Abstract
Background and Purpose
Early retinal neurodegeneration occurs as one of the complications of diabetes even before clinically detectable diabetic vascular retinopathy. The pathogenesis of retinal diabetic neuropathy is still not well understood. We investigated the serial changes or fluctuations in intraocular pressure (IOP) and examined their roles in the pathogenesis of neuronal degeneration in diabetic retina.
Experimental Approach
Male Sprague Dawley rats with streptozotocin‐induced diabetes were treated with ophthalmic preparations of brinzolamide, latanoprost, both drugs (combined treatment) or saline for 8 weeks. IOP was measured daily under general anaesthesia using a rebound tonometer. Antegrade axoplasmic flow in the optic nerve was assessed with a fluorescent substrate. Immunohistochemical staining, TUNEL assays and western blots were also used.
Key Results
The fluctuation of IOP was higher in the diabetes group than in the normal control or the combined treatment group. Diabetes‐induced apoptosis of retinal ganglion cells was decreased by combined treatment. Increased expression of glial fibrillary acidic protein or Iba‐1 in the retina or optic nerve head, induced by diabetes, was attenuated only by the combined treatment. Intercellular adhesion molecule‐1 was increased in diabetic rats but not in the combined treatment group. Diabetes‐induced loss of antegrade axoplasmic transport was partially relieved with combined treatment.
Conclusion and Implications
Elevated IOP fluctuations seemed to be associated with the gliosis, neuroinflammation, and neurodegeneration induced by diabetes. The loss of retinal ganglion cells might be relieved by IOP‐lowering medication. The improvement of unstable perfusion pressure could play a role in neuroprotection in the diabetic retina.
Abbreviations
- GCL
ganglion cell layer
- GFAP
glial fibrillary acidic protein
- GSL1
Griffonia simplicifolia Lectin I
- IB4
isolectin B4
- Iba‐1
ionized calcium binding adaptor molecule
- ICAM
intercellular adhesion molecule
- INL
inner nuclear layer
- iNOS
inducible NOS
- IOP
intraocular pressure
- NG2
neural glial antigen 2
- ONH
optic nerve head
- RGC
retinal ganglion cell
- RNFL
retinal nerve fibre layer
- STZ
streptozotocin
What is already known
Early retinal neurodegeneration often occurs even before clinically detectable diabetic vascular retinopathy.
What does this study add
IOP‐lowering medicine seemed to decrease diabetic retinal neurodegeneration.
What is the clinical significance
Topical application of IOP‐lowering drugs might be another therapeutic strategy for retinal neuroprotection in diabetes.
1. INTRODUCTION
Diabetic retinopathy, a leading cause of blindness, is one of the major microvascular complications of diabetes (Antonetti, Klein, & Gardner, 2012; Cheung, Mitchell, & Wong, 2010). The retina is not only a system of blood vessels but also includes a neurovascular‐multicellular unit (Antonetti et al., 2012; Stitt et al., 2016). A growing body of evidence suggests that early retinal neurodegeneration, also called retinal diabetic neuropathy, occurs even before clinically detectable diabetic vascular retinopathy (El‐Fayoumi, Badr Eldine, Esmael, Ghalwash, & Soliman, 2016; Park, Kim, & Park, 2011; Simo et al., 2014; Sohn et al., 2016). We previously found apoptosis of retinal ganglion cells (RGCs) in diabetic rats, although other retinal cellular damage was also reported (Barber et al., 1998; Jung, Kim, Park, & Park, 2013; Park et al., 2003; Park, Kim, & Park, 2014; Simo, Stitt, & Gardner, 2018). Retinal neural degeneration is now regarded as one of the critical pathological characteristics of the ocular complications of diabetes (Stitt et al., 2016).
Neural degeneration may contribute to impaired psychophysical visual function, such as electrophysiological measurements or contrast sensitivity (Georgakopoulos et al., 2011; Prager, Garcia, Mincher, Mishra, & Chu, 1990; Verrotti et al., 2000). Visual dysfunction might be clinically subtle in the early retinal neurodegeneration, whereas proliferative diabetic retinopathy or diabetic macular oedema leads to a substantial decrease in vision. However, the loss of RGCs is critical because it is irreversible. The pathogenesis of RGC degeneration in diabetes is still not fully elucidated, although inflammation, oxidative stress, or exposure to advanced glycation end products, excitotoxicity, and an imbalanced level of growth factors have been suggested (Kern & Barber, 2008; Stitt et al., 2016). Clinical data have demonstrated that microalbuminuria, a well‐known marker for vascular endothelial dysfunction, was a risk factor for retinal nerve fibre layer (RNFL) defects, independent of the presence of diabetic microvasculopathy (Choi et al., 2015). Autonomic dysfunction was also related to RNFL loss in young diabetic patients (Jeon, Park, Lee, & Park, 2018). However, why RGCs are the most vulnerable to stimuli related to diabetes remains a critical unanswered question.
A meta‐analysis revealed that diabetes was a risk factor for glaucoma, which is characterized by a selective loss of RGCs and typical visual field damage, even though several studies on the association between diabetes and glaucoma have reported inconsistent results (Zhao, Cho, Kim, Friedman, & Guallar, 2015). High intraocular pressure (IOP) or a fluctuation in IOP is one of the most important risk factors for the development and progression of glaucoma, even though glaucoma can develop within the normal range of IOP (Caprioli & Coleman, 2008; Coleman & Miglior, 2008; Ernest et al., 2013; Kim & Caprioli, 2018; Tojo, Abe, Ishida, Yagou, & Hayashi, 2017). Diabetes was correlated with a slightly higher IOP (Singh & Heong, 1986; Soto et al., 2014; Zhao, Cho, Kim, Friedman, & Guallar, 2015). A significant postural increase in IOP was found in diabetic patients (Singh & Heong, 1986; Williams, Peart, & Letley, 1980). The unstable regulation of local adrenoceptors, which is significant for maintaining the homeostasis of IOP, has been suggested as the mechanism underlying the abnormal response of the IOP (Singh & Heong, 1986).
Even a small increase or fluctuation in IOP could make RGCs vulnerable to stress in the diabetic retina, in which the capacity for autoregulation might be reduced (Wong, Bui, & Vingrys, 2011). There has been no assessment of the specific role for an increase or fluctuation in IOP in the pathogenesis of diabetic retinal neurodegeneration. Therefore, we measured the serial change in IOP and its variation after the induction of diabetes in rats. We also investigated whether IOP‐lowering medication can be neuroprotective in diabetic retinal neurodegeneration.
2. METHODS
2.1. Animals
All animal care and research procedures were in accordance with the Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Guidelines and Policies for Rodent Experiments provided by the Institutional Animal Care and Use Committee in the School of Medicine, The Catholic University of Korea Institutional Animal Care and Use Committee. The Department of Laboratory Animals, The Catholic University of Korea, Songeui Campus, acquired AAALAC International full accreditation in 2018. Experimental protocols complied with the Association for Research in Vision and Ophthalmology Statement on the Use of Animals in Ophthalmic and Vision Research. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath & Lilley, 2015) and with the recommendations made by the British Journal of Pharmacology.
We used adult male Sprague Dawley rats (7–8 weeks, 200–300 g) and these rats were originally obtained from Charles River Laboratories (MA, USA) and were purchased from a local breeder (Orient Bio, Korea).
2.2. Induction of diabetes
Diabetes mellitus was triggered by one i.p. injection of streptozotocin (STZ; Sigma‐Aldrich; 60 mg·kg−1 body weight) in a 0.1‐M citrate buffer solution (pH 4.5). Age‐matched control rats were treated with the same volume of citrate buffer solution. Blood glucose values were measured using an automated Accu‐Check glucometer (Roche Diagnostics Ltd.) at 3 days after the induction of diabetes. Rats with plasma glucose levels over 3.5 mg·ml−1 were determined to be diabetic and used for further experimentation. Body weight and plasma glucose levels were recorded every week after the induction of diabetes.
2.3. Measurement of IOP
IOP was assessed under general anaesthesia using isoflurane‐nitrous oxide at baseline and everyday between 10:00 a.m. and 12:00 p.m. before applying the drugs to each animal after the induction of diabetes using a rebound tonometer (TonoVet®, Icare, Helsinki, Finland). IOP was measured five times. Mean IOP measurements per week were compared among the groups.
2.4. Allocation of groups and drug treatments
From the day when the induction of diabetes was confirmed, the rats were given one drop of 1% brinzolamide ophthalmic suspension (Azopt®) twice a day, at about 10:00 a.m. and 6:00 p.m., 0.005% latanoprost ophthalmic solution (Xalatan®) once a day between 10:00 a.m. and 12:00 p.m. or saline once a day between 10:00 a.m. and 12:00 p.m. for 8 weeks in both eyes. The combined treatment groups were treated with both brinzolamide and latanoprost. Each animal was treated with the same drug or saline in both eyes to avoid the crossover effect of topical eye drops. Ten animals were randomly assigned to each treatment group except for the experiments on visualization of antegrade axoplasmic flow (five animals for each group), with the experimenter blinded to drug treatment.
2.5. Visualization of antegrade axoplasmic flow
General anaesthesia was performed with isoflurane‐nitrous oxide, and local anaesthesia was performed with a drop of proparacaine hydrochloride (Alcaine 0.5%). To observe antegrade axoplasmic flow, rats were intravitreally injected with 5‐μl cholera toxin subunit B conjugated with Alexa Fluor 488 (1 mg·ml−1 in PBS; Thermo Fisher Scientific) using a 31‐gauge insulin syringe (Becton, Dickinson and Company). The needle was kept in place for 1 min to block the leakage of the injected cholera toxin B conjugates. Three days later, all the animals were deeply anaesthetized with overdose of isoflurane‐nitrous oxide and then killed by inhalation of CO2. After that, eye enucleation was performed with a 360‐degree‐periotomy, dissection of Tenon's capsule away from the globe in the four quadrants, disinsertion of rectus muscles, and cutting of optic nerve. Eyeballs were fixed in 4% paraformaldehyde in 0.1‐M phosphate buffer for 20 min. The posterior segments of the eyes were fixed in 4% paraformaldehyde in 0.1‐M phosphate buffer for 50 min and cryopreserved in 0.1‐M phosphate buffer containing 30% sucrose at 4°C overnight. The posterior eyecups were embedded in optimal cutting temperature compound (Leica Biosystems) and cut into cryostat sections (10 μm). Alexa Fluor 488 was visualized using a confocal laser scanning microscope (Zeiss).
2.6. Immunofluorescence staining
Eye enucleation was performed in rats that were deeply anaesthetized with overdose of isoflurane‐nitrous oxide and then killed by inhalation of CO2, and the eyes were then prepared for immunofluorescence staining. Enucleated eyes were washed with PBS. The whole eye was fixed in 4% paraformaldehyde in 0.1‐M phosphate buffer for 20 min. After removing the anterior segment, the posterior eyecups were fixed in 4% paraformaldehyde in 0.1‐M phosphate buffer for 1 hr at 4°C. The posterior eyecups were washed with PBS and cryopreserved in 0.1‐M phosphate buffer containing 25% sucrose at 4°C overnight. After washing with PBS, the posterior eyecups were embedded in optimal cutting temperature compound and frozen with liquid nitrogen. The samples were cut into cryostat sections (12 μm) at the orientation from superior to inferior optic nerve and stored at −20°C until subsequent use.
Retinal sections were thawed, air‐dried, and washed with 0.1‐M PBS. The antibody‐based procedures used in this study comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018). The sections were permeabilized with 3% Triton for 30 min and blocked with 10% normal donkey serum for 1 hr at room temperature. The slides were incubated with anti‐glial fibrillary acidic protein (GFAP, Millipore, 1:400, RRID:AB_11212597), anti‐VEGF (Abcam, 200, RRID:AB_2212642), anti‐intercellular adhesion molecule 1 (ICAM‐1, Santa Cruz Biotechnology, 1:100, RRID:AB_627120), anti‐ionized calcium binding adaptor molecule (Iba‐1, Wako Pure Chemical, 1:500, RRID:AB_839504), anti‐neural glial antigen 2 (NG2, Abcam, 1:500, RRID:AB_881569), Biotinylated Griffonia simplicifolia Lectin I (GSL I), solectin B4 (IB4, Vector Laboratories, Burlingame, 1:500, RRID:AB_2314661) overnight at 4°C. The sections were then incubated with Alexa Fluor 488‐labelled goat anti‐mouse IgG (Thermo Fisher Scientific, RRID:AB_2534069) or streptavidin Alexa Fluor 488 conjugate or Alexa Fluor 546‐labelled goat anti‐mouse IgG (Thermo Fisher Scientific, RRID:AB_2534071) or Alexa Fluor 546‐labelled goat anti‐rabbit IgG (Thermo Fisher Scientific Cat# A‐11010, RRID:AB_2534077) for 1 hr at room temperature. The sections were mounted using VECTASHIELD mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Using a confocal laser scanning microscope, the slides were visualized. Each eyecup was divided into two mid central (1.5 mm from the optic nerve) and two peripheral (3.5 mm from the optic nerve) areas (Figure S1). All image analyses were performed for the four areas. Images were analysed using ImageJ software (ImageJ, RRID:SCR_003070). Multi‐colour images are split into single channels and which are converted to grayscale before processing. We measured the fluorescent intensity above the threshold using the “set measurements” tool.
2.7. TUNEL
To evaluate apoptotic cells, a TUNEL assay was conducted in accordance with the manufacturer's instructions (In Situ Cell Detection Kit, Roche). After washing, cryostat sections were incubated in TUNEL reaction mix, rinsed, and mounted with VECTASHIELD mounting medium with DAPI. For double‐labelled staining, the sections were incubated with mouse anti‐NeuN antibody (NeuN, Millipore, 1:200, RRID:AB_2298772), followed by incubation with Alexa Fluor 488‐labelled goat anti‐mouse IgG (Thermo Fisher Scientific Cat# A‐11001, RRID:AB_2534069) for 1 hr at room temperature. The slides were imaged using a confocal laser scanning microscope. The number of TUNEL‐positive cells was divided by DAPI‐positive cells in retinal ganglion cell layer (GCL) and then multiplied by 100 to calculate the percentage of TUNEL‐positive cells.
2.8. Western blot analysis
Extraction of retinal proteins and an immunoblot assay were performed as previously described (Jung, Kim, Park, & Park, 2013). Retinal tissues were homogenized in RIPA buffer, and the total protein level was quantified using a standard bicinchoninic acid assay (Pierce). Sample buffer was added to retinal samples containing 30 μg of total protein per sample. The protein mixture was separated by SDS‐PAGE and immobilized onto a nitrocellulose membrane. The blots were washed and blocked with 5% skim milk‐1× Tris‐buffered saline/Tween 20 buffer for 1 hr at room temperature. Then, the membranes were incubated with antibodies against GFAP (Millipore, 1:1,000, RRID:AB_11212597), ICAM‐1 (Santa Cruz, 1:500, RRID:AB_627120) or inducible NOS (iNOS; Abcam, 1:250, RRID:AB_301857), and actin (Santa Cruz, 1:200, RRID:AB_2714189) overnight at 4°C. The membranes were incubated with an HRP‐conjugated goat anti‐rabbit IgG (GenDEPOT Cat# SA002‐500, USA) or goat anti‐mouse IgG (GenDEPOT Cat# SA001‐500, USA) as the secondary antibody for 1 hr. Immunoreactive proteins were detected by ECL Western Blotting Substrate (Thermo Scientific, Waltham, MA, USA), and then protein bands were observed by an Image Analyzer System (Syngene, UK). Quantification was performed using ImageJ software (ImageJ, RRID:SCR_003070).
2.9. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). The group size shown (n) is the number of independent values used for statistical analysis. Experiments were designed to generate groups of equal size without performing a formal power analysis. Group size selection was based on our previous experience or studies using similar experimental models. Data analysis was carried out without knowledge of which group the samples came from.Statistical analysis was performed only for data from experimental groups where each group size was at least n = 5. For immunofluorescence staining, seven retinal sections per animal were used in the analysis. For western blot analysis, one whole retina per animal was analysed. To observe antegrade axoplasmic flow, one optic nerve head (ONH) per animal was analysed. No outliers were excluded from the data. All data are indicated as the mean ± SD. Statistical analyses were carried out using SPSS software (SPSS, RRID:SCR_002865). Multiple comparisons among the groups were made using Kruskal–Wallis and post hoc Dunn's test (one‐tailed). Values of P < .05 were considered statistically significant.
2.1. Materials
The latanoprost ophthalmic solution (Xalatan®) was supplied by Pfizer, (New York, NY, USA) and the brinzolamide ophthalmic suspension (Azopt®) was fromAlcon Laboratories, Inc., (Ft. Worth, TX, USA)
2.2. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Fabbro et al., 2019).
3. RESULTS
3.1. Body weight and blood glucose
Body weight was significantly lower in the diabetes group, with or without drug treatment, than in the normal control group from 3 weeks to 8 weeks after STZ injection (Figure S2). Blood glucose was higher in the diabetes group than in the normal control group from 1 week to 8 weeks after STZ injection.
3.2. IOP fluctuation
The mean IOP in the diabetes group was higher than in the normal control group, the latanoprost or brinzolamide, or the combined treatment group (Figure 1). The SD of IOP was higher in the diabetes group than in the normal control. The combined treatment with brinzolamide and latanoprost lowered the IOP fluctuation increased by diabetes.
FIGURE 1.
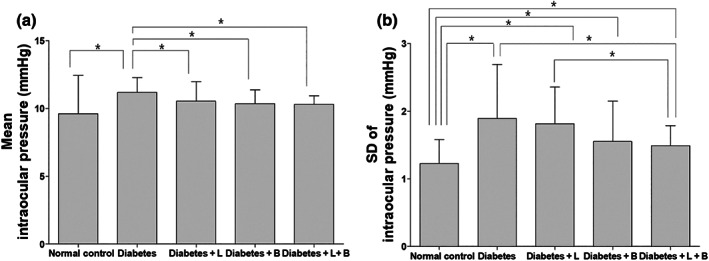
The average value (a) of and variation (b) in intraocular pressure (IOP). The average IOP in the diabetes group was higher than in the normal control group, the latanoprost (L) or brinzolamide (B), or the combined treatment (L + B) group. The SD of mean IOP was higher in the diabetes group than in the normal control. The combined treatment lowered the increased IOP fluctuation induced by diabetes. *P < .05, significantly different as indicated; Kruskal–Wallis and post hoc Dunn's test (one‐tailed)
Serial IOP data are presented in Figure S3. Baseline IOP did not show any significant difference among groups (n = 10 per group). At 3, 5, 7, and 8 weeks after STZ injection, the diabetes group showed higher IOP than did the normal control group. The combined treatment group (latanoprost and brinzolamide) or the brinzolamide group displayed lower IOP than that in the diabetes group treated with saline at 3, 5, and 7 weeks after STZ injection. The combined treatment group showed lower IOP than did the diabetes group treated with saline also at 8 weeks after STZ injection.
3.3. Apoptotic death of RGCs
A representative image of each group is shown in Figure 2. TUNEL‐positive cells were observed in the GCL at 8 weeks after the induction of diabetes (Figure 2). Most of the TUNEL‐positive cells were colocalized with an anti‐NeuN antibody, which labels RGCs in the diabetic retina (Figure 2b). The percentage of TUNEL‐positive cells was higher in the diabetes group than in the normal control group (n = 10 per group). The combined treatment with both brinzolamide and latanoprost decreased the proportion of apoptotic cells in the diabetic retina (n = 10 per group).
FIGURE 2.
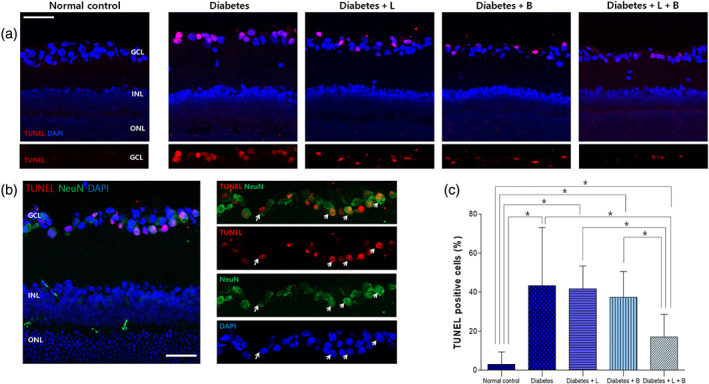
Apoptosis in the retina after the induction of diabetes at 8 weeks after streptozotocin injection. (a) TUNEL‐positive cells were observed in the ganglion cell layer (GCL) at 8 weeks after the induction of diabetes. (b) Most TUNEL‐positive cells were colocalized with the anti‐NeuN antibody, which labels retinal ganglion cells (RGCs) in the diabetic retina. (c) The proportion of apoptotic cells was greater in diabetic rats then in normal control rats. The combined treatment with latanoprost and brinzolamide reduced the proportion of TUNEL‐positive cells in the diabetic retina (n = 10 per group). *P < .05, significantly different as indicated; Kruskal–Wallis and post hoc Dunn's test (one‐tailed)
3.4. VEGF and blood vessels
In normal control rats, immunofluorescence staining with the anti‐VEGF antibody was scarcely found in the GCL (Figure 3). VEGF expression increased in the GCL mostly and in the inner nuclear layer (INL) at 8 weeks after the induction of diabetes (n = 10 per group). Brinzolamide or the combined treatment attenuated this elevated VEGF expression.
FIGURE 3.
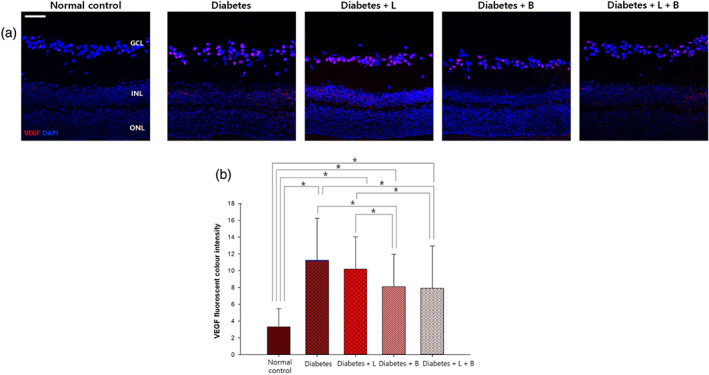
VEGF expression in the retina at 8 weeks after streptozotocin injection. (a) After the injection of streptozotocin, VEGF expression increased mostly in the ganglion cell layer (GCL) and the inner nuclear layer (INL). (b) VEGF immunostaining decreased by brinzolamide or the combined treatment with latanoprost or brinzolamide (n = 10 per group). *P < .05, significantly different as indicated; Kruskal–Wallis and post hoc Dunn's test (one‐tailed)
We performed immunofluorescence staining with an anti‐NG2 antibody to evaluate the anatomical changes in pericytes and an anti‐GSL‐IB4 antibody to measure the microvessel density in the retina at 8 weeks after the induction of diabetes. There was no significant difference in the degree of anti‐NG2 antibody or with anti‐GSL‐IB4 antibody among the groups (n = 10 per group, Figure S4).
3.5. Glial activation in the retina and ONH
In vertical sections of the control retina, GFAP immunofluorescence staining was limited to astrocytes and the end feet of Müller cells at the inner limiting membrane (ILM; Figure 4). At 8 weeks after the induction of diabetes, GFAP expression extended to the outer plexiform layer (n = 10 per group). Among the diabetic rats with drug treatment, brinzolamide or the combined treatment significantly decreased GFAP immunofluorescence staining. Western blot analysis showed that the elevated GFAP expression in the diabetes group was attenuated in the combined treatment group
FIGURE 4.
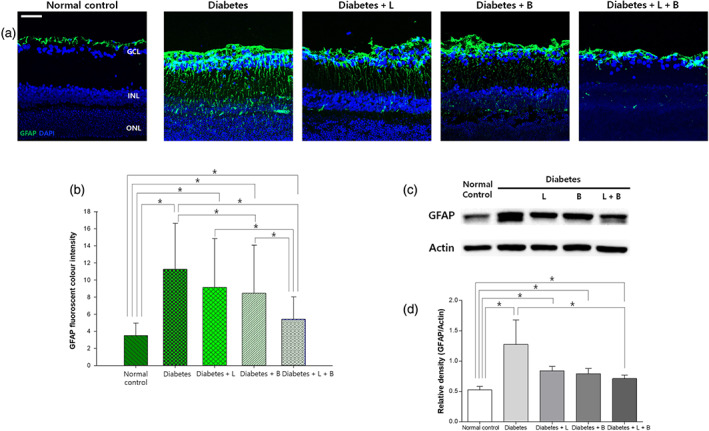
Macroglial activation in the diabetic retina at 8 weeks after STZ (streptozotocin) injection. (a) Glial fibrillary acidic protein (GFAP) immunoreactivity spanned the entire retina vertically to the outer plexiform layer in the diabetes group, and this effect was attenuated by the combined treatment. (b) Increased GFAP immunostaining was reduced by the brinzolamide or the combined treatment (n = 10 per group). (c) In western blot analysis, elevated GFAP expression in the diabetes group decreased in the combined treatment group (n = 10 per group). *P < .05, significantly different as indicated; Kruskal–Wallis and post hoc Dunn's test (one‐tailed)
Immunostaining of Iba‐1, a marker for activated microglia, was weak in the GCL in the control group (Figure 5). At 8 weeks after the induction of diabetes, microglial cells stained with Iba‐1 showed an amoeboid shape with some branches, and Iba‐1 expression was increased in the GCL and extended to the INL (n = 10 per group). The combined treatment with latanoprost and brinzolamide or treatment with brinzolamide alone lowered the expression of Iba‐1 in the diabetic retina.
FIGURE 5.
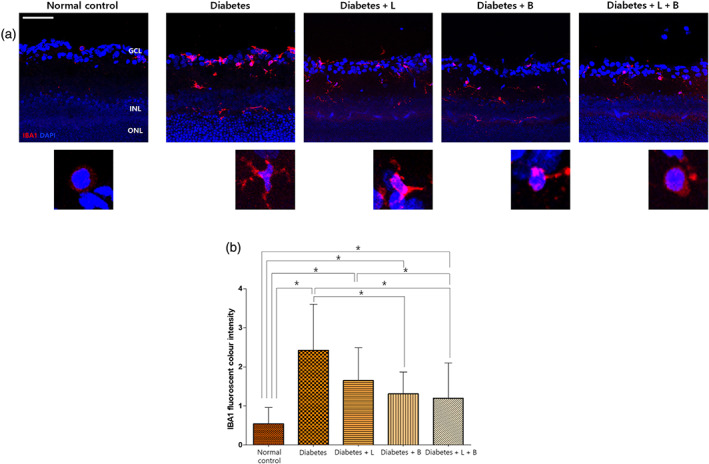
Microglial expression in the retina at 8 weeks after streptozotocin injection. (a) Ionized calcium binding adaptor molecule (Iba‐1) immunostaining showed an amoeboid shape with some branches, and Iba‐1 expression increased in the ganglion cell layer and extended to the inner nuclear layer (INL) after the injection of streptozotocin. (b) Brinzolamide or combined treatment lowered the expression of Iba‐1 increased by diabetes (n = 10 per group). *P < .05, significantly different as indicated; Kruskal–Wallis and post hoc Dunn's test (one‐tailed)
In the ONH, the immunofluorescent staining for GFAP was weak and scant in the normal control group (n = 10 per group, Figure 6). The diabetic rats expressed intense GFAP labelling, indicating gliosis of astrocytes in the ONH at 8 weeks after the induction of diabetes. Treatment with brinzolamide or the combined treatment decreased GFAP immunostaining.
FIGURE 6.
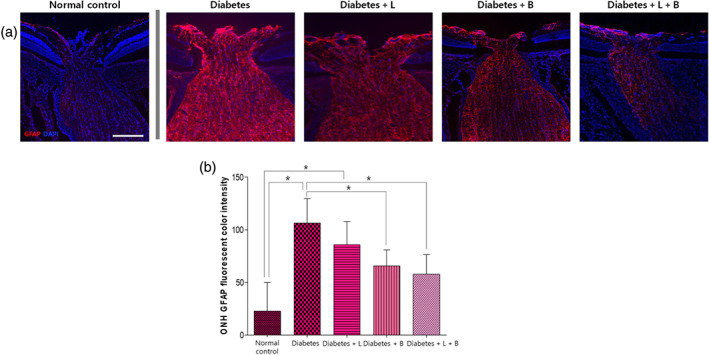
Macroglial immunostaining in the optic nerve head (ONH) at 8 weeks after streptozotocin injection. (a) The diabetes group showed increased glial fibrillary acidic protein (GFAP) expression and the gliosis of astrocytes compared to that in the normal control group. (b) In diabetic rats, brinzolamide or the combined treatment reduced GFAP immunostaining in the ONH (n = 10 per group). *P < .05, significantly different as indicated; Kruskal–Wallis and post hoc Dunn's test (one‐tailed)
3.6. Neuroinflammation
To evaluate the neuroinflammation, we examined the expression of ICAM‐1 and iNOS in the retina. In the normal control group, weak and sparse ICAM‐1 immunostaining was found in the GCL and INL (Figure 7). In the diabetes group, the expression of ICAM‐1 increased mostly in the INL at 8 weeks after the induction of diabetes. The up‐regulated expression of ICAM‐1 was attenuated by the combined treatment (n = 10 per group). Western blot analysis also showed similar results. iNOS expression analysed by western blotting was up‐regulated in the diabetes group compared to that in the control group at 8 weeks after the induction of diabetes, and this expression was attenuated by the brinzolamide or the combined treatment.
FIGURE 7.
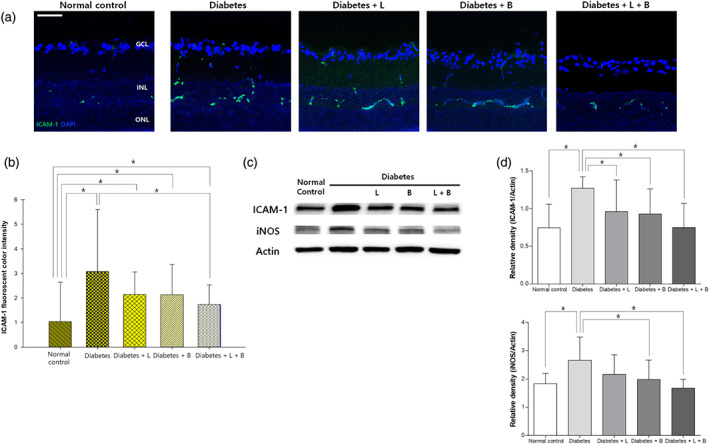
Expression of intercellular adhesion molecule 1 (ICAM‐1) in the retina at 8 weeks after STZ (streptozotocin) injection. (a) In the diabetes group, the expression of ICAM‐1 increased mostly in the inner nuclear layer. (b) Increased immunofluorescence staining of ICAM‐1 by diabetes was reduced by the combined treatment (n = 10 per group). (c) ICAM‐1 and inducible NOS (iNOS) expression analysed by western blot were up‐regulated in the diabetes group compared to that in the control group, and this effect was attenuated by brinzolamide alone or the combined treatment (n = 10 per group). *P < .05, significantly different as indicated; Kruskal–Wallis and post hoc Dunn's test (one‐tailed)
3.7. Antegrade axoplasmic flow in the ONH
Active antegrade axoplasmic transport of RGC through the ONH was visualized in the normal control group (Figure 8). In the diabetes group, some antegrade axoplasmic flow was blocked in the lamina region at 8 weeks after the induction of diabetes, and overall expression of the injected cholera toxin subunit B was lower in the diabetes group than in the control group (n = 5 per group). The loss of antegrade axoplasmic transport was partly reversed with the combined latanoprost and brinzolamide treatment.
FIGURE 8.
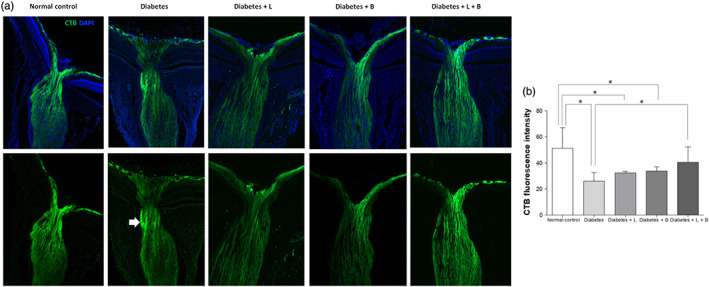
Antegrade axoplasmic flow in the optic nerve head at 8 weeks after streptozotocin injection. In the diabetes group, some antegrade axoplasmic flow was blocked in the lamina region (arrow) compared to that in the normal control group. The overall expression of cholera toxin subunit B was lower in the diabetes group than the control group (n = 5 per group). The blockade of antegrade axoplasmic transport was partly alleviated with combined latanoprost and brinzolamide treatment (n = 5 per group). *P < .05, significantly different as indicated; Kruskal–Wallis and post hoc Dunn's test (one‐tailed)
4. DISCUSSION
In diabetic rats, increased IOP and higher fluctuations in IOP readings were observed. A combination of drugs known to lower IOP, latanoprost and brinzolamide, reduced IOP fluctuation. Degeneration of RGCs was found at 8 weeks after the induction of diabetes, and the combined treatment decreased this effect. There is a possibility that the reduction of IOP fluctuation by IOP‐lowering drugs might decrease intermittent mechanical stress, glial activation, axoplasmic flow, and eventually neurodegeneration, although further studies are needed to elucidate a direct causal relationship between IOP changes and neuroprotection.
Clinically, diabetic patients often develop defects in the RNFL (Choi et al., 2015; van Dijk et al., 2010). Recent evidence suggests that the diabetic neurodegeneration of RGCs precedes microvasculopathy and is progressive over time (El‐Fayoumi, Badr Eldine, Esmael, Ghalwash, & Soliman, 2016; Jung, Kim, Park, & Park, 2013; Park, Kim, & Park, 2014; Sohn et al., 2016). Progressive retinal neurodegeneration was independent of HbA1C and the progression of diabetic retinopathy (Sohn et al., 2016). There have been no guidelines to manage diabetic patients with RNFL loss because the precise mechanism of diabetic retinal neuropathy is not fully understood.
Diabetic rats demonstrated greater fluctuation and average IOP than in the normal control rats. There is a mildly elevated IOP in patients with diabetes, approximately 2–3 mmHg higher than in those without diabetes, although this is not a universal finding (Chan et al., 2016; Kawase et al., 2008; Wong, Bui, & Vingrys, 2011). To our knowledge, there has been few studies regarding the variability of IOP in diabetes. The mechanism in terms of the diabetes‐induced elevation in IOP is not clear. Autonomic dysfunction or hyperglycaemia disturbing the performance of the trabecular meshwork might lead to increase or variability of IOP (Sato & Roy, 2002; Zhao, Cho, Kim, Friedman, & Guallar, 2015). Diabetic patients showed a higher postural change in IOP than those in normal control subjects (Singh & Heong, 1986; Williams, Peart, & Letley, 1980). Unusual alterations in IOP might result from the inadequate control of local adrenoceptors important for maintaining the homeostasis of IOP (Singh & Heong, 1986). Our group previously found that autonomic dysfunction was also a risk factor for RNFL loss in diabetic patients (Jeon, Park, Lee, & Park, 2018).
The increased fluctuation in IOP might be involved in the pathophysiology of diabetic retinal neurodegeneration because the retinal neurodegeneration was attenuated when the IOP fluctuations were reduced with IOP‐lowering drugs. From the first‐line IOP‐lowering medications for glaucoma, we employed a PG analogue (latanoprost) and a carbonic anhydrase inhibitor (brinzolamide) as each IOP‐lowering treatment arm in this study, because topical β‐adrenoceptor antagonists and α2‐ adrenoceptor agonists also reduce BP, thereby affecting perfusion pressure (Quaranta et al., 2006). To boost the effects of IOP‐lowering treatments in a step‐wise manner, we added the combined treatment arm with the use of both latanoprost and brinzolamide. Each drug treatment slightly lowered IOP, but the effects were more marked in the combined treatment group. The combined IOP‐lowering treatment (latanoprost or brinzolamide) reduced the variability of IOP and the mean IOP after the induction of diabetes. Latanoprost or brinzolamide alone decreases IOP mostly in mice or rats, even though the effects can vary depending on the method of IOP measurements or the use of an ocular hypertension model that mimics glaucoma (Kanamori, Naka, Fukuda, Nakamura, & Negi, 2009; Seki et al., 2005; Yang et al., 2012). Diabetic rats showed a slightly elevated IOP, but all IOPs were under 20 mmHg, which is definitely lower than the IOPs in the chronic ocular hypertension model. The degree of IOP reduction by IOP‐lowering treatment is lower when the IOP is within the normal range than when the IOP is abnormally high. General anaesthesia using isoflurane, as used in this study, could also lower the measured IOP (Jia, Cepurna, Johnson, & Morrison, 2000). Therefore, it seemed that the IOP‐lowering effects of each treatment were too small to make a significant difference in our diabetic rat model. Only the combined treatment decreased IOP fluctuation, which was up‐regulated in diabetic rats.
At 8 weeks after diabetes induction, the rats showed no anatomical alterations in pericytes, which are the earliest detectable microvascular markers, or in the capillaries, corresponding to the study by Sohn et al. (2016). However, VEGF expression was increased in the diabetic group compared to that in the control group, which also coincides with previous studies (Jung, Kim, Park, & Park, 2013; Lv et al., 2018). Hypoxia is one of main inducers of VEGF gene transcription (Ferrara, 2004). Inflammation, advanced glycation end products, oxidative stress, or hyperglycaemia itself also could be the factors accounting for the high levels of VEGF in diabetic retina, especially in the setting of diabetic macular oedema (Das, 2016; Simo et al., 2014). The up‐regulated expression of VEGF might indicate a decreased ability to maintain blood flow homeostasis, relatively mild hypoxic status, or low‐grade inflammation in the diabetic retina (Romero‐Aroca et al., 2016). Our group previously reported that vascular endothelial dysfunction could have relevance to RNFL defects in diabetic patients (Choi et al., 2015). It may be possible that the dysfunction of pericytes or the endothelium is related to elevated VEGF levels, even though there is no definite loss of pericytes in the retina. Further studies are needed to elucidate this hypothesis.
Greater IOP fluctuation induced by diabetes might be associated with intermittent mechanical stress to the ONH or retina, even though physiological IOP fluctuation occurs normally (Tojo, Abe, Ishida, Yagou, & Hayashi, 2017). Damage to retinal neurons by abnormally high IOP fluctuations may be worsened if normal compensatory mechanisms are compromised (Caprioli & Coleman, 2008). A growing body of evidence shows that eyes, in the presence of diabetes, display a decreased potential to autoregulate blood flow when confronted by lowered or unstable ocular perfusion pressure (the balance between BP and IOP; Wong, Bui, & Vingrys, 2011). In this study, VEGF was up‐regulated in the diabetic retina and this finding could be evidence of unstable blood flow. A higher fluctuation in IOP simultaneously with unstable blood flow homeostasis could result in unstable ocular perfusion and thereby chronic low‐grade ischaemia and reperfusion damage in the diabetic retina (Flammer et al., 2002; Joos, Li, & Sappington, 2010; Wong, Bui, & Vingrys, 2011).
Macroglial and microglial activation was found in the retina or ONH in our diabetes model. In this study, Iba‐1 was used as a marker of activated microglia because it is microglia‐/macrophage‐specific calcium binding protein with the actin‐bundling activity (Ohsawa, Imai, Sasaki, & Kohsaka, 2004). Iba‐1 is up‐regulated and participates in phagocytosis in activated microglia. (Ohsawa, Imai, Sasaki, & Kohsaka, 2004) The effects of intermittent IOP elevation on glial activation has been reported previously (Joos, Li, & Sappington, 2010). Using an ischaemic/reperfusion injury model, our group also previously found a loss of neuronal cells and gliosis in the retina and ONH (Cho, Kim, Park, & Park, 2011). Reactive gliosis early after retinal injury can be cytoprotective, but the prolonged activation of glial cells can be detrimental to neurons (Bringmann et al., 2009). Triggered by prolonged proinflammatory stimulus, chronically and excessively stimulated microglia might contribute to neuroinflammation by increasing inflammatory factors (Block, Zecca, & Hong, 2007; Cuenca et al., 2014).
The expression of ICAM‐1, which assists the adhesion of macrophages to retinal capillary walls to sustain chronic inflammation, was elevated in our diabetic model (Grigsby et al., 2014). The increased expression of iNOS was found in this study. In a diabetes model, Zheng et al. (2007) reported that elevated iNOS might play a role in the inflammation‐like process of diabetic retinopathy. The combined IOP‐lowering treatment reduced glial activation and the expression of inflammatory‐related proteins, which were elevated in diabetic rats. Unstable IOP fluctuation and possibly insecure perfusion pressure seemed to have relevance to gliosis and neuroinflammation in the diabetic retina.
Disturbed antegrade axoplasmic transport concurrently with gliosis was found in the ONH after the induction of diabetes, corresponding to the study of Fernandez, Pasquini, Dorfman, Aldana Marcos, and Rosenstein (2012). The systematically arranged and mechanosensitive ONH astrocytes might primarily sense and react to mechanical strain from IOP fluctuations (Tehrani et al., 2016). Repeated IOP elevations might render astrocytes more reactive to further IOP fluctuations or elevations considering the astrocytic structural and molecular changes (Tehrani et al., 2016). Axoplasmic transport disturbance induced by elevated IOP fluctuations might also contribute to neurodegeneration in the diabetic retina because the combined treatment which reduced IOP variability, also decreased ONH gliosis and restored the disturbed antegrade axoplasmic transport.
In this study, we could not completely exclude non‐IOP‐dependent effects of latanoprost or brinzolamide on the attenuation of diabetic retinal neurodegeneration. Neurodegeneration models such as optic nerve crush or glutamate excitotoxicity, or in vitro study reported the potential neuroprotective effects of latanoprost independently of their effects on IOP (Drago et al., 2001; Kanamori, Naka, Fukuda, Nakamura, & Negi, 2009; Nakanishi et al., 2006; Yamagishi, Aihara, & Araie, 2011; Zheng et al., 2011). The effects of PG analogues or brinzolamide on intraocular haemodynamics have been also suggested as a secondary neuroprotective mechanism (Boltz et al., 2011; Kurashima et al., 2010; Weiwei & Hu, 2009). However, the IOP‐independent effects of IOP‐lowering agents cannot be eliminated totally from studies in vivo. A surgical approach which makes fistula could be considered to decrease IOP, but postoperative complications such as hypotony, inflammation, or hyphema can occur. With regard to the effects of latanoprost on retinal neuro‐glial cells in diabetic rats, Nakanishi et al. (2006) reported that latanoprost reduced apoptosis by inhibiting caspase‐3. However, IOP was not measured in diabetic rats, and latanoprost was used for only 5 days. Recently, the EUROCONDOR clinical trial reported that topical administration of brimonidine, one of the IOP‐lowering drugs, and somatostatin might play a role in preventing the progression of pre‐existing retinal neurodysfunction in the subset of diabetic patients with some degree of retinal neurodegeneration, even though it did not arrest retinal microvasculopathy (Simo et al., 2019).
IOP fluctuation can be analysed by different measurements, such as peak, range, and SD (Caprioli & Coleman, 2008). We chose SD because it considers the total number of measurement values and may be less influenced by outliers (Caprioli & Coleman, 2008). Although serial IOPs at each week for 8 weeks were compared between the groups without correction for multiple comparisons for each time point to minimize the risk of Type II errors and presented as supporting information, it is one of limitations of this study. Larger animal studies or clinical human studies are needed to confirm the effects of the IOP‐lowering drugs on serial IOPs after induction of diabetes. The lack of observation on anterograde axoplasmic transport in the superior colliculus could be one of limitations in this study. In addition, we analysed the microglia morphology in the vertical sections of retina, not in the retinal whole mount preparations. That could be one of the limitations in our study.
Initially, diabetic neuropathy might considerably affect the inner retina, especially the RGCs, as selective RGC loss occurs in glaucoma (Wong, Bui, & Vingrys, 2011). Elevated IOP fluctuations in the diabetic retina seemed to induce repetitive low‐grade ischaemic/reperfusion injury through unstable perfusion pressure and intermittent mechanical stress in both the retina and ONH (Figure S5). This effect was followed by glial activation, neuroinflammation, axoplasmic transport disturbance, and neurodegeneration. This study did not reveal whether a reduction of IOP fluctuation could delay the progression of diabetic microvascular retinopathy. In diabetic patients, additional oral medication may be a burden as these drugs are frequently prescribed to reduce the complications of diabetes. Topical treatment may minimize the systemic effects and lessen safety issues because topical brinzolamide or latanoprost has been widely used for the treatment of glaucoma since they were approved by the FDA in the late 1990s.
In conclusion, the IOP fluctuation appeared to be associated with the retinal neurodegeneration induced by diabetes, even though the direct causality between them needs further investigation. The IOP‐lowering topical treatment to reduce IOP fluctuation might be considered as another therapeutic strategy for neuroprotection in diabetes. Further human clinical studies are needed to confirm our findings.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers, and other organisations engaged with supporting research.
AUTHOR CONTRIBUTIONS
K.I.J. designed the study. K.I.J. and J.E.W. conducted the study. K.I.J. and J.E.W. collected the data. K.I.J., C.K.P., and J.E.W. analysed and interpreted the data. K.I.J. did the statistical expertise. K.I.J. wrote the article. C.K.P. did the critical revision of the article.
Supporting information
Figure S1. Representative posterior eyecup image stained with DAPI for analysis. Each eyecup was divided into 2 mid central (1.5 mm from the optic nerve), 2 peripheral areas (3.5 mm from the optic nerve) for image analysis
Figure S2 Body weight and blood glucose level in the control and diabetic rats. Body weight was lighter in the streptozotocin injected group than the normal control group (P < 0.001) at 3 weeks after streptozotocin injection. Serum glucose was elevated in the diabetes group compared with that in the normal control group, except at the baseline, at 8 weeks after streptozotocin injection (all P < 0.001) (n = 10 per group). * P < 0.05 by using Kruskal‐Wallis and post hoc Dunn's test (one‐tailed)
Figure S3 Serial change in intraocular pressure (IOP). The diabetes group (n = 10) showed higher IOP than did the normal control group (n = 10) at 3, 5, 7, and 8 weeks after Streptozotocin (STZ) injection. The combined treatment group (latanoprost and brinzolamide) (n = 10) or the brinzolamide group (n = 10) displayed lower IOP than did the diabetes group treated with saline at 3, 5, and 7 weeks after STZ injection. The combined treatment group showed lower IOP than did the diabetes group treated with saline also at 8 weeks after STZ injection. * P < 0.05 by using Kruskal‐Wallis and post hoc Dunn's test (one‐tailed)
Figure S3 Serial change in intraocular pressure (IOP). The diabetes group (n = 10) showed higher IOP than did the normal control group (n = 10) at 3, 5, 7, and 8 weeks after Streptozotocin (STZ) injection. The combined treatment group (latanoprost and brinzolamide) (n = 10) or the brinzolamide group (n = 10) displayed lower IOP than did the diabetes group treated with saline at 3, 5, and 7 weeks after STZ injection. The combined treatment group showed lower IOP than did the diabetes group treated with saline also at 8 weeks after STZ injection. * P < 0.05 by using Kruskal‐Wallis and post hoc Dunn's test (one‐tailed)
Figure S4 Immunofluorescence staining for pericyte and microvessel in retina. A. immunofluorescence staining of anti‐NG2 antibody to measure pericyte and anti‐GSL‐IB4 antibody to evaluate microvessel was observed in the ganglion cell layer, inner plexiform layer, and inner nuclear layer. B. Expression of anti‐NG2 antibody merged well with anti‐GSL‐IB4 antibody. C. There was no significant difference in the degree of anti‐NG2 antibody or with anti‐GSL‐IB4 antibody staining among the groups (P = 0.625 for anti‐NG2 antibody, P = 0.514 for anti‐GSL‐IB4 antibody) (n = 10 per group). * P < 0.05 by using Kruskal‐Wallis and post hoc Dunn's test (one‐tailed)
Figure S5 Hypothetical overview of the relationship between fluctuation of intraocular pressure and neurodegeneration in diabetic retina
IOP, intraocular pressure
ACKNOWLEDGEMENTS
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant HI15C1940). The authors declare that no competing interests exist with the funder. The funder had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Jung KI, Woo JE, Park CK. Intraocular pressure fluctuation and neurodegeneration in the diabetic rat retina. Br J Pharmacol. 2020;177:3046–3059. 10.1111/bph.15033
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … Collaborators, C. G. T. P. (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … Collaborators, C. G. T. P. (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonetti, D. A. , Klein, R. , & Gardner, T. W. (2012). Diabetic retinopathy. The New England Journal of Medicine, 366, 1227–1239. 10.1056/NEJMra1005073 [DOI] [PubMed] [Google Scholar]
- Barber, A. J. , Lieth, E. , Khin, S. A. , Antonetti, D. A. , Buchanan, A. G. , & Gardner, T. W. (1998). Neural apoptosis in the retina during experimental and human diabetes. Early Onset and Effect of Insulin. The Journal of Clinical Investigation, 102, 783–791. 10.1172/JCI2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block, M. L. , Zecca, L. , & Hong, J. S. (2007). Microglia‐mediated neurotoxicity: Uncovering the molecular mechanisms. Nature Reviews. Neuroscience, 8, 57–69. 10.1038/nrn2038 [DOI] [PubMed] [Google Scholar]
- Boltz, A. , Schmidl, D. , Weigert, G. , Lasta, M. , Pemp, B. , Resch, H. , … Schmetterer, L. (2011). Effect of latanoprost on choroidal blood flow regulation in healthy subjects. Investigative Ophthalmology & Visual Science, 52, 4410–4415. 10.1167/iovs.11-7263 [DOI] [PubMed] [Google Scholar]
- Bringmann, A. , Iandiev, I. , Pannicke, T. , Wurm, A. , Hollborn, M. , Wiedemann, P. , … Reichenbach, A. (2009). Cellular signaling and factors involved in Muller cell gliosis: Neuroprotective and detrimental effects. Progress in Retinal and Eye Research, 28, 423–451. 10.1016/j.preteyeres.2009.07.001 [DOI] [PubMed] [Google Scholar]
- Caprioli, J. , & Coleman, A. L. (2008). Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology, 115, 1123, e1123–1129. [DOI] [PubMed] [Google Scholar]
- Chan, M. P. , Grossi, C. M. , Khawaja, A. P. , Yip, J. L. , Khaw, K. T. , Patel, P. J. , … UK Biobank Eye and Vision Consortium . (2016). Associations with intraocular pressure in a large cohort: Results from the UK biobank. Ophthalmology, 123, 771–782. 10.1016/j.ophtha.2015.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, N. , Mitchell, P. , & Wong, T. Y. (2010). Diabetic retinopathy. Lancet, 376, 124–136. 10.1016/S0140-6736(09)62124-3 [DOI] [PubMed] [Google Scholar]
- Cho, K. J. , Kim, J. H. , Park, H. Y. , & Park, C. K. (2011). Glial cell response and iNOS expression in the optic nerve head and retina of the rat following acute high IOP ischemia‐reperfusion. Brain Research, 1403, 67–77. 10.1016/j.brainres.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Choi, J. A. , Ko, S. H. , Park, Y. R. , Jee, D. H. , Ko, S. H. , & Park, C. K. (2015). Retinal nerve fiber layer loss is associated with urinary albumin excretion in patients with type 2 diabetes. Ophthalmology, 122, 976–981. 10.1016/j.ophtha.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Coleman, A. L. , & Miglior, S. (2008). Risk factors for glaucoma onset and progression. Survey of Ophthalmology, 53(Suppl1), S3–S10. 10.1016/j.survophthal.2008.08.006 [DOI] [PubMed] [Google Scholar]
- Cuenca, N. , Fernandez‐Sanchez, L. , Campello, L. , Maneu, V. , De la Villa, P. , Lax, P. , & Pinilla, I. (2014). Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Progress in Retinal and Eye Research, 43, 17–75. 10.1016/j.preteyeres.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, A. (2016). Diabetic retinopathy: Battling the global epidemic. Investigative Ophthalmology & Visual Science, 57, 6669–6682. 10.1167/iovs.16-21031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk, H. W. , Verbraak, F. D. , Kok, P. H. , Garvin, M. K. , Sonka, M. , Lee, K. , … Abràmoff, M. D. (2010). Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Investigative Ophthalmology & Visual Science, 51, 3660–3665. 10.1167/iovs.09-5041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago, F. , Valzelli, S. , Emmi, I. , Marino, A. , Scalia, C. C. , & Marino, V. (2001). Latanoprost exerts neuroprotective activity in vitro and in vivo. Experimental Eye Research, 72, 479–486. 10.1006/exer.2000.0975 [DOI] [PubMed] [Google Scholar]
- El‐Fayoumi, D. , Badr Eldine, N. M. , Esmael, A. F. , Ghalwash, D. , & Soliman, H. M. (2016). Retinal nerve fiber layer and ganglion cell complex thicknesses are reduced in children with type 1 diabetes with no evidence of vascular retinopathy. Investigative Ophthalmology & Visual Science, 57, 5355–5360. 10.1167/iovs.16-19988 [DOI] [PubMed] [Google Scholar]
- Ernest, P. J. , Schouten, J. S. , Beckers, H. J. , Hendrikse, F. , Prins, M. H. , & Webers, C. A. (2013). An evidence‐based review of prognostic factors for glaucomatous visual field progression. Ophthalmology, 120, 512–519. 10.1016/j.ophtha.2012.09.005 [DOI] [PubMed] [Google Scholar]
- Fernandez, D. C. , Pasquini, L. A. , Dorfman, D. , Aldana Marcos, H. J. , & Rosenstein, R. E. (2012). Early distal axonopathy of the visual pathway in experimental diabetes. The American Journal of Pathology, 180, 303–313. 10.1016/j.ajpath.2011.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara, N. (2004). Vascular endothelial growth factor: Basic science and clinical progress. Endocrine Reviews, 25, 581–611. 10.1210/er.2003-0027 [DOI] [PubMed] [Google Scholar]
- Flammer, J. , Orgul, S. , Costa, V. P. , Orzalesi, N. , Krieglstein, G. K. , Serra, L. M. , … Stefánsson, E. (2002). The impact of ocular blood flow in glaucoma. Progress in Retinal and Eye Research, 21, 359–393. 10.1016/s1350-9462(02)00008-3 [DOI] [PubMed] [Google Scholar]
- Georgakopoulos, C. D. , Eliopoulou, M. I. , Exarchou, A. M. , Tzimis, V. , Pharmakakis, N. M. , & Spiliotis, B. E. (2011). Decreased contrast sensitivity in children and adolescents with type 1 diabetes mellitus. Journal of Pediatric Ophthalmology and Strabismus, 48, 92–97. 10.3928/01913913-20100420-02 [DOI] [PubMed] [Google Scholar]
- Grigsby, J. G. , Cardona, S. M. , Pouw, C. E. , Muniz, A. , Mendiola, A. S. , Tsin, A. T. , … Cardona, A. E. (2014). The role of microglia in diabetic retinopathy. Journal of Ophthalmology, 2014, 1–15. 705783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, S. J. , Park, H. L. , Lee, J. H. , & Park, C. K. (2018). Relationship between systemic vascular characteristics and retinal nerve fiber layer loss in patients with type 2 diabetes. Scientific Reports, 8, 10510 10.1038/s41598-018-28985-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, L. , Cepurna, W. O. , Johnson, E. C. , & Morrison, J. C. (2000). Effect of general anesthetics on IOP in rats with experimental aqueous outflow obstruction. Investigative Ophthalmology & Visual Science, 41, 3415–3419. [PubMed] [Google Scholar]
- Joos, K. M. , Li, C. , & Sappington, R. M. (2010). Morphometric changes in the rat optic nerve following short‐term intermittent elevations in intraocular pressure. Investigative Ophthalmology & Visual Science, 51, 6431–6440. 10.1167/iovs.10-5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K. I. , Kim, J. H. , Park, H. Y. , & Park, C. K. (2013). Neuroprotective effects of cilostazol on retinal ganglion cell damage in diabetic rats. The Journal of Pharmacology and Experimental Therapeutics, 345, 457–463. 10.1124/jpet.113.203067 [DOI] [PubMed] [Google Scholar]
- Kanamori, A. , Naka, M. , Fukuda, M. , Nakamura, M. , & Negi, A. (2009). Latanoprost protects rat retinal ganglion cells from apoptosis in vitro and in vivo. Experimental Eye Research, 88, 535–541. 10.1016/j.exer.2008.11.012 [DOI] [PubMed] [Google Scholar]
- Kawase, K. , Tomidokoro, A. , Araie, M. , Iwase, A. , Yamamoto, T. , Tajimi Study G , & Japan Glaucoma Society . (2008). Ocular and systemic factors related to intraocular pressure in Japanese adults: The Tajimi study. The British Journal of Ophthalmology, 92, 1175–1179. 10.1136/bjo.2007.128819 [DOI] [PubMed] [Google Scholar]
- Kern, T. S. , & Barber, A. J. (2008). Retinal ganglion cells in diabetes. The Journal of Physiology, 586, 4401–4408. 10.1113/jphysiol.2008.156695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , Altman, D. G. , & Group NCRRGW . (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. H. , & Caprioli, J. (2018). Intraocular pressure fluctuation: Is it important? J. Ophthalmic vis. Res., 13, 170–174. 10.4103/jovr.jovr_35_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashima, H. , Watabe, H. , Sato, N. , Abe, S. , Ishida, N. , & Yoshitomi, T. (2010). Effects of prostaglandin F2α analogues on endothelin‐1‐induced impairment of rabbit ocular blood flow: Comparison among tafluprost, travoprost, and latanoprost. Experimental Eye Research, 91, 853–859. 10.1016/j.exer.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Lv, J. , Chen, M. M. , Mu, Z. H. , Wang, F. , Qian, Z. Y. , Zhou, L. , … Yang, Z. H. (2018). Intravitreal bevacizumab injection attenuates diabetic retinopathy in adult rats with experimentally induced diabetes in the early stage. Journal Diabetes Research, 2018, 1–18. 9216791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, J. C. , & Lilley, E. (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. British Journal of Pharmacology, 172, 3189–3193. 10.1111/bph.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi, Y. , Nakamura, M. , Mukuno, H. , Kanamori, A. , Seigel, G. M. , & Negi, A. (2006). Latanoprost rescues retinal neuro‐glial cells from apoptosis by inhibiting caspase‐3, which is mediated by p44/p42 mitogen‐activated protein kinase. Experimental Eye Research, 83, 1108–1117. 10.1016/j.exer.2006.05.018 [DOI] [PubMed] [Google Scholar]
- Ohsawa, K. , Imai, Y. , Sasaki, Y. , & Kohsaka, S. (2004). Microglia/macrophage‐specific protein Iba1 binds to fimbrin and enhances its actin‐bundling activity. Journal of Neurochemistry, 88, 844–856. 10.1046/j.1471-4159.2003.02213.x [DOI] [PubMed] [Google Scholar]
- Park, H. Y. , Kim, I. T. , & Park, C. K. (2011). Early diabetic changes in the nerve fibre layer at the macula detected by spectral domain optical coherence tomography. The British Journal of Ophthalmology, 95, 1223–1228. 10.1136/bjo.2010.191841 [DOI] [PubMed] [Google Scholar]
- Park, H.‐Y. L. , Kim, J. H. , & Park, C. K. (2014). Neuronal cell death in the inner retina and the influence of vascular endothelial growth factor inhibition in a diabetic rat model. The American Journal of Pathology, 184, 1752–1762. 10.1016/j.ajpath.2014.02.016 [DOI] [PubMed] [Google Scholar]
- Park, S. H. , Park, J. W. , Park, S. J. , Kim, K. Y. , Chung, J. W. , Chun, M. H. , & Oh, S. J. (2003). Apoptotic death of photoreceptors in the streptozotocin‐induced diabetic rat retina. Diabetologia, 46, 1260–1268. 10.1007/s00125-003-1177-6 [DOI] [PubMed] [Google Scholar]
- Prager, T. C. , Garcia, C. A. , Mincher, C. A. , Mishra, J. , & Chu, H. H. (1990). The pattern electroretinogram in diabetes. American Journal of Ophthalmology, 109, 279–284. 10.1016/s0002-9394(14)74550-7 [DOI] [PubMed] [Google Scholar]
- Quaranta, L. , Gandolfo, F. , Turano, R. , Rovida, F. , Pizzolante, T. , Musig, A. , & Gandolfo, E. (2006). Effects of topical hypotensive drugs on circadian IOP, blood pressure, and calculated diastolic ocular perfusion pressure in patients with glaucoma. Investigative Ophthalmology & Visual Science, 47, 2917–2923. 10.1167/iovs.05-1253 [DOI] [PubMed] [Google Scholar]
- Romero‐Aroca, P. , Baget‐Bernaldiz, M. , Pareja‐Rios, A. , Lopez‐Galvez, M. , Navarro‐Gil, R. , & Verges, R. (2016). Diabetic macular edema pathophysiology: Vasogenic versus inflammatory. Journal Diabetes Research, 2016, 1–17. 2156273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, T. , & Roy, S. (2002). Effect of high glucose on fibronectin expression and cell proliferation in trabecular meshwork cells. Investigative Ophthalmology & Visual Science, 43, 170–175. [PubMed] [Google Scholar]
- Seki, M. , Tanaka, T. , Matsuda, H. , Togano, T. , Hashimoto, K. , Ueda, J. , … Abe, H. (2005). Topically administered timolol and dorzolamide reduce intraocular pressure and protect retinal ganglion cells in a rat experimental glaucoma model. The British Journal of Ophthalmology, 89, 504–507. 10.1136/bjo.2004.052860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo, R. , Hernandez, C. , & European Consortium for the Early Treatment of Diabetic R . (2014). Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends in Endocrinology and Metabolism, 25, 23–33. [DOI] [PubMed] [Google Scholar]
- Simo, R. , Hernandez, C. , Porta, M. , Bandello, F. , Grauslund, J. , Harding, S. P. , … Gibson, J. (2019). Effects of topically administered neuroprotective drugs in early stages of diabetic retinopathy: Results of the EUROCONDOR clinical trial. Diabetes, 68, 457–463. 10.2337/db18-0682 [DOI] [PubMed] [Google Scholar]
- Simo, R. , Stitt, A. W. , & Gardner, T. W. (2018). Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia, 61, 1902–1912. 10.1007/s00125-018-4692-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, M. , & Heong, S. C. (1986). Postural behaviour of intraocular pressure in diabetics. The British Journal of Ophthalmology, 70, 456–459. 10.1136/bjo.70.6.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, E. H. , van Dijk, H. W. , Jiao, C. , Kok, P. H. , Jeong, W. , Demirkaya, N. , … Abràmoff, M. D. (2016). Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proceedings of the National Academy of Sciences of the United States of America, 113, E2655–E2664. 10.1073/pnas.1522014113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto, I. , Howell, G. R. , John, C. W. , Kief, J. L. , Libby, R. T. , & John, S. W. (2014). DBA/2J mice are susceptible to diabetic nephropathy and diabetic exacerbation of IOP elevation. PLoS ONE, 9, e107291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt, A. W. , Curtis, T. M. , Chen, M. , Medina, R. J. , McKay, G. J. , Jenkins, A. , … Lois, N. (2016). The progress in understanding and treatment of diabetic retinopathy. Progress in Retinal and Eye Research, 51, 156–186. [DOI] [PubMed] [Google Scholar]
- Tehrani, S. , Davis, L. , Cepurna, W. O. , Choe, T. E. , Lozano, D. C. , Monfared, A. , … Morrison, J. C. (2016). Astrocyte structural and molecular response to elevated intraocular pressure occurs rapidly and precedes axonal tubulin rearrangement within the optic nerve head in a rat model. PLoS ONE, 11, e0167364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo, N. , Abe, S. , Ishida, M. , Yagou, T. , & Hayashi, A. (2017). The fluctuation of intraocular pressure measured by a contact lens sensor in normal‐tension glaucoma patients and nonglaucoma subjects. Journal of Glaucoma, 26, 195–200. 10.1097/IJG.0000000000000517 [DOI] [PubMed] [Google Scholar]
- Verrotti, A. , Lobefalo, L. , Trotta, D. , Della Loggia, G. , Chiarelli, F. , Luigi, C. , … Gallenga, P. (2000). Visual evoked potentials in young persons with newly diagnosed diabetes: A long‐term follow‐up. Developmental Medicine and Child Neurology, 42, 240–244. 10.1017/s0012162200000414 [DOI] [PubMed] [Google Scholar]
- Weiwei, Z. , & Hu, R. (2009). Targeting carbonic anhydrase to treat diabetic retinopathy: Emerging evidences and encouraging results. Biochemical and Biophysical Research Communications, 390, 368–371. 10.1016/j.bbrc.2009.10.031 [DOI] [PubMed] [Google Scholar]
- Williams, B. I. , Peart, W. S. , & Letley, E. (1980). Abnormal intraocular pressure control in systemic hypertension and diabetic mellitus. The British Journal of Ophthalmology, 64, 845–851. 10.1136/bjo.64.11.845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, V. H. , Bui, B. V. , & Vingrys, A. J. (2011). Clinical and experimental links between diabetes and glaucoma. Clinical & Experimental Optometry, 94, 4–23. 10.1111/j.1444-0938.2010.00546.x [DOI] [PubMed] [Google Scholar]
- Yamagishi, R. , Aihara, M. , & Araie, M. (2011). Neuroprotective effects of prostaglandin analogues on retinal ganglion cell death independent of intraocular pressure reduction. Experimental Eye Research, 93, 265–270. 10.1016/j.exer.2011.06.022 [DOI] [PubMed] [Google Scholar]
- Yang, Q. , Cho, K. S. , Chen, H. , Yu, D. , Wang, W. H. , Luo, G. , … Chen, D. F. (2012). Microbead‐induced ocular hypertensive mouse model for screening and testing of aqueous production suppressants for glaucoma. Investigative Ophthalmology & Visual Science, 53, 3733–3741. 10.1167/iovs.12-9814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, D. , Cho, J. , Kim, M. H. , Friedman, D. S. , & Guallar, E. (2015). Diabetes, fasting glucose, and the risk of glaucoma: A meta‐analysis. Ophthalmology, 122, 72–78. 10.1016/j.ophtha.2014.07.051 [DOI] [PubMed] [Google Scholar]
- Zheng, J. , Feng, X. , Hou, L. , Cui, Y. , Zhu, L. , Ma, J. , … Chen, H. (2011). Latanoprost promotes neurite outgrowth in differentiated RGC‐5 cells via the PI3K‐Akt‐mTOR signaling pathway. Cellular and Molecular Neurobiology, 31, 597–604. 10.1007/s10571-011-9653-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, L. , Du, Y. , Miller, C. , Gubitosi‐Klug, R. A. , Kern, T. S. , Ball, S. , & Berkowitz, B. A. (2007). Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin‐induced diabetes. Diabetologia, 50, 1987–1996. 10.1007/s00125-007-0734-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Representative posterior eyecup image stained with DAPI for analysis. Each eyecup was divided into 2 mid central (1.5 mm from the optic nerve), 2 peripheral areas (3.5 mm from the optic nerve) for image analysis
Figure S2 Body weight and blood glucose level in the control and diabetic rats. Body weight was lighter in the streptozotocin injected group than the normal control group (P < 0.001) at 3 weeks after streptozotocin injection. Serum glucose was elevated in the diabetes group compared with that in the normal control group, except at the baseline, at 8 weeks after streptozotocin injection (all P < 0.001) (n = 10 per group). * P < 0.05 by using Kruskal‐Wallis and post hoc Dunn's test (one‐tailed)
Figure S3 Serial change in intraocular pressure (IOP). The diabetes group (n = 10) showed higher IOP than did the normal control group (n = 10) at 3, 5, 7, and 8 weeks after Streptozotocin (STZ) injection. The combined treatment group (latanoprost and brinzolamide) (n = 10) or the brinzolamide group (n = 10) displayed lower IOP than did the diabetes group treated with saline at 3, 5, and 7 weeks after STZ injection. The combined treatment group showed lower IOP than did the diabetes group treated with saline also at 8 weeks after STZ injection. * P < 0.05 by using Kruskal‐Wallis and post hoc Dunn's test (one‐tailed)
Figure S3 Serial change in intraocular pressure (IOP). The diabetes group (n = 10) showed higher IOP than did the normal control group (n = 10) at 3, 5, 7, and 8 weeks after Streptozotocin (STZ) injection. The combined treatment group (latanoprost and brinzolamide) (n = 10) or the brinzolamide group (n = 10) displayed lower IOP than did the diabetes group treated with saline at 3, 5, and 7 weeks after STZ injection. The combined treatment group showed lower IOP than did the diabetes group treated with saline also at 8 weeks after STZ injection. * P < 0.05 by using Kruskal‐Wallis and post hoc Dunn's test (one‐tailed)
Figure S4 Immunofluorescence staining for pericyte and microvessel in retina. A. immunofluorescence staining of anti‐NG2 antibody to measure pericyte and anti‐GSL‐IB4 antibody to evaluate microvessel was observed in the ganglion cell layer, inner plexiform layer, and inner nuclear layer. B. Expression of anti‐NG2 antibody merged well with anti‐GSL‐IB4 antibody. C. There was no significant difference in the degree of anti‐NG2 antibody or with anti‐GSL‐IB4 antibody staining among the groups (P = 0.625 for anti‐NG2 antibody, P = 0.514 for anti‐GSL‐IB4 antibody) (n = 10 per group). * P < 0.05 by using Kruskal‐Wallis and post hoc Dunn's test (one‐tailed)
Figure S5 Hypothetical overview of the relationship between fluctuation of intraocular pressure and neurodegeneration in diabetic retina
IOP, intraocular pressure


