plan quality for planners with different planning experiences in left-sided breast cancer patients. Radiat Oncol. 2017;12(1):85. doi: 10.1186/s13014-017-0822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogliata A, Nicolini G, Clivio A, Vanetti E, Laksar S, Tozzi A, Scorsetti M, Cozzi L. A broad scope knowledge-based model for optimization of VMAT in esophageal cancer: validation and assessment of plan quality among different treatment centers. Radiat Oncol. 2015;10:220. doi: 10.1186/s13014-015-0530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamima T, Ueda Y, Fukunaga JI, Shimizu Y, Tamura M, Ishikawa K, Monzen H. Multi-institutional evaluation of knowledge-based planning performance of volumetric modulated arc therapy (VMAT) for head and neck cancer. Phys Med. 2019;64:174–181. doi: 10.1016/j.ejmp.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Ueda Y, Fukunaga JI, Kamima T, Adachi Y, Nakamatsu K, Monzen H. Evaluation of multiple institutions’ models for knowledge-based planning of volumetric modulated arc therapy (VMAT) for prostate cancer. Radiat Oncol. 2018;13(1):46. doi: 10.1186/s13014-018-0994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura M, Monzen H, Matsumoto K, Kubo K, Otsuka M, Inada M, Doi H, Ishikawa K, Nakamatsu K, Sumida I, Mizuno H, Yoon DK, Nishimura Y. Mechanical performance of a commercial knowledge-based VMAT planning for prostate cancer. Radiat Oncol. 2018;13(1):163. doi: 10.1186/s13014-018-1114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubo K, Monzen H, Ishii K, Tamura M, Kawamorita R, Sumida I, Mizuno H, Nishimura Y. Dosimetric comparison of RapidPlan and manually optimized plans in volumetric modulated arc therapy for prostate cancer. Phys Med. 2017;44:199–204. doi: 10.1016/j.ejmp.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Breast cancer contouring atlas. Available at: https://www.rtog.org/CoreLab/ContouringAtlases/BreastCancerAtlas.aspx [Last accessed 05/02/2020]
- 21.Das IJ, Cheng CW, Chopra KL, Mitra RK, Srivastava SP, Glatstein E. Intensity-modulated radiation therapy dose prescription, recording, and delivery: patterns of variability among institutions and treatment planning systems. J Natl Cancer Inst. 2008;100(5):300–307. doi: 10.1093/jnci/djn020. [DOI] [PubMed] [Google Scholar]
- 22.Hussein M, South CP, Barry MA, Adams EJ, Jordan TJ, Stewart AJ, Nisbet A. Clinical validation and benchmarking of knowledge-based IMRT and VMAT treatment planning in pelvic anatomy. Radiother Oncol. 2016;120(3):473–479. doi: 10.1016/j.radonc.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE, Bogart JA, Dobelbower MC, Bosch W, Galvin JM, Kavadi VS, Narayan S, Iyengar P, Robinson CG, Wynn RB, Raben A, Augspurger ME, MacRae RM, Paulus R, Bradley JD. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: A secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 25.Abouegylah M, Braunstein LZ, Alm El-Din MA, Niemierko A, Salama L, Elebrashi M, Edgington SK, Remillard K, Napolitano B, Naoum GE, Sayegh HE, Gillespie T, Farouk M, Ismail AA, Taghian AG. Evaluation of radiation-induced cardiac toxicity in breast cancer patients treated with trastuzumab-based chemotherapy. Breast Cancer Res Treat. 2019;174(1):179–185. doi: 10.1007/s10549-018-5053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubo K, Monzen H, Ishii K, Tamura M, Nakasaka Y, Kusawake M, Kishimoto S, Nakahara R, Matsuda S, Nakajima T, Kawamorita R. Inter-planner variation in treatment-plan quality of plans created with a knowledge-based treatment planning system. Phys Med. 2019;67:132–140. doi: 10.1016/j.ejmp.2019.10.032. [DOI] [PubMed] [Google Scholar]
- 27.Uehara T, Monzen H, Tamura M, Ishikawa K, Doi H, Nishimura Y. Dose-volume histogram analysis and clinical evaluation of knowledge-based plans with manual objective constraints for pharyngeal cancer. J Radiat Res. doi: 10.1093/jrr/rraa021. [DOI] [PMC free article] [PubMed] [Google Scholar]