Abstract
Objective
Tracheal suctioning can cause pain and physiological indicator variations in patients with traumatic brain injury (TBI). The aim of the present study was to compare pain severity and physiological indicator variations during the closed tracheal suction system (CTSS) and open tracheal suction system (OTSS) in patients with TBI.
Methods
This study was a clinical trial. Samples included all ventilated patients with TBI. The patients were randomly divided into the OTSS and CTSS groups. In both groups, the Critical Care Pain Observation Tool (CPOT) and physiological indicators were recorded by three nurses prior to suctioning, the end of suctioning and 5 min after suction completion. Data were analysed using the independent t-test and repeated measurement tests.
Results
A total of 112 patients participated in the present study. Before the interventions, the mean value of the Glasgow Coma Scale was 6.45±1.13, blood pressure 128.33±20.54, saturated oxygen in arterial blood (SpO2) 96.74±2.76, respiratory rate (RR) 15.06±3.98, end-tidal CO2 (EtCO2) 36.2±21.98, heart rate 82.18±42.33 and CPOT-based pain 0.43±0.94 in the patients. Independent t-test was used to compare CTSS and OTSS, suggesting significant differences with respect to the mean values of SpO2, RR and EtCO2 immediately after suctioning. This test showed significant differences between the two groups with respect to pain intensity at all three points of measurement. The two groups were also found to be significantly different with respect to RR measured 5 min after suctioning (p<0.05).
Conclusion
Compared to OTSS, CTSS can cause higher reductions in pain levels during and after suctioning in patients with head traumas and can also cause higher improvements in physiological indicators, such as RR, O2 saturation and EtCO2.
Keywords: Close tracheal suction system, open tracheal suction system, pain, physiological indicators, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major health problem worldwide (1). Patients with TBI are candidates for tracheal intubation owing to their reduced consciousness, respiratory rates (RRs) and Glasgow Coma Scale (GCS) and also their loss of laryngeal reflexes (2). Given the patients’ inability to spontaneously clean their airways, airway intubation and the use of mechanical ventilation are major factors contributing to defects in airway clearance. The use of artificial airways causes excessive mucus membrane stimulation and secretion. Moreover, patients with TBI cannot void airway secretions due to their inability to cough and their lack of increased intrathoracic pressure. To solve this problem, intubated patients who are connected to the ventilator in the intensive care unit (ICU) should intermittently undergo sterilised tracheal suctioning. Suction procedures have been reported to be very painful and cause uneasiness. Airway suctioning is a painful event with life-threatening complications (3–6). Jeong et al. (7) and Javadi et al. (8) showed that tracheal suctioning can cause tracheal mucosal injury and pain in patients hospitalised in ICUs.
Acute pain associated with tracheal suctioning cause long-term stress on the biological system and may affect the patients’ treatment outcomes and quality of life even after their discharge (9). Pain also increase intracranial pressure (ICP) by creating agitation, increasing metabolic needs and increasing cerebral blood flow (10–12). In addition to being painful, the airway suctioning procedure may affect physiological indicators, such as blood pressure (BP), PaO2, O2 saturation, heart rate (HR), RR, ICP and GCS (12, 13).
Selecting an appropriate tracheal suctioning method is one way for minimising the complications of tracheal suctioning. Open and closed suctioning are two methods for performing sterile suctioning in intubated patients. Open tracheal suction system (OTSS) is the most commonly used method for tracheal suctioning. Using this method involves patient disconnection from the ventilator during the suction procedure. However, endotracheal suctioning is performed in a closed tracheal suction system (CTSS), while maintaining the pulmonary volume and ensuring continuous oxygenation without disconnecting the ventilator from the patient (14). Some researchers have contrasted OTSS and CTSS and investigated their effects on respiratory parameters, such as oxygenation and heart rhythm and ICP increase. Most of these studies have confirmed that CTSS minimises the oxygenation reduction. CTSS is also believed to cause fewer reductions in arterial oxygen pressure than OTSS. Furthermore, cardiac complications, such as tachycardia and rhythm disorder, are more prevalent in patients undergoing OTSS (15). In a meta-analysis conducted by Jongerden, 15 clinical trials were reviewed on the effects of CTSS and OTSS in patients receiving mechanical ventilation, concluding that OTSS can increase the mean arterial pressure (MAP) more than CTSS (16). A study conducted by Lasocki et al. (17) on 18 patients hospitalised in the ICU showed that using CTSS causes an 18% reduction in arterial oxygen saturation potentially caused by shorter suctioning duration and lower levels of the sympathetic stimulation in CTSS. Ayfer and Suzan (18) found the pain score to be slightly higher in newborns undergoing OTSS than in those undergoing CTSS. In contrast, a meta-analysis by Jongerden et al. (16) showed no preference between CTSS and OTSS.
To the best of the author’s knowledge, the pain caused by these two types of suction has not been compared in patients with head traumas. Given the importance of pain and physiological indicators in patients with TBI and the inevitability of performing suctioning in these patients and also the unknown range of variations in pain intensity and physiological indicators in CTSS and OTSS, the present study was conducted to compare pain severity and variations in physiological indicators during CTSS and OTSS in patients with TBI.
Methods
The present paralleled clinical trial (IRCTID: IRCT2016052311399N4) was performed on a population comprising all ventilated patients with TBI hospitalised in the ICU between May 22, 2016 and June 21, 2018. The present study was approved by the ethics committee of Semnan University of Medical Sciences (no. IR.SEMUMS. REC.1395.31). Participation was voluntary and confidential. The research sample included eligible patients. The inclusion criteria consisted of having TBIs, a maximum GCS score of 8, being intubated with an oral tracheal tube, absence of pain prior to suctioning, not using analgesics during the previous 6 h and not using neuromuscular blocking agents. The exclusion criteria comprised the emergence of dangerous cardiac arrhythmias in patient monitoring during suctioning and detection of blood in suction secretions.
The data collection tools included a demographic questionnaire, a physiological parameters form and the Critical Care Pain Observation Tool (CPOT), which is a behavioural tool for assessing pain in patients unable to speak. This tool comprises four items, namely facial expression, body movements, muscle tension and compliance with the ventilator for intubated patients and vocalisation for extubated patients. Each item is scored 0–2, giving a total scale score of 0–8. This tool has been used in 255 patients hospitalised in the ICU, and its validity and reliability have been confirmed (19). A Cronbach’s alpha of 0.89 also confirmed the reliability of the tool in the present study. The sample size was calculated as 116 (n=58 in each group) based on an effect size of 0.529, a power of 80%, a confidence interval of 95% and the sample size determination formula for comparing means in two independent groups in G*Power-3.0.10.
Written informed consent was obtained from a first-degree relative of the eligible patients. Demographic information and physiological parameters were recorded. The patients were then randomly divided into two groups: A and B groups. To ensure randomisation, letter A was written on 30 cards and letter B was written on 30 other cards, and the cards were placed in a bag. Samples were selected by asking one of the nurses of the ward to take out one card at a time from the bag, and the patients were assigned to group A or B depending on the letter written on the card. The withdrawn cards were not returned to the bag to preserve the number of samples required for each group. Patients in group A underwent OTSS, and those in group B underwent CTSS.
The patients in both groups received 100% oxygen by the ventilator for 2 min before undergoing suctioning. The end-tidal CO2 (EtCO2) device was then attached to the patient’s tracheal tube. The ventilator was disconnected from the patients in group A before undergoing suctioning. In group B, the ventilator was not disconnected from the patients, and suctioning was performed by inserting the suction catheter into the tracheal tube through a Y-connector between the tracheal tube and the ventilator tube. Suctioning was performed in both groups for 14 s using catheter no. 14 with a pressure <120 mmHg. The tracheal tube was connected to the ventilator immediately after suctioning, and the patients underwent 100% oxygen therapy for 2 min using the ventilator.
A digital timer was placed where all the samplers could easily see it to aid with the data collection before suctioning. The timer started as soon as 100% oxygen was delivered and showed the required time points to the samplers. In both groups, CPOT-based pain intensity, systolic and diastolic BP, MAP, HR, RR and saturated oxygen in arterial blood (SpO2) and EtCO2 levels were recorded by three nurses during oxygenation prior to suctioning (1 min before suctioning initiation), at the end of suctioning (14 s after suctioning initiation), at the end of oxygenation following suction completion (2 min and 14 s after suctioning initiation) and 5 min after suction completion (5 min and 14 s after suctioning initiation). A nurse was in charge of recording BP, RR and pulse rate, and another nurse was in charge of recording SpO2 and EtCO2. A trained nurse was also in charge of recording pain intensity based on CPOT. This nurse was trained to be able to accurately complete the CPOT within 10 s.
Given the quantitative nature of the dependent variables, the two groups were compared before and after the intervention using the independent t-test. Given the repeated measures obtained, the generalised linear model (GLM) was used to investigate and adjust the potential effect of the underlying variables and interactive effects. A P value <0.05 was set as the level of statistical significance in all the tests used.
Results
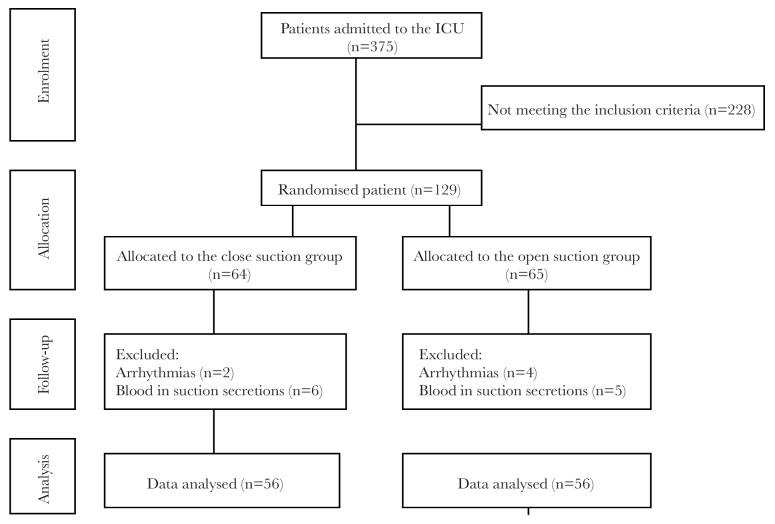
Of the 275 patients hospitalised in the ICU during sampling, 129 were found to be eligible. During the sampling process, 6 patients were excluded due to the emergence of arrhythmia, and 11 patients were excluded due to the detection of blood in their suction secretions. Data associated with 112 patients were ultimately analysed (Figure 1).
Figure 1.
Flowchart of patients through the trial
The mean age of the participating patients was 50.96±18.61 years. Sixty-nine (61.6%) patients were male, and the rest were female. Forty-nine (43.7%) patients had a history of hypertension, and 32 (28.57%) patients had a history of diabetes. Before the interventions, the mean value of GCS was 6.45±1.13, BP 128.33±20.54, SpO2 96.74±2.76, RR 15.06±3.98, EtCO2 36.2±21.98, HR 82.18±42.33 and CPOT-based pain 0.43±0.94 in the patients. The chi-square test found the two groups to be insignificantly different before the interventions with respect to all the variables except for gender distribution (p=0.033) (Table 1).
Table 1.
Background parameters of patients in the open and close suction groups
| Background parameters | Close suction group (n=56) | Open suction group (n=56) | p |
|---|---|---|---|
|
| |||
| Age (year) | 52.59±18.87 | 49.34±18.38 | 0.385 |
| Gender (male/female) | 27/29 | 16/40 | 0.033* |
| HTN history (yes/no) | 35/21 | 28/28 | 0.182 |
| Diabetes history (yes/no) | 37/19 | 43/13 | 0.209 |
| BP (mmHg) | 129.70±18.40 | 126.96±22.56 | 0.484 |
| HR (bpm) | 84.04±17.19 | 80.80±19.43 | 0.353 |
| RR | 14.75±3.92 | 15.38±4.04 | 0.408 |
| O2 saturation | 96.57±2.85 | 96.91±2.67 | 0.518 |
| EtCO2 | 36.29±2.84 | 36.13±3.14 | 0.777 |
| GCS | 6.32±1.16 | 6.57±1.09 | 0.308 |
| Pain based on CPOT | 0.35±0.66 | 0.31±0.13 | 0.506 |
| Frequency of suction per day | 3.58±1.14 | 3.55±1.45 | 0.575 |
| Ventilation mode (IPPV/SIMV) | 40/16 | 36/20 | 0.198 |
Data are shown as mean±standard deviation or n. HTN: hypertension; BP: blood pressure; HR: heart rate; bpm: beat per minute; RR: respiratory rate; EtCO2: end-tidal CO2; GCS: Glasgow Coma Scale; CPOT: Critical Care Pain Observation Tool; IPPV: intermittent positive-pressure ventilation; SIMV: synchronised intermittent mandatory ventilation
Independent t-test was used to compare CTSS and OTSS, suggesting significant differences with respect to the mean values of SpO2, RR and EtCO2 immediately after suctioning. This test showed significant differences between the two groups with respect to pain intensity at all three points of measurement. The two groups were also found to be significantly different with respect to RR measured 5 min after suctioning (p<0.05) (Table 2).
Table 2.
Pain and physiological parameter changes during and after suction in the close and open groups
| Parameters (min after start suction) | Close suction group (mean±SD) | Open suction group (mean±SD) | p |
|---|---|---|---|
|
| |||
| Pain based on CPOT (1′) | 0.30±0.76 | 0.82±1.49 | 0.023* |
| Pain based on CPOT (3′) | 2.39±1.63 | 4.64±2.38 | 0.000* |
| Pain based on CPOT (7′) | 0.39±0.86 | 1.27±1.76 | 0.001* |
| BP (1′) | 128.68±16.48 | 124.38±22.50 | 0.251 |
| BP (3′) | 134.29±19.32 | 136.25±23.83 | 0.633 |
| BP (7′) | 132.34±16.63 | 138.13±22.26 | 0.122 |
| HR (1′) | 83.93±17.65 | 81.79±19.13 | 0.539 |
| HR (3′) | 86.23±17.78 | 91.05±22.62 | 0.213 |
| HR (7′) | 88.13±17.25 | 92.91±22.77 | 0.213 |
| RR (1′) | 14.61±3.80 | 15.21±4.00 | 0.412 |
| RR (3′) | 16.61±4.82 | 19.20±5.25 | 0.008* |
| RR (7′) | 15.43±4.89 | 18.48±6.44 | 0.006* |
| O2 saturation (1′) | 97.84±2.26 | 97.77±2.09 | 0.863 |
| O2 saturation (3′) | 96.39±2.87 | 94.80±3.48 | 0.010* |
| O2 saturation (7′) | 96.23±3.15 | 92.93±4.58 | 0.000* |
| EtCO2 (1′) | 36.34±2.49 | 36.02±2.93 | 0.533 |
| EtCO2 (3′) | 35.50±2.89 | 33.70±3.34 | 0.003* |
| EtCO2 (7′) | 35.77±2.69 | 34.98±3.03 | 0.150 |
Independent t-test showed that there is a significant difference between the two groups.
CPOT: Critical Care Pain Observation Tool; BP: blood pressure; HR: heart rate; RR: respiratory rate; EtCO2: end-tidal CO2
The fit of the GLM with gender included showed that the variations of all the dependent variables were significant over time in both the OTSS and CTSS groups (p<0.001). The principle and interactive effect of gender on all the dependent variables was insignificant (p>0.05), suggesting that the disproportionate distribution of gender between the two groups did not affect the study results. The model-derived tests also showed that the interactive effects of time on the dependent variables were significant between the two groups (p<0.05). The P-values obtained from the model of intra-group effects showed that dependent variables of SpO2 and pain were significantly different between the two groups after adjusting the main effects of intra-group changes and two-way and three-way interactive effects with grouping and gender. In other words, pain was significantly higher, and blood oxygen levels were significantly lower in OTSS than in CTSS (Table 2).
Discussion
The present study found the mean pain severity of the upper airways to be 0.35±0.66 in mechanically ventilated patients with head traumas undergoing CTS and 0.31±0.13 in those undergoing OTS, suggesting no significant differences between the two groups before suctioning, which is in line with the observations made by Dastdadeh et al. (20) between the two groups of candidates for CTS and OTS. This finding suggests that the presence of a tracheal tube in the upper airways causes slight pain in patients with head traumas, which should be considered by the medical team.
The results showed that pain recovery and approaching the baseline were faster in the CTSS group than in the OTSS group. In fact, 5 min after suction completion, the CTSS group experienced pain almost at the same level as the baseline, whereas pain was approximately 4 times as severe as the baseline in the OTSS group. The findings also showed that the mean pain score was lower in the CTSS group than in the OTSS group immediately after and 2 and 5 min after suctioning, suggesting significant differences between the two groups based on statistical tests. Ayfer and Suzan (18) confirmed this finding by showing that the pain caused by CTS was less than that caused by OTS. In contrast, separately conducted studies by Dastdadeh et al. (20) and Mohammadpour et al. (3) showed no significant differences in the pain caused by tracheal tube suctioning in the CTS and OTS groups. This discrepancy of results can be explained by the difference in the study samples, suction duration, method of administering suctioning, failing to use 100% oxygen before and after suctioning and the difference in methods used in other studies. In the present study, the higher pain levels in the OTSS group after suctioning appear to be caused by more manipulation of the tracheal tube during suctioning, since the tracheal tube should be disconnected from the ventilator in OTSS, causing the tracheal tube to move and stimulate the trachea and subsequently increase the pain. Moreover, in the OTSS group, the airway diameter might be reduced, and the contact surface of the tracheal tube tip with the tracheal wall increases due to a sudden reduction in the airway pressure as a result of the complete disconnection of the patient’s tracheal tube from the ventilator. Furthermore, the contact of the tracheal tube and the suction catheter with the tracheal wall can cause laryngeal spasm and pain even up to 5 min after suction completion. The nurse may also rush to perform suctioning resulting in a more invasive procedure caused by the ventilator being disconnected from the patient and fears of hypoxia in the patient undergoing OTSS.
In the present study, the means of all the physiological indicators (BP, HR, RR, O2 saturation and EtCO2) were within the normal range except for GCS, suggesting no significant differences between the two groups before the intervention. Similarly, Dastdadeh et al. (20) and Jongerden et al. (15) found the two groups of CTSS and OTSS not to be significantly different before suctioning as all physiological indicators were within the normal range. This finding shows that the stabilisation of physiological indicators in the ICUs is important for the treatment team, and any factor disrupting the stability of these indicators, including airway suctioning, should be identified and controlled.
Although the mean BP and HR increased after suctioning in both the study groups, the two groups were found not to be significantly different with respect to mean BP and HR at any measurement points. Similarly, Jongerden et al. (15) showed no significant differences in the mean HR and MAP of the patients after CTS and OTS. In contrast, Subhash Mengar (21) and Afshari et al. (22) reported significantly higher values for HR in the OTS group than in the CTS group. This finding suggests that OTSS and CTSS similarly affect BP and HR in ventilated patients with head traumas. Given that increased BP and HR can increase ICP in patients with head traumas, measures should be taken to prevent BP and HR from increasing during suctioning.
After completing suctioning, the mean RR increased, and the mean O2 saturation and EtCO2 decreased in both the study groups. Statistical tests also showed statistically significant differences in the mean RR and O2 saturation between the two groups 2 and 5 min after the suction completion and in the mean EtCO2 2 min after the suction completion. Moreover, Afshari et al. (22) and Subhash Mengar (21) reported statistically significant differences in O2 saturation values between the CTS and OTS groups. However, Dastdadeh et al. (20) found no significant differences in the mean O2 saturation between the CTS and OTS groups. Higher reductions in O2 saturation in the OTS group than in the CTS group appear to be caused by a sudden reduction in pulmonary oxygen pressure and the discharge of large amounts of oxygen from the patient’s lungs following the disconnection of the ventilator from the patients’ tracheal tube. Higher levels of EtCO2 observed in the OTS group than in the CTS group can also be exaplined by the ventilation disconnection and the patient’s inability of exhaling CO2.
Conclusion
Compared to OTSS, CTSS can cause higher reductions in pain levels during and after suctioning in patients with head traumas and can also cause higher improvements in physiological indicators, such as RR, O2 saturation and EtCO2. Moreover, physiological variables, such as BP and HR, increase during suctioning irrespective of the type of the method used. Therefore, CTSS is recommended to be used in suctioning of the upper airways in patients with head traumas.
Main Points.
Close tracheal suction system (CTSS) should be used for mechanically-ventilated patients with head traumas.
CTSS can reductions in pain levels during and after suctioning in patients with head traumas.
CTSS can improvements the physiological indicators after suctioning in patients with head traumas.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Semnan University of Medical Sciences (no. IR.SEMUMS.REC.1395.31).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.E.; Design – A.E., H.D.; Supervision – A.E.; Resources – A.E.; Materials – H.D.; Data Collection and/or Processing – H.D.; Analysis and/or Interpretation – F.P., M.T.; Literature Search – M.T., F.P.; Writing Manuscript – M.T., F.P.; Critical Review – A.E., H.D.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 2.John PA, Dominic B, Justin M. Neurocritical Care A Guide to Practical Management. Springer; London Dordrecht Heidelberg New York: 2010. p. 1. [Google Scholar]
- 3.Mohammadpour A, Amini S, Shakeri MT, Mirzaei S. Comparing the effect of open and closed endotracheal suctioning on pain and oxygenation in post CABG patients under mechanical ventilation. Iran J Nurs Midwifery Res. 2015;20:195–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen CM, Rosendahl-Nielsen M, Hjermind J, Egerod I. Endotracheal suctioning of the adult intubated patient. Intensive Crit Care Nurs. 2009;25:21–30. doi: 10.1016/j.iccn.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Juneja D, Javeri Y, Singh O, Nasa P, Pandey R, Uniyal B. Comparing influence of intermittent subglottic secretions drainage with/without closed suction systems on the incidence of ventilator associated pneumonia. Indian J Crit Care Med. 2011;15:168–72. doi: 10.4103/0972-5229.84902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seguin PH, Perrichet H, Le Pabic E, Launey Y, Tiercin M, Corre R, et al. Effect of continuous versus intermittent subglottic suctioning on tracheal mucosa by the mallinckrodt taper-guard evac oral tracheal tube in intensive care unit ventilated patients: A prospective randomized study. Indian J Crit Care Med. 2018;22:1–4. doi: 10.4103/ijccm.IJCCM_350_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong JH, Nam SJ, Cho YJ, Lee YJ, Kim SJ, Song IA, et al. A Closed-Suction Catheter with a Pressure Valve Can Reduce Tracheal Mucosal Injury in Intubated Patients. Korean J Crit Care Med. 2014;29:7–12. doi: 10.4266/kjccm.2014.29.1.7. [DOI] [Google Scholar]
- 8.Javadi M, Hejr H, Zolad M, Khalili A, Paymard A. Comparing the effect of endotracheal tube suction using open method with two different size catheters 12 and 14 on discharge secretion, pain, heart rate, blood pressure, and arterial oxygen saturation of patients in the intensive care unit: A randomized clinical trial. Annals of Tropical Medicine and Public Health. 2017;10:1312–7. doi: 10.4103/ATMPH.ATMPH_181_17. [DOI] [Google Scholar]
- 9.Stamenkovic DM, Laycock H, Karanikolas M, Ladjevic NG, Neskovic V, Bantel C. Chronic Pain and Chronic Opioid Use After Intensive Care Discharge - Is It Time to Change Practice? Front Pharmacol. 2019;10:23. doi: 10.3389/fphar.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddad SH, Arabi YM. Critical care management of severe traumatic brain injury in adults. Scand J Trauma Resusc Emerg Med. 2012;20:12. doi: 10.1186/1757-7241-20-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobscha SK, Clark ME, Morasco BJ, Freeman M, Campbell R, Helfand M. Systematic review of the literature on pain in patients with polytrauma including traumatic brain injury. Pain Med. 2009;10:1200–17. doi: 10.1111/j.1526-4637.2009.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ugras GA, Aksoy G. The effects of open and closed endotracheal suctioning on intracranial pressure and cerebral perfusion pressure: a crossover, single-blind clinical trial. J Neurosci Nurs. 2012;44:E1–8. doi: 10.1097/JNN.0b013e3182682f69. [DOI] [PubMed] [Google Scholar]
- 13.Chen HJ, Chen YM. Pain Assessment: Validation of the Physiologic Indicators in the Ventilated Adult Patient. Pain Manag Nurs. 2015;16:105–11. doi: 10.1016/j.pmn.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Kuriyama A, Umakoshi N, Fujinaga J, Takada T. Impact of closed versus open tracheal suctioning systems for mechanically ventilated adults: a systematic review and meta-analysis. Intensive Care Med. 2015;41:402–11. doi: 10.1007/s00134-014-3565-4. [DOI] [PubMed] [Google Scholar]
- 15.Jongerden Irene P, Kesecioglu J, Speelberg B, Buiting Anton G, Leverstein-van Hall Maurine A, Bonten Marc J. Changes in heart rate, mean arterial pressure, and oxygen saturation after open and closed endotracheal suctioning: A prospective observational study. J Crit Care. 2012;27:647–54. doi: 10.1016/j.jcrc.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Jongerden Irene P, Rovers Maroeska M, Grypdonck Mieke H, Bonten Marc J. Open and closed endotracheal suction systems in mechanically ventilated intensive care patients: A meta-analysis. Crit Care Med. 2007;35:260–70. doi: 10.1097/01.CCM.0000251126.45980.E8. [DOI] [PubMed] [Google Scholar]
- 17.Lasocki S, Lu Q, Sartorius A, Fouillat D, Remerand F, Rouby JJ. Open and Closed-Circuit Endotracheal Suctioning in Acute Lung Injury: Efficiency and Effects on Gas Exchange. Anesthesiology. 2006;104:9. doi: 10.1097/00000542-200601000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Ayfer A, Suzan Y. Effects of Open and Closed Suctioning Systems on Pain in Newborns Treated with Mechanical Ventilation. American Society for Pain Management Nursing. 2016;15:11. doi: 10.1016/j.pmn.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Gelinas C, Johnston C. Pain assessment in the critically ill ventilated adult: validation of the critical-care pain observation tool and physiologic indicators. Clin J Pain. 2007;23:497–505. doi: 10.1097/AJP.0b013e31806a23fb. [DOI] [PubMed] [Google Scholar]
- 20.Dastdadeh R, Ebadi A, Vahedian-Azimi A. Comparison of the Effect of Open and Closed Endotracheal Suctioning Methods on Pain and Agitation in Medical ICU Patients: A Clinical Trial. Anesth Pain Med. 2016;6:1–8. doi: 10.5812/aapm.38337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subhash Mengar R. A comparative Study to Assess the Effectiveness of Open tracheal Suction System and closed tracheal Suction System on Physiological Parameters. Int J Nurs Education. 2018;10:109–12. doi: 10.5958/0974-9357.2018.00114.9. [DOI] [Google Scholar]
- 22.Afshari A, Safari M, Oshvandi K, Soltanian AR. The Effect of the Open and Closed System Suctions on Cardiopulmonary Parameters: Time and Costs in Patients Under Mechanical Ventilation. Nurs Midwifery Stud. 2014;3:1–6. doi: 10.5812/nms.14097. [DOI] [PMC free article] [PubMed] [Google Scholar]