Abstract
Cytokine release syndrome (CRS) is a systemic inflammatory response that can be triggered by many factors such as infections. CRS in patients with coronavirus disease 2019 (COVID-19) is life-threatening and can occur very rapidly after COVID-19 diagnosis. Tocilizumab (TCZ), an interleukin-6 (IL-6) inhibitor, may ameliorate the CRS associated with severe COVID-19 and thus improve clinical outcomes. We present a case of life-threatening CRS caused by COVID-19 infection successfully treated with TCZ.
LEARNING POINTS
Cytokine release syndrome (CRS) is a systemic inflammatory response that can be triggered by COVID-19.
CRS can be life-threatening in severe COVID-19.
Tocilizumab may have a role in treating severe COVID-19 patients with CRS.
Keywords: Tocilizumab, COVID-19, cytokine release syndrome
INTRODUCTION
SARS-CoV-2 causes a mild to moderate illness characterized by fever and respiratory symptoms, with or without evidence of pneumonia. However, up to 10% of patients with COVID-19 may develop severe pneumonia with hypoxia, cytokine release syndrome (CRS) and multiorgan failure [1]. Tocilizumab (TCZ) is a humanized monoclonal inhibitor of the proinflammatory cytokine interleukin-6 (IL-6) and is licensed for use in the clinical management of CRS [2]. Peer-reviewed data on the clinical use of TCZ in severe COVID-19 are very limited. We describe a case of CRS caused by severe COVID-19 infection with a favourable response to TCZ therapy.
CASE DESCRIPTION
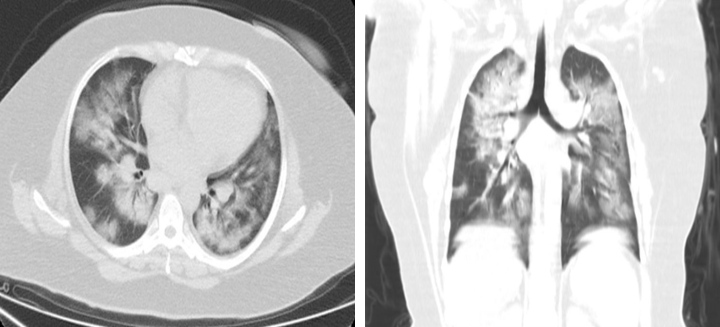
A 41-year-old woman with a history of hypertension, presented with fever, productive cough and general fatigue. She denied any recent travel but had a history of contact with sick people. In the emergency department she was tachypnoeic (respiratory rate of 22 breaths/min), tachycardiac (130 bpm), febrile (39°C) and hypoxic (oxygen saturation (SpO2) of 88% on room air). Physical examination of the lungs revealed bilateral crackles and wheeze. The white cell count was normal and the absolute lymphocyte count was low (0.61–103/μl), while the erythrocyte sedimentation rate (77; range, 0–20 mm/h) and C-reactive protein levels (110; range, 0–5 mg/l) were increased. D-dimer was 1657 ng/ml and procalcitonin was normal. Assays for influenza viruses and a respiratory syncytial virus were all negative. A nasopharyngeal swab was positive for SARS-CoV-2 on RT-PCR assay. Computed tomography (CT) at the time of admission revealed bilateral ground-glass opacities and consolidation (Fig. 1). The diagnosis of COVID-19 pneumonia was made and treatment with oseltamivir, hydroxychloroquine and low-molecular-weight heparin was initiated. The patient received supplemental oxygen through a nasal cannula at a rate of 4 l/min. Blood and urine cultures were negative.
Figure 1.
CT scan showing ground-glass opacities and consolidation
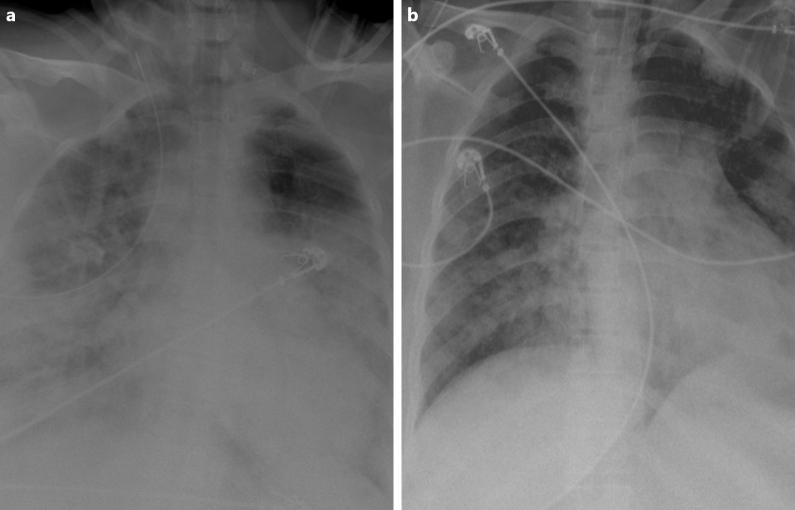
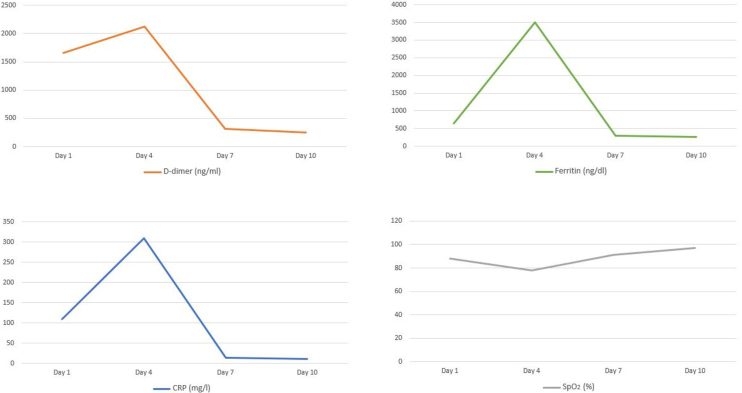
After 3 days of hospitalization the patient’s clinical condition deteriorated (respiratory rate of 26 breaths/min and SpO2 decreased to 78%) and she required intensive care unit (ICU) admission. A chest x-ray at the time of ICU admission revealed hazy bilateral lobe opacity (Fig. 2a). The patient was intubated and diagnosed with CRS. She was given a single intravenous (IV) dose of 400 mg TCZ and IV methylprednisone 60 mg daily for 3 days. Ventilatory support requirements reduced day-by-day. C-reactive protein, ferritin and D-dimer levels dropped from 310 mg/l, 3500 ng/dl and 2123 ng/ml on the day of ICU admission to 13.2 mg/l, 300 ng/dl and 320 ng/ml after 3 days, respectively. A chest x-ray showed that the lesions significantly improved within 3 days of TCZ administration (Fig. 2b). The patient was successfully extubated 5 days after treatment with TCZ. No adverse events were described. On day 10, a clear improvement in the patient’s general condition was observed, with an SpO2 of 97% without any need for supplemental oxygen (Fig. 3).
Figure 2.
(a) X-ray of the lung, at the time of ICU admission, revealed hazy bilateral lobe opacity. (b) X-ray after treatment showed significant improvement
Figure 3.
Laboratory and SpO2 data
DISCUSSION
COVID-19 is associated with increased proinflammatory cytokines. The elevated cytokine levels may also be responsible for the lethal complications of COVID-19. Moreover, histopathological examination of lung tissue from deceased patients with severe COVID-19 showed evidence of extensive alveolar oedema, proteinaceous exudate and patchy inflammatory cellular infiltration. These findings suggest that severe COVID-19 infection is associated with a cytokine storm and pulmonary inflammation secondary to a dysregulated host immune response [3]. IL-6 contributes to host defence against infection; however, exaggerated synthesis of IL-6 while fighting environmental stress leads to an acute severe systemic inflammatory response. In the subset of patients with severe COVID-19 infection, this cytokine activation presents with recognized features such as high plasma levels of C-reactive protein, D-dimer and ferritin, and a decreased lymphocyte count. TCZ is a monoclonal antibody against IL-6 and is currently approved to treat chronic inflammatory conditions such as rheumatoid arthritis, giant cell arteritis and polyarticular juvenile idiopathic arthritis [4]. CRS in a patient with COVID-19 is life-threatening and can take place very rapidly after COVID-19 diagnosis. Currently, there is no established treatment for COVID-19-associated CRS. TCZ is an option for use in the clinical management of COVID-19 patients with CRS and is associated with a dramatic improvement in inflammatory markers, radiological changes and hypo-oxygenemia.
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interests.
REFERENCES
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23(8):943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020 Apr 10;:102452. doi: 10.1016/j.jaut.2020.102452. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]