Abstract
Immune checkpoint inhibitors have revolutionized cancer therapy leading to exceptional success. However, there is still the need to improve their efficacy in non‐responder patients. Natural killer (NK) cells represent the first line of defence against tumours, due to their ability to release immunomodulatory cytokines and kill target cells that have undergone malignant transformation. Harnessing NK cell response will open new possibilities to improve control of tumour growth. In this respect inhibitory checkpoints expressed on these innate lymphocytes represents a promising target for next‐generation immunotherapy. In this review, we will summarize recent evidences on the expression of NK cells receptors in cancer, with a focus on the inhibitory checkpoint programmed cell death protein 1 (PD‐1). We will also highlight the strength and limitations of the blockade of PD‐1 inhibitory pathway and suggest new combination strategies that may help to unleash more efficiently NK cell anti‐tumour response.
Abbreviations
- AbE
abscopal effect
- AML
acute myeloid leukaemia
- BM
bone marrow
- CAR
chimeric antigen receptor
- CTLA‐4
cytotoxic T‐lymphocyte‐associated protein 4
- DCs
dendritic cells
- Gal‐9
galectin‐9
- GvDH
graft versus host disease
- HMGB‐1
high‐mobility group box 1
- ICIs
immune checkpoint inhibitors
- ITAM
immunoreceptor tyrosine‐based activation motif
- ITIM
immunoreceptor tyrosine‐based inhibitory motif
- ITSM
immunoreceptor tyrosine‐based switch motif
- IDO
indoleamine 2,3‐dioxygenase
- iPSCs
induced pluripotent stem cells
- NKG2A
killer cell lection‐like receptor C1
- KIRs
killer immunoglobulin (Ig)‐like receptors
- LILRB
leukocyte Ig‐like receptor subfamily B
- LAG‐3
lymphocyte activation gene‐3
- MHC‐I
major histocompatibility complex class I
- mAbs
monoclonal antibodies
- NCRs
natural cytotoxicity receptors
- NSCLC
non‐small‐cell lung cancer
- PB
peripheral blood
- PtdSer
phospatidylserine
- PD‐L1
programmed cell death 1 ligand 1
- PD‐L2
programmed cell death 1 ligand 2
- PD‐1
programmed cell death protein 1
- RT
radiotherapy
- SHP‐1 and SHP‐2
SH2 domain‐containing phosphatase
- sPD‐1
soluble form of PD‐1
- SCCHN
squamous cell carcinomas of the head and neck
- TIGIT
T‐cell immunoglobulin and ITIM domain
- TIM3
T‐cell immunoglobulin and mucin domain‐containing protein 3
- TAAs
tumour‐associated antigens
- TILs
tumour‐infiltrating lymphocytes
- TMB
tumour mutational burden
- UCB
umbilical cord blood
1. INTRODUCTION
Natural Killer (NK) cells are potent effector cells that play a pivotal role in the innate response against infections by viruses and, more importantly, against tumours growth, preventing tumour spreading and metastases. Upon activation, NK cells elicit a strong cytolytic activity and release chemokines and cytokines able to orchestrate early inflammatory responses. Thus, NK cells have an essential role in the first‐line defence of the innate immune responses and modulate the subsequent activation of the adaptive immune system (Moretta, Bottino, Mingari, Biassoni, & Moretta, 2002; Moretta et al., 2004; Sivori, Vacca, et al., 2019). Originally, NK cells were thought to reside primarily in peripheral blood, bone marrow and spleen but recent evidences could demonstrate their presence in lymph nodes and other non‐lymphoid organs such as the uterus, liver and lung (Shi, Ljunggren, La Cava, & Van Kaer, 2011).
The mechanisms of action of NK cells remained a mystery for many years until the ‘missing self’ hypothesis, proposed in the late 1980s, revealed that NK cells, by sensing the absence of major histocompatibility complex class I (MHC‐I) on target cells, are able to discriminate between healthy and virus‐infected or tumour cells (Ljunggren & Karre, 1990). This hypothesis was confirmed by the discovery, in mice and human NK cells, of MHC‐specific receptors able to deliver inhibitory signals that block NK cell cytotoxicity (Moretta et al., 1990; Ciccone et al., 1992; Moretta et al., 1993; Moretta, Bottino, et al., 1996). Recognition of self‐MHC‐I molecules represents the most important mechanism to protect self‐cells from NK cell killing. The discovery that ‘off’ signals are required to prevent NK‐mediated autoreactivity suggested that ‘on’ signals should be present as well and be responsible for NK cell activation. Indeed, several surface receptors able to promote NK cell cytotoxicity were subsequently identified and characterized (Moretta et al., 2001; Moretta et al., 2004). Triggering of NK activating receptors occurs through binding with specific (non‐MHC) ligands de novo expressed or overexpressed in stressed cells and, more importantly, in virus‐infected or tumour‐transformed cells. However, both tumour cells and tumour micro‐environment can dampen NK cell‐mediated anti‐tumour activity by modulating the membrane expression of activating receptors (see below). The following paragraphs will analyse the NK cell receptors with particular regard to the inhibitory checkpoints and their important role as attractive therapeutic targets to enhance anti‐tumour immune responses. In addition, we will discuss recent data indicating that different combined immunotherapies may represent new therapeutic approaches.
2. NATURAL KILLER CELL RECEPTORS
2.1. Inhibitory and activating receptors
NK cell function is regulated by an array of inhibitory and activating receptors. As mentioned before, the inhibitory receptors specific for human leukocyte antigen class I (HLA‐I) molecules provide the most important regulation of NK cells activity. Two main different types of HLA‐I‐specific inhibitory receptors have been identified in NK cells and are represented by the CD94/killer cell lectin‐like receptor C1 (NKG2A) heterodimer and the members of the killer immunoglobulin (Ig)‐like receptor (KIR) family (Moretta et al., 2014). Killer cell lectin like receptor C1 (NKG2A), as designated by International Union of Pharmacology (IUPHAR) guide to IMMUNOPHARMACOLOGY (Armstrong et al., 2020), is a type II transmembrane protein containing a lectin‐type domain in the extracellular portion, which specifically recognizes HLA‐E, a non‐classical HLA‐I molecule with a limited polymorphism (Lopez‐Botet et al., 1997). KIRs are type I transmembrane receptors that specifically recognize shared polymorphic determinants of HLA‐A, HLA‐B and HLA‐C molecules (Moretta et al., 1993). Importantly, KIR and NKG2A may be expressed also by T lymphocytes upon prolonged activation (Mingari et al., 1995; Mingari et al., 1996; Mingari, Moretta, & Moretta, 1998; Mingari, Ponte, et al., 1998) or exposure to TGFβ (Bertone et al., 1999). Their expression may limit Tcell function, a critical event in tumour‐infiltrating T lymphocytes.
LIR‐1 (LILRB1), another type I transmembrane molecule, belonging to the Ig‐like receptor superfamily is another HLA‐specific receptor interacting with both classical (HLA‐A, HLA‐B and HLA‐C) and non‐classical (HLA‐G) HLA‐I ligands (Cosman et al., 1997; Ponte et al., 1999; Vitale et al., 1999). Despite molecular differences, these inhibitory receptors, upon interaction with HLA‐I ligands, activate common downstream pathways that transduce inhibitory signals determining a strong inhibition of the polarization and release of lytic granules (Das & Long, 2010). Indeed, they share the cytoplasmic domain called immunoreceptor tyrosine‐based inhibitory motif (ITIM) that is phosphorylated upon ligand interaction and recruits the tyrosine phosphatase SH2 domain‐containing phosphatase (SHP)‐1 and (SHP)‐2 (Lanier, Corliss, Wu, Leong, & Phillips, 1998; Moretta, Bottino, et al., 1996). In turn, these phosphatases activate a downstream cascade to turn off NK cell function. It must be noted that at least one receptor specific for classical HLA‐I molecules is expressed by NK cells allowing the whole NK cell pool to detect the loss of even a single allele on self‐cell, an event frequently occurring in transformed tumour cells (Garrido, 2019).
The activity of inhibitory receptors is required to counterbalance the function of activating receptors. The major activating NK receptors are represented by the Natural Cytotoxicity Receptors (NCRs) that consist of three molecules named NKp46 (Pessino et al., 1998; Sivori et al., 1997), NKp44 (Vitale et al., 1998) and NKp30 (Pende et al., 1999). NCRs are type I transmembrane receptors, belonging to the immunoglobulin‐like family and their transmembrane domains contain a positively charged amino acid required for the interaction with the Immunoreceptor tyrosine‐based activation motif (ITAM) sequence present in adaptor polypeptides (Barrow, Martin, & Colonna, 2019). NCRs, selectively expressed by NK cells (with the exception of NCR+ ILC3), represent the most reliable markers to identify these cells and play a pivotal role in NK‐mediated tumour cells killing. In addition, it has been demonstrated that the magnitude of the cytolytic activity towards NK‐susceptible target cells correlates with NCRs surface density on NK cells (Sivori et al., 2000). Different ligands, such as virus‐derived molecules and/or intracellular proteins (i.e. the nuclear protein PCNA) that are expressed in response to stress or during tumour transformation, have been shown to interact with NCRs extracellular regions. In addition, it has been recently demonstrated that also the extracellular ligand Nidogen‐1 and the soluble plasma glycoprotein called complement factor P/properdin can interact with NKp44 and NKp46, respectively (Gaggero et al., 2018; Narni‐Mancinelli et al., 2017; Sivori, Vacca, et al., 2019).
NKG2D (HER2), a type II transmembrane and C‐type lectin‐like molecule, represents another important NK cell‐activating receptor. Also, its ligands, namely, ULBPs and MICA/B, are overexpressed in infected and tumour cells (Lanier, 2015). Other triggering surface molecules are represented by co‐receptors that include 2B4 and NTB‐A, as well as DNAM‐1, NKp80 and CD59 whose function is to amplify NK cell triggering (Moretta et al., 2014). However, to exert their activity, they rely on the simultaneous engagement of one or another triggering receptor (such as NCRs and/or NKG2D).
Thus, it can be concluded that the balance of signals derived from activating and inhibitory receptors determines if NK cells will exert their cytotoxic activity or remain inactive. As mentioned before, the inhibitory receptors specific for HLA‐I, KIRs and NKG2A, control NK cells autoreactivity. Under normal conditions, the interaction between inhibitory receptors and their ligands inactivates NK cells, preventing killing of healthy cells, with the remarkable exception of the NK‐mediated editing of Dendritic cells (DCs), which failed to undergo proper maturation (Moretta et al., 2002). During virus infection or tumour progression, transformed cells down‐regulate or even lose HLA‐I expression, becoming susceptible to NK‐mediated cell lysis. For example, this is particularly important during the initial phase of viral infection, when T lymphocytes have not expanded sufficiently to mediate protection and NK cells play a primary role in limiting virus spreading. It is well known that also tumour cells may express low levels of HLA‐I molecules. However, loss of HLA‐I expression is not sufficient to induce NK‐mediated cell killing, which will occur only if triggering receptors have also been properly engaged by specific ligands present on the surface of target cells (Moretta et al., 2004). Indeed, such ligands for activating receptors are usually absent or expressed at low levels in resting healthy cells, while they are up‐regulated or even expressed de novo at the surface of stressed normal cells or virus‐infected or transformed tumour cells. In this context, while stressed healthy cells express normal or increased levels of HLA‐I molecules, which efficiently engage the inhibitory receptors, in virus‐infected or tumour cells, the activating signal will overcome the signalling mediated by the inhibitory receptors promoting target cell killing and cytokine release.
Notably, KIRs and the other HLA‐I‐specific inhibitory receptors should be considered as the first discovered inhibitory checkpoints controlling NK cells and, in some instances, T‐cell function (relevant to anti‐tumour defence).
2.2. Inhibitory checkpoint receptors
In addition to the inhibitory receptors specific for HLA‐I molecules, additional inhibitory checkpoints (including PD‐1, TIM‐3, LAG‐3, etc.), primarily involved in the maintenance of immune cell homeostasis, have been described in NK cells. Most of these inhibitory checkpoints are not expressed by resting NK cells. However, under pathological conditions, their expression can be de novo induced upon interaction with specific ligands frequently expressed on the surface of tumour cells. In this context, checkpoint receptors can affect NK cell function facilitating tumour immune escape. A major inhibitory checkpoint is represented by programmed cell death protein 1 (PD‐1) that was originally discovered to be expressed on T cells and to induce programmed cell death during T‐cell thymic selection (Ishida, Agata, Shibahara, & Honjo, 1992). Even though PD‐1 was initially described on T, B and myeloid cells, it has been recently described also on NK cells (see below) (Pesce et al., 2017) (Huang, Francois, McGray, Miliotto, & Odunsi, 2017). PD‐1 is a type I transmembrane glycoprotein composed of an IgV‐type extracellular domain, which belongs to the CD28/cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4) subfamily of the Ig superfamily (Chamoto, Al‐Habsi, & Honjo, 2017). Structural and biochemical analyses demonstrated that, due to the lack of membrane‐proximal cysteine residues required for homodimerization, PD‐1 is monomeric in solution as well as on cell surface (Zhang et al., 2004). Its cytoplasmic domain contains two tyrosine residues, the membrane‐proximal one constitutes the ITIM region, while the other domain is represented by the immunoreceptor tyrosine‐based switch motif (ITSM) (Okazaki & Honjo, 2007) (Figure 1). Despite ITIM is often associated with inhibitory receptors (including KIRs and NKG2A) being required for the activation of the inhibitory cascade, the tyrosine residue in the ITSM has been shown to be essential for PD‐1 inhibitory function (Okazaki, Maeda, Nishimura, Kurosaki, & Honjo, 2001). Indeed, upon antigen stimulation, this tyrosine residue is phosphorylated and recruits SHP‐2, which through dephosphorylation of downstream effector targets, initiates the inhibitory cascade. The PD‐1 ligands are represented by the type I transmembrane glycoprotein programmed cell death 1 ligand 1 (PD‐L1) and programmed cell death 1 ligand 2 (PD‐L2). Expression of PD‐L1 is detectable on haematopoietic cells such as T and B cells, macrophages and bone marrow‐derived mast cells. In addition, PD‐L1 may be expressed also in non‐haematopoietic cells like mesenchymal stem cells, lung, keratinocytes and vascular endothelium cells (Keir, Butte, Freeman, & Sharpe, 2008). On the contrary, PD‐L2 shows a more restricted expression which is limited to macrophages, bone marrow‐derived mast cells, dendritic cells and peritoneal B1 cells (Zhong, Tumang, Gao, Bai, & Rothstein, 2007). Importantly, the expression of PD‐L1 and PD‐L2 in both lymphoid and non‐lymphoid tissues suggests that this pathway could regulate immune responses not only in target organs but also in secondary lymphoid organs. In normal condition, the engagement of the PD‐1 pathway regulates immune cell activation and ensures peripheral tolerance. However, PD‐1 ligands (PD‐L1 and PD‐L2) have been shown to be often expressed also by cells of different tumours. In this context, the interaction of PD‐1 with its ligands inhibits NK cell‐mediated killing, thus favouring tumour immune escape. Considering that the PD‐1/PD‐Ls axis is widely adopted by different tumour cells to escape the immune system, it is not surprising that this pathway has been extensively studied to develop new therapeutic approaches aimed to restore the anti‐tumour immune response (see below). Of notice is also the discovery of a soluble form of PD‐1 (sPD‐1); recently different studies have been focused on sPD‐1 possible role as an anti‐tumour agent (Elhag et al., 2012; Nielsen, Ohm‐Laursen, Barington, Husby, & Lillevang, 2005). In this context, clinical studies have investigated the presence of sPD‐1 and its correlation with the overall survival of patients with different cancers (Kruger et al., 2017; Sorensen, Demuth, Weber, Sorensen, & Meldgaard, 2016).
FIGURE 1.
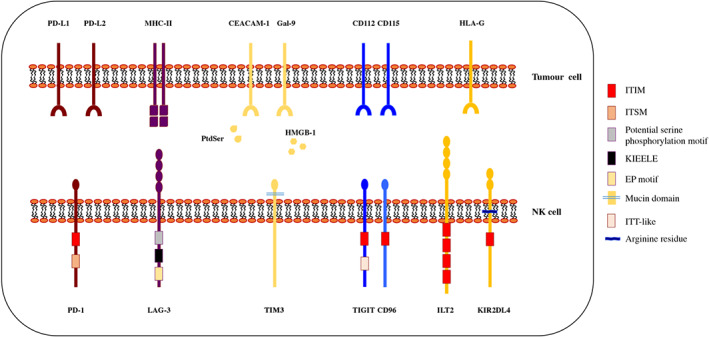
Schematic representation of inhibitory checkpoints and their ligands. NK cells express multiple inhibitory checkpoint receptors such as PD‐1, TIM3, LAG‐3, TIGIT, CD96, ILT2 and KIR2DL4 that interact with specific ligands expressed on virus‐infected or tumour‐transformed cells. The different extracellular and intracellular domains reported illustrate the molecular heterogeneity of these inhibitory checkpoints. The classical ITIM domain is shared between PD‐1, TIGIT, CD96, ILT2 and KIR2DL4, while the ITSM‐ and ITT‐like regions are present only in PD‐1 and TIGIT, respectively. LAG‐3 has an unusual cytoplasmic region containing a potential serine phosphorylation motif (S454), a glutamic acid–proline (EP) repeat and the conserved KIEELE motif that is indispensable for its inhibitory function. TIM3 presents a mucin domain in the extracellular region while lacks any conserved motifs in the intracytoplasmic tail. KIR2DL4 presents an arginine residue, in the transmembrane domain, able to interact with FcεRI
In humans, in addition to PD‐1, other inhibitory checkpoints may be expressed on the surface of NK cells. Lymphocyte activation gene‐3 (LAG‐3) is a type 1 transmembrane protein with significant homology to CD4. Compared with PD‐1, LAG‐3 has a larger extracellular domain that consists of four Ig‐like domains and it is also characterized by an unusual cytoplasmic tail which contains domains not found in other inhibitory checkpoints (De Sousa, Leitner, Grabmeier‐Pfistershammer, & Steinberger, 2018) (Figure 1). LAG‐3 binds MHC class II molecules promoting an exhausted profile observed in tumour‐infiltrating lymphocytes (TILs). While its role in the modulation of T‐cell proliferation, activation and homeostasis has been extensively studied, LAG‐3 effects on NK cell function are still to be clarified (Kim & Kim, 2018). As for PD‐1, a soluble form of LAG‐3, acting as an immune adjuvant has been identified and clinical‐grade sLAG3 has been analysed in phase I and II clinical trials to favour stimulation of the immune system (Chiossone & Vivier, 2017).
Tcell immunoglobulin and mucin domain‐containing protein 3 (TIM3), a member of the TIM family, is a type I transmembrane protein containing an N‐terminal IgV‐like domain and a mucin domain (Figure 1). Similarly, to LAG‐3, TIM3 does not contain classical inhibitory motifs, like ITIM, however phosphorylation of two tyrosine residues present in its cytoplasmic tail mediates the intracellular signalling pathway (De Sousa et al., 2018). Several ligands have been found to associate with TIM3 such as the high‐mobility group box 1 (HMGB‐1), galectin‐9 (Gal‐9), phospatidylserine (PtdSer) and CEACAM‐1 (Figure 1). TIM3 expression has been detected in different lymphoid cells and it has been suggested to be a marker for mature and/or activated NK cells. An increase of TIM3 expression in NK cells has been observed in patients with advanced melanoma and has been correlated with the NK cell exhaustion phenotype (Gallois, Silva, Osman, & Bhardwaj, 2014). Of note, TIM3 is often co‐expressed with PD‐1 during cancer progression and chronic infection.
Other widely studied inhibitory checkpoints are represented by the T‐cell immunoglobulin and ITIM domain (TIGIT) and CD96 (Figure 1). TIGIT and CD96 belong to the Ig superfamily, that includes also the activating co‐receptor DNAM‐1. Both receptors contain an ITIM sequence in their cytoplasmic domains that initiates the inhibitory cascade leading to inhibition of NK cell function, including granule polarization, cytotoxicity and cytokine secretion (Stanietsky et al., 2009). TIGIT and CD96 corresponding ligands are represented by the cell surface nectin and nectin‐like CD115 (PVR) and CD112 (PVRL2, nectin‐2) molecules that are up‐regulated in different tumours. Considering that CD115 and CD112 are also DNAM‐1‐ligands, it is evident that both these inhibitory checkpoints and the activating co‐receptor may compete for the same ligands. Therefore, TIGIT engagement with CD115 inhibits NK cell function by counterbalancing the DNAM‐1‐mediated activating signals. TIGIT can be detected in resting NK cells and its expression is up‐regulated upon NK cell activation and in tumour‐associated NK cells of patients with colon cancer (Zhang et al., 2018). Indeed, high expression of TIGIT on tumour‐infiltrating NK cells, has been shown to correlate with reduced cytotoxicity and cytokine release.
The immunoglobulin‐like transcript 2, ILT2 (LILRB1) and KIR2DL4 represent two other important NK cell inhibitory receptors (Lin & Yan, 2018). While ILT2 ( (LILRB1) is expressed on NK cells, T cells, monocytes, B cells, dendritic cells and myeloid‐derived suppressor cells (MDSCs), KIR2DL4 is predominantly found on decidual NK cells and cannot be detected on the surface of peripheral blood resting NK cells (Koopman et al., 2003; Rajagopalan & Long, 2012). Regulation of NK cell function by KIR2DL4 is quite complicated because it is characterized by one ITIM domain in its intracellular region and, by an arginine residue, in its transmembrane domain able to interact with FcεRI. Thus it presents features of both inhibitory and stimulatory receptors (Rajagopalan & Long, 2012). ILT2 (LILRB1) belongs to the leukocyte Ig‐like receptor subfamily B (LILRB), a group of type I transmembrane glycoprotein, and its extracellular region consists of four immunoglobulin domains, while the cytoplasmic tail is characterized by four ITIM sequences (Kang et al., 2016). Both ILT2 and KIR2DL4 interact with the HLA‐G molecule. HLA‐G in normal condition is expressed at the fetal–maternal interface and few healthy adult tissues like cornea, pancreatic isles and thymic medulla (Carosella, Rouas‐Freiss, Tronik‐Le Roux, Moreau, & LeMaoult, 2015; Lin & Yan, 2018). However, its expression can be switched on in pathological conditions, such as cancer, viral infection autoimmune and inflammatory diseases (Lin & Yan, 2018). Indeed, aberrant HLA‐G expression has been detected in several tumours like ovarian carcinoma, gastric cancer, breast and lung cancer, and colorectal cancer (Carosella et al., 2015), and it has been correlated with metastatic status, advanced tumour stage and poor disease outcome. Indeed, overexpression of HLA‐G is a well‐known mechanism used by tumour cells to avoid and inhibit both the innate and adaptive immune responses (Li et al., 2017). Thus, HLA‐G/ILT2 interaction impairs NK cells chemotaxis, cytotoxicity, IFN‐γ production and MICA/NKG2D‐induced activation (Menier, Riteau, Carosella, & Rouas‐Freiss, 2002; Morandi et al., 2011). For this reason, the HLA‐G/ILT signalling pathway is considered as a new immune checkpoint and a promising immunotherapy target.
3. INHIBITORY CHECKPOINTS AS TARGETS OF IMMUNOTHERAPY
Immunotherapy through immune checkpoint inhibitors (ICIs) results in increased activation of the immune response and has recently led to the approval of several new drugs. The first developed approach was aimed at blocking two key inhibitory receptors that critically affect peripheral T‐cell tolerance and T‐cell function: CTLA‐4 and PD‐1. CTLA‐4 is a CD28 homologue but with much higher affinity for B7. Thus, the inhibitory signal is mostly due to the ‘subtraction’ of B7 to the costimulatory signal physiologically provided by CD28:B7 binding and required for T‐cell receptor (TCR)‐mediated T‐cell activation (Walunas et al., 1994). Anti‐CTLA4 blockade with ipilimumab was the first treatment to prolong the overall survival in patients with advanced melanoma in randomized trials (Hodi et al., 2010; Robert et al., 2011). More recently, strategies aimed at blocking PD‐1 have been developed. PD‐1 blockade has been shown to improve the survival and the progression‐free survival in patients with metastatic melanoma and in patients with metastatic squamous and non‐squamous non‐small‐cell lung cancer (NSCLC) who have been previously treated with standard therapies. Recently, immunotherapy with monoclonal antibodies (mAbs) that target the PD‐1/PD‐L1 axis has successfully improved cancer treatment and has been approved in a variety of cancers, including several solid tumours (Baitsch et al., 2011; Hamid et al., 2013; Rizvi et al., 2015; Topalian et al., 2012). Both CTLA‐4 and PD‐1 have similar negative effects on T‐cell activation, however, while CTLA‐4 is rapidly expressed on the surface of T cells following activation, thus compromising early T‐cell response, PD‐1 is expressed on T cells upon exhaustion and acts later on long‐term tolerance maintenance. Although both CTLA‐4‐ and PD‐1‐deficient mice develop autoimmune phenotypes (Nishimura, Nose, Hiai, Minato, & Honjo, 1999; Walunas, Bakker, & Bluestone, 1996), the severity and timing is different, suggesting that the two receptors differentially shape the T‐cell repertoire and the immune response. While PD‐1‐deficient mice take several months to develop autoimmunity, which it is targeted to specific organs, CTLA‐4‐deficient mice develop multi‐organ disease and die within 3 weeks, consistent with the critical physiological role of this receptor for T‐cell homeostatic expansion. Another important difference between the two checkpoints is that CTLA‐4 is expressed by T cells only, while PD‐1 is expressed by other immune cells, also including NK cells (Fife & Bluestone, 2008). Thus, PD‐1 checkpoint blockade allows broadening the efficacy of immunotherapy by acting on multiple effector lymphocytes and, in particular, on the NK cell compartment, which has recently become the focus of interest for therapeutic interventions (Andre et al., 2018; Cerwenka & Lanier, 2018; Mariotti, Quatrini, Munari, Vacca, & Moretta, 2019; Mingari, Pietra, & Moretta, 2019; Pesce et al., 2019). In this context, a common strategy to elude T‐cell‐mediated immune responses is the down‐regulation or loss of expression of HLA‐I molecules, which as has been documented for a large range of epithelial cell cancers and melanomas (Garrido, 2019; So et al., 2005; Zitvogel, Tesniere, & Kroemer, 2006). Therefore, while initially immunotherapy was mainly focused on enhancing Tcell responses, targeting immune checkpoints expressed on NK cells is now extremely important for advanced tumours that become ‘invisible’ to T cells. Among the NK cell checkpoints, PD‐1 expression has been documented in many tumour settings and will be the focus of the next paragraphs.
4. PD‐1/PD‐L AXIS BLOCKADE: AN EXAMPLE OF IMMUNOTHERAPY TARGETING AN NK CELL INHIBITORY CHECKPOINT
4.1. PD‐1/PD‐L axis in tumours
The PD‐1/PD‐L1 axis plays a fundamental role in the inhibition of anti‐tumour effector function of CD8+ T cells. Different from T cells, which express high levels of PD‐1 at exhaustion, PD‐1 is absent or expressed at very low levels on peripheral blood NK cells of healthy donors, with the exception of a minor fraction of cytomegalovirus (CMV)‐seropositive individuals (Pesce et al., 2017). In mice, it was shown that endogenous glucocorticoids released upon infection with murine CMV (MCMV) induce PD‐1 expression on spleen NK cells (Quatrini et al., 2018). Regarding human NK cells, recent data would suggest that persistent stimulation by tumour cells expressing ligands for activating NK receptors or soluble factors present in the TME may induce PD‐1 surface expression (Pesce et al., 2019) with consequent impairment of anti‐tumour NK effector function. Notably, human NK cells have been shown to display an intracytoplasmatic pool of PD‐1 mRNA and PD‐1 protein localized in the Golgi (Mariotti et al., 2019); however, the stimuli required for its expression on the membrane are still undefined.
There is clear evidence that tumour cells express the natural ligands of PD‐1 (PD‐Ls), as a mean to evade immune attack (Munari, Zamboni, Lunardi, Marconi, et al., 2018). Recently, different independent studies provided evidence for the expression of PD‐1 on human tumour‐infiltrating NK cells including multiple myeloma (Benson et al., 2010), Kaposi sarcoma (Beldi‐Ferchiou et al., 2016), ovarian carcinoma (Pesce et al., 2017) and digestive and lung cancers (Liu et al., 2017; Tumino et al., 2019). Of note, in malignant pleural effusions derived from patients with primary or metastatic lung tumours, PD‐1 expression is not only confined to NK cells but is also expressed by tumour‐infiltrating ILC3s (Tumino et al., 2019). It has been shown that NK cells isolated from pleural effusions could release cytokines and efficiently kill tumour cells (Croxatto et al., 2017; Vacca, Martini, Mingari, & Moretta, 2013). However, when these PD‐1+ NK cells interacted with tumour cells expressing PD‐Ls, their cytokine production was sharply inhibited (Tumino et al., 2019).
4.2. Strengths and limitations of monoclonal antibodies targeting the PD‐1/PD‐L axis in cancer
Monoclonal antibodies acting through PD‐1 blockade represent a major breakthrough in oncology, showing significant clinical success in the treatment of several types of cancer. There are currently five approved therapeutic agents on the market targeting the PD‐1/PD‐L1 pathway: Nivolumab and pembrolizumab are humanized IgG monoclonal antibodies directed at the PD‐1 receptor, whereas atezolizumab, durvalumab and avelumab are humanized IgG monoclonal antibodies directed at the PD‐L1 ligand. Several large‐scale clinical trials showed significant response rates and improvements in overall survival in a variety of patients with solid tumours including NSCLC, gastrointestinal carcinomas, squamous cell carcinomas of the head and neck (SCCHN), renal cell carcinomas, urothelial carcinomas, cervical carcinomas and breast carcinomas, as well as in patients with lymphoma or melanoma. For this reason, all of these five antibodies have been approved for use as the first‐ or second‐line treatment (Garon et al., 2015; Reck et al., 2016) (Borghaei et al., 2015; Rizvi et al., 2015). Each of these drugs binds to a different epitope on their respective target and their mechanisms of action consist in blocking the PD‐1/PD‐L1 receptor–ligand interaction. A novel class of anti‐PD‐1 antibodies was recently identified with a different mechanism of action but with an antagonistic activity comparable with that of pembrolizumab and nivolumab. These antibodies bind to the opposite face of the PD‐1 protein relative to the PD‐1–PD‐L1 interaction site and act predominantly through the restoration of the positive signalling transduced by the simultaneous engagement of CD28 co‐receptor (Fenwick et al., 2019). Although the synergistic effect of these non‐blocking anti‐PD‐1 antibodies with blocking PD‐1 antibodies has only been demonstrated in vitro and in a mouse tumour model in vivo, their distinct mechanism of action suggests that they may complement classical immunotherapy by enhancing the recovery of anti‐tumour activity of cytotoxic lymphocytes.
Despite the successes of the above‐mentioned Food and Drug Administration (FDA)‐approved antibodies, only ∼30–40% of patients show a response to anti‐PD‐1 immunotherapy. Response rates vary widely between cancer types and there is a particular need for predictive biomarkers that can be used to identify patients more likely to respond to immune checkpoint modulators. To this end, different tumour features are evaluated prior to the design of the therapy. These include assays to analyse the level of PD‐L1 expression in tumour cells and DNA sequencing to estimate tumour mutational burden (TMB) (Havel, Chowell, & Chan, 2019). One important critical limitation that may influence the response to the PD‐1/PD‐L1 axis blockade is the screening of tumours based on the expression of PD‐L1, which is routinely evaluated by immunohistochemical staining using different methods (tumour proportion score, combined positive score and tumour‐infiltrating immune cells). This diagnostic test may guide the decision of the appropriate therapeutic strategy to be adopted and helps to predict benefits in subgroups of patients, depending on the degree of biomarker expression at different cut‐off (Sholl et al., 2016) (Figure 2). Higher PD‐L1 expression on tumour cells generally correlates with higher rates of clinical response to treatment with PD‐1/PD‐L1 inhibitors. However, this is not always the case, as clinical responses are also observed in patients with tumours negative for PD‐L1. It is reasonable to think that such discrepancy may be at least in part explained by the fact that a number of tumours could be misclassified, in terms of PD‐L1 expression, due to different factors including expression heterogeneity in the tumours, interclone differences and interobserver/intraobserver variability, as demonstrated in several reports (Munari et al., 2017; Munari, Rossi, Zamboni, Lunardi, et al., 2018; Munari, Zamboni, Lunardi, Marchionni, et al., 2018).
FIGURE 2.
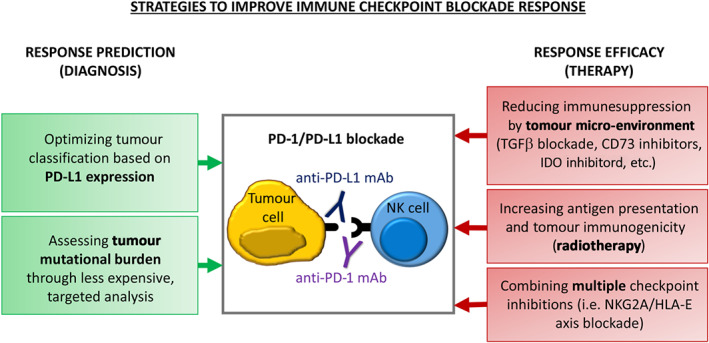
Different approaches to enhance immune response. Despite the development of new therapeutic approaches targeting the inhibitory checkpoints, the percentage of patients responding to anti‐PD‐1 immunotherapy remained low. Different strategies can be adopted to improve both the immune response prediction and efficacy. A more in‐depth analysis of tumour mutational burden (TMB) and a better characterization of PD‐L1 expression at tumour site will allow a more accurate diagnosis. In addition, improvement of immune checkpoint blockade response can be obtained combining different therapeutic approaches
High tumour mutational load has been correlated to better response to immune checkpoint blockade, because it may generate novel antigens that are not subject to immune tolerance, thus improving T‐cell‐mediated adaptive immune responses (Yarchoan, Hopkins, & Jaffee, 2017). Therefore, TMB has emerged as a promising novel biomarker to further aid in patient selection (Figure 2). High mutation burden is typically observed in carcinogen‐driven tumours such as lung cancer and melanoma and in tumours caused by germline mutations in genes encoding for proteins involved in DNA repair and replication. The main limitation of TMB analysis is represented by the high costs of the whole genome sequencing; therefore, it is limited to malignancies that are more likely to be highly mutated, and it is often analysed indirectly through less expensive techniques. Indirect TMB assessment includes analysis of microsatellite instability status, chromosomal structural analysis and mutational analysis of selected genes (Galuppini et al., 2019).
The limitations of the efficacy of immune checkpoint blockade therapies highlight the need for improving predictive strategies and finding novel synergistic therapeutic targets to increase the response rate and/or the number of patients that can be treated.
5. COMBINED THERAPIES TO IMPROVE IMMUNE CHECKPOINT BLOCKADE EFFICACY
A great effort has been done recently to explore the possible advantages of combining the blockade of multiple inhibitory signals at the same time, targeting NK cells together with T cells, neutralizing inhibitory molecules present at the tumour site or increasing tumour immunogenicity through radiotherapy (RT).
5.1. Immune checkpoint inhibitor (ICI) and tumour immunosuppressive micro‐environment
A major role in the failure of immune checkpoint therapies is played by the TME. For instance, hypoxia is responsible for the generation of adenosine, which decreases proliferation and effector function of cytotoxic lymphocytes (Cekic, Day, Sag, & Linden, 2014). Adenosine is generated by the sequential degradation of ATP to AMP, and AMP dephosphorylation, by CD39 and CD73, respectively. A combination of anti‐CD73 and anti‐PD‐1 treatments has been tested in patients with advanced solid tumours and anti‐tumour activity has been preliminary reported (Siu et al., 2018), and several other molecules that target the adenosine releasing pathway are currently in clinical development (Perrot et al., 2019). Another enzyme present in the TME is the indoleamine 2,3‐dioxygenase (IDO), which catalyses tryptophan degradation leading to deprivation of this amino acid and kynurenine production. Both these events contribute to tumour tolerance acting on the differentiation of regulatory T cells (Tregs) and directly suppressing Tand NK cell function and proliferation (Della Chiesa et al., 2006; Munn & Mellor, 2007). Indeed, it was shown that IDO mediates resistance to anti‐CTLA4 and PD‐1 therapies (Holmgaard, Zamarin, Munn, Wolchok, & Allison, 2013), but to date, no significant benefit has been revealed by combined IDO and PD‐1/PD‐L1 axis inhibition in melanoma (Long et al., 2019). Another component of the TME that contributes to the evasion of immune surveillance and to the failure of immune checkpoint blockade is TGFβ (Mariathasan et al., 2018). TGFβ excludes immune cells from the tumour bed (Tauriello et al., 2018) and inhibits both T cells and NK cell cytotoxicity (Castriconi et al., 2013; Gao et al., 2017; Viel et al., 2016). A first‐in‐class bifunctional checkpoint inhibitor (the fusion protein M7824) comprising the extracellular domain of human TGFβRII (TGFβ Trap) linked to the C‐terminus of human anti‐PD‐L1 heavy chain (αPD‐L1) has been developed and has proved to be efficient in promoting T‐cell and NK cell activation in mouse tumour models (Knudson et al., 2018). These findings prove that simultaneous targeting of TGFβ and PD‐L1/PD‐1 immunosuppressive pathways may represent a promising tool to promote anti‐tumour responses (Figure 2).
5.2. Combination of ICI targeting different pathways
Another strategy to improve the efficacy of checkpoint blockade is to target multiple checkpoints at the same time. Indeed, several studies demonstrated that the combined blockade of multiple inhibitory checkpoints resulted to be more effective in controlling tumour growth compared with blockade of a single receptor (Nirschl & Drake, 2013). These data confirmed the hypothesis that combined immune checkpoint blockade could be an innovative and potential treatment strategy to be applied to a wide variety of cancers (Figure 2). In addition, this notion revealed that inhibitory checkpoints might synergize to down‐modulate the overall antit‐umour immune response. Among the inhibitory receptors expressed by NK cells, a promising target for combined therapy is NKG2A, which has the advantage of being expressed also by activated T cells (Bertone et al., 1999; Mingari, Ponte, et al., 1998) and whose ligand (HLA‐E) is frequently overexpressed in tumours. NKG2A blockade with the mAbs monalizumab in combination with the blockade of the PD‐1/PD‐L1 axis was proved to enhance both T‐cell and NK cell responses (Andre et al., 2018). Moreover, blocking NKG2A increased NK cell antibody‐dependent cell‐mediated cytotoxicity, thus enhancing the efficacy of cetuximab (an anti‐estimated GFR [anti‐EGFR] antibody) treatment in patients with squamous cell carcinoma of the head and neck SCCHN (Andre et al., 2018). These findings suggest that targeting NKG2A may represent a complementary tool to unleash NK cell response by combining checkpoint inhibitors with monoclonal antibodies targeting tumour antigens. In addition, combined immunotherapies targeting PD‐1 and other inhibitory checkpoints are under investigation. Thus, IMP321, a clinical‐grade sLAG‐3 protein, is currently used in combination with anti‐PD‐1 therapy in phase II clinical trials. Moreover, the efficacy of an anti‐LAG3 monoclonal antibodies (relatlimab) is currently being investigated in several types of cancer (NCT02061761; NCT02658981). As mentioned before, TIM3 and PD‐1 are often found to be co‐expressed on NK cells from cancer patients. Thus, anti‐TIM3‐blocking monoclonal antibodies have been used in combination with anti‐PD‐1 in phase I clinical trials to block tumour growth and restore the anti‐tumour immune response (Chiossone & Vivier, 2017). Moreover, the inhibitory receptor T‐cell immunoglobulin and ITIM domain has been shown to act in synergy with both PD‐1 and TIM3 (Kurtulus et al., 2015). Indeed, combined blockade of TIGIT and TIM3 or PD‐1 resulted in tumour regression and increased anti‐tumour immunity. In this context, anti‐PD‐1 therapy combined with a fully human anti‐TIGIT monoclonal antibody (MTIG7192A, RG6058) is tested in a phase I clinical trial towards different solid tumours.
Moreover, due to the potent inhibitory effect exerted by KIRs on NK cell function, a human monoclonal antibodies blocking KIR2DL1, KIR2DL2 and KIR2DL3 on NK cells, namely, lirilumab, was developed. In clinical trials for acute myeloid leukaemia (AML), myeloma or solid tumours, lirilumab showed, however, limited anti‐tumour efficacy (Carlsten et al., 2016) (Vey et al., 2018). By contrast, IPH4102, a first‐in‐class monoclonal antibodies targeting KIR3DL2 is showing encouraging clinical activity in patients with cutaneous T‐cell lymphoma (Bagot et al., 2019). In addition, several approaches have been developed to block the HLA‐G/ILT signalling pathway, which take into account also the different mechanism regulating HLA‐G surface expression. These approaches include HLA‐G antagonists, blocking antibodies against HLA‐G or its receptors, siRNA, inhibition of its intercellular transfer and HLA‐G as target for drug delivery (Li et al., 2017; Lin & Yan, 2019; Zhang et al., 2014). Therefore, KIRs and HLA‐G may also represent promising targets for combined therapy.
5.3. ICI and radiotherapy
Radiotherapy (RT) is an essential treatment for cancer patients (Ngwa et al., 2018). It has been widely demonstrated that RT is able to modulate the anti‐tumour response acting, through different mechanisms, on the TME. Indeed, it reduces the overall tumour burden increasing systemic responses to immunotherapy, and in addition, preclinical models showed several immune‐stimulatory effects of ionizing radiations. These include activation of pro‐death signalling in tumour cells and release of damage‐associated molecular patterns and ‘eat me’ signals. Of note, RT can also serve as an in situ vaccine, promoting the release of tumour‐associated antigens (TAAs) and enhancing the number of APC cells in both the tumour micro‐environment and lymph nodes with consequent enhanced dendritic cell activation, T‐cell priming and recruitment of CD8+ T lymphocytes (Hwang, Pike, Royce, Mahal, & Loeffler, 2018). In addition, RT can modulate the innate immune response, increasing NK cell‐mediated cytotoxicity through the up‐regulation of NK cell‐activating receptor, NKG2D and NKp30, expression (Kim et al., 2006) (Matta et al., 2013). Moreover, it has been demonstrated that the interaction of RT with patient immune system is essential to promote the abscopal effect (AbE), which represents a promising tool to use abscopal effect for the treatment of not only localized tumours but also metastases (Ngwa et al., 2018). AbE was first observed in 1953 and it describes the systemic anti‐tumour reactions upon RT treatment that can lead to regression of lesions in metastases outside the radiation field (Mole, 1953). However, on the other side, RT may also impair the anti‐tumour immune responses increasing the influx of MDSC and Tregs and promoting the release of inflammatory cytokines, which can also induce PD‐L1 expression (Gandhi et al., 2015) (Deng et al., 2014). Thus, considering the important achievements obtained with ICI in targeting the immune response and, at the same time, the fact that not all patients benefit of ICI or RT treatment alone, increase interest raised for the integration of immune checkpoint blockade with RT for the treatment of patients with numerically and spatially restricted areas of metastasis (Pitroda, Chmura, & Weichselbaum, 2019). More importantly, combination of RT and ICI represents a great opportunity to boost AbE rates. Indeed, preclinical studies demonstrated that regression of non‐irradiated lesions can only be observed in immunocompetent mice, indicating that despite the increase of tumour immunogenicity, RT alone is not sufficient to induce AbE (Demaria et al., 2004). Several reports indicated that AbE is mainly observed in highly immunogenic tumours, such as malignant melanoma, renal cell carcinoma, hepatocellular carcinoma and NSCLC. Importantly, several immunotherapeutic agents, targeting different mechanisms of immune‐mediated responses, are able to boost AbE (Ngwa et al., 2018). In particular, preclinical studies indicate that PD‐1/PD‐L1 blockade is optimal in combination with RT. Indeed, an enhancement of anti‐tumour effects and significant reduction of secondary non‐irradiated tumours was observed in mice model, bearing solid or haematological tumours, treated with in‐field RT in combination with PD‐1 or PD‐L1 blockers (Gong, Le, Massarelli, Hendifar, & Tuli, 2018). Clinical studies confirmed that combination of anti‐PD‐1 or anti‐PD‐L1 immunotherapies with RT was associated with clinical significant and durable tumour responses in different cancer patients (Haymaker et al., 2017) (Yuan et al., 2017) (Takamori et al., 2018) (Xie, Gu, Zhang, Chen, & Wu, 2017).
However, some limitations have also emerged from experimental and clinical data (Ngwa et al., 2018). Despite the boost of the immune response, the presence of immune suppressive environment (i.e. MDSC and Treg cells and/or immunosuppressive cytokines) or tolerance at the tumour sites can repress AbE. Immune‐related adverse events can be also due to the overlapping toxicities derived from RT and ICI combination (Vanneman & Dranoff, 2012). More importantly, despite the numerous preclinical and clinical studies, the mechanisms underlying AbE have not yet been identified. Therefore, it is essential to develop new strategies to overcome these limitations and improve our understanding of the biological pathway regulating AbE. In this context, numerous preclinical studies have explored a range of radiation regimens combined with immune checkpoint blockade to optimize synergy with immunotherapy (Patel & Minn, 2018) (Figure 2). However, since the radiation dose, fractionation, timing and target organs are still not well defined, additional clinical studies are needed to fully realize the potential of integrated radio‐immunotherapies. Under investigation studies, aimed to minimize the effect of immunosuppression, use different approaches to improve T‐cell stimulation primed by RT and/or immunotherapy. Moreover, considering that AbE is more often associated to highly immunogenic tumours, it is essential to develop new strategies to increase immunogenicity. In this context, preclinical models demonstrated that RT, ICI and anticancer vaccine combination could increase abscopal effect response rates (Zheng et al., 2016). Overall, despite the multiple challenges in overcoming cancer immunosuppression, there is an increasing interest in developing new strategies to boost AbE in order to improve patient outcomes.
6. CHIMERIC ANTIGEN RECEPTOR‐ENGINEERED NK CELLS
In the field of tumour immunotherapy, a complementary approach to unleash cytotoxic lymphocytes through ICI is represented by the development of chimeric antigen receptor (CAR)‐cells. This approach is based on engineering cells with synthetic receptors in order to combine their high anti‐tumour activity with the capability of tumour antigen recognition of antibodies. This promising technique was first used to engineer T cells generating CAR‐T cells (Singh & McGuirk, 2020). The expression of synthetic receptors instead of the physiological TCR allows CAR‐T cells to recognize any cell surface molecule independently of MHC presentation, thus broadening the array of antigens recognized by T cells. Several approaches to generate CAR‐T cells have been developed in order to maximize their activity, persistence and expansion (Depil, Duchateau, Grupp, Mufti, & Poirot, 2020). Since their development, adoptive cell transfer of chimeric antigen receptor T cells revealed successful in the treatment of high‐risk B‐cell lymphoma and lymphoblastic leukaemia (Neelapu et al., 2017) (Park et al., 2018). Despite the important success in the treatment of haematological malignancies, similar results have not been obtained so far in solid tumours. However, in this context, it is worth to mention the recent development of a CAR‐T cells against the HLA‐G molecule in order to elicit immune response against HLA‐G+ solid tumours (presentation at the ESMO Immuno‐Oncology Congress 2019, Geneva, Switzerland Volume 30, Supplement 11, Loustau, M. Annals of Oncology, Volume 30, xi12). Nevertheless, some obstacles limit chimeric antigen receptor T clinical application, such as: high cost, need of personalized production that requires a substantial delay between patient enrolment and treatment, cytokine release syndrome and neurotoxicity (Sivori et al., 2019). On the contrary, NK cells engineering could represent an appealing solution. Indeed, chimeric antigen receptor NK cells do not require HLA matching and can be administered in allogenic settings without causing graft versus host disease (GvDH) providing a cost‐effective ‘off‐the‐shelf’ product (Burger et al., 2019) (Zhang, Hu, & Shi, 2020). In addition, due to the expression of activating receptors, such as NCR, NKG2D, DNAM‐1 and CD16, NK cells are able to induce cytotoxicity in both CAR‐dependent and ‐independent manners reducing the eventuality of loss of CAR‐targeting antigen‐related relapses and increasing their killing activity (Hu, Tian, & Zhang, 2018). Different sources for the isolation and generation of genetically engineered NK cells exist, including peripheral blood and umbilical cord blood (UCB) NK cells, various human NK cell lines (NK92, YT, KHYG, etc.) and induced pluripotent stem cell (iPSC)‐derived NK cells. Several clinical trials are evaluating the efficiency of chimeric antigen receptor NK92 cells in haematological malignancies and multiple myeloma as well as in glioblastoma (Chu et al., 2014) (Han et al., 2015) (Zhang et al., 2020). However, irradiated NK92 cannot expand in vivo after infusion, and lacking the expression of CD16 and NKp44, the ADCC and the natural cytotoxicity, proper of activated primary NK cells, are compromised, making the NK92 cell line not the most reliable source for NK cell engineering (Sivori, Meazza, et al., 2019). For this reason, UCB‐, PB‐ and iPSC‐derived NK cells have also been used as possible source for the development of CAR‐NK cells. Several attempts have been made to identify the best protocol for PB‐NK expansion, and an innovative strategy, which does not require a feeder layer, has been developed and successfully tested for CAR‐CD19 NK cells (Quintarelli et al., 2020). CAR‐NK cells derived from iPSC also demonstrated potent ability to kill, both in vitro and in vivo, mesothelin‐expressing tumours (Li, Hermanson, Moriarity, & Kaufman, 2018). iPSC‐NK cells represent an innovative and particularly promising tool because they efficiently kill both haematological malignancies and solid tumours, and most importantly, they are reproducible and can be easily genetically modified and expanded on the clinical scale (Zhang et al., 2020).
However, low in vivo persistence, immunosuppression exerted by TME and difficulty to infiltrate into solid tumours represent other challenges for CAR‐NK clinical applications (Zhang et al., 2020). Thus, CAR constructs have been optimized according to NK cell features. NKG2D‐expressing CAR‐NK cells gave encouraging results in a mouse model of osteosarcoma, as they could recognize different tumour cells as well as immunosuppressive cells (MDSC and Tregs) present in the TME (Parihar et al., 2019). Moreover, a chimeric PD‐1–NKG2D construct was also used to increase NK92 killing against various tumour cells (Guo et al., 2019). CAR‐NK cells can also be engineered with IL‐15 and/or chemokine receptors in order to increase specificity, efficacy and safety properties.
Even though there are still relevant challenges that impair a widespread use of CAR‐NK cell‐based therapies including improvement of ex vivo expansion, CAR construct optimization and development of new strategies to dampen the TME immunosuppression, NK cell therapy represent a promising and powerful tool for a more affordable ‘off‐the‐shelf’ cancer immunotherapy.
7. CONCLUDING REMARKS
Recent years have witnessed an extraordinary progress in cancer therapy. The major advances are related to the use of checkpoint inhibitors, which dramatically changed the clinical outcome of cancer patients. Notably, the most efficient responses have been obtained in highly aggressive, metastatic tumours, primarily melanoma and NSCLC. These results have been explained with the high rate of genetic mutations of these tumours. Indeed, a direct correlation has been established between responses to checkpoint inhibitory treatment and mutation rates of different tumour types. A likely explanation is that genetic mutations, upon altering proteins, generate new tumour antigens recognized by T cells in an HLA‐restricted fashion. However, HLA‐I partial or total losses are a major mechanism of tumour escape and selection of tumour cells clones that became ‘invisible’ to T lymphocytes. In this respect, NK cells, different from T cells, efficiently kill HLA‐I‐deficient tumour cells, representing useful effectors to attack these tumours. In this context, harnessing NK cell function should represent a major focus of present and future immunotherapy. Indeed, the function of inhibitory checkpoints expressed by NK cells, their biology and role in tumour immunotherapy, particularly in combination therapies, should be further investigated. Other strategies should also be examined in depth. A more promising approach is represented by NK cells that have been genetically modified to express CAR directed to tumour surface antigens. Different from chimeric antigen receptor T cells that must be used in an autologous setting (allogenic T cells would cause a severe GVHD), allogenic NK cells do not cause GVHD. Thus, donor‐derived CAR‐NK cells may be safely used for cancer treatment. This current technology allows obtaining large number of CAR‐NK cells in vitro with a 4‐log expansion, while their survival and expansion in vivo may be overcome by the additional transfection with IL‐15‐inducing gene. Notably, CAR‐NK cells may represent optimal ‘off‐the‐shelf’ donor‐derived products, since they can be prepared in large scale, with markedly reduced costs. In addition, they are immediately available to cancer patients in need of treatment and may be administered according to well‐defined protocols, as to the timing and the number of cell infused. The possible benefit of CAR‐NK approach in combination with checkpoint inhibitors should also be examined.
In conclusion, both major discoveries in the past three decades and the continuous technological advances have provided a large armamentarium that allowed and will allow improving treatment of high‐risk leukaemia and solid tumours. In this context, NK‐based therapies may play a fundamental role.
7.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in https://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Armstrong et al., 2020) and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/2020 (Alexander et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The present study has been supported by the following grants: Associazione Italiana per la Ricerca sul Cancro (AIRC) IG 2017 Id.19920 (L.M.), AIRC Special Program ‘Metastatic disease: the key unmet need in oncology’ Special Project 5x1000 Id. 21147 (L.M.), Ricerca Corrente OPBG (L.M. and P.V.) and Ministero della Salute (Italian Ministry of Health; GR‐2013‐02356568 [P.V.], RF‐2016‐02362288 [G.P.] and 5x1000 2015 [M.C.M.]). L.Q. has received funding from AIRC and from the European Union's Horizon 2020 Framework Programme under the Marie Skłodowska Curie Grant Agreement No. 800924. F.R.M. is a recipient of Fondazione Umberto Veronesi fellowship, and N.T. is a recipient of an AIRC fellowship.
Mariotti FR, Quatrini L, Munari E, et al. Inhibitory checkpoints in human natural killer cells: IUPHAR Review 28. Br J Pharmacol. 2020;177:2889–2903. 10.1111/bph.15081
F. R. Mariotti and L. Quatrini contributed equally to this work.
REFERENCES
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. ,CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: Introduction and other protein targets. British Journal of Pharmacology, 176(Suppl 1), S1–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre, P. , Denis, C. , Soulas, C. , Bourbon‐Caillet, C. , Lopez, J. , Arnoux, T. , Vivier, E. (2018). Anti‐NKG2A mAb is a checkpoint inhibitor that promotes anti‐tumor immunity by unleashing both T and NK cells. Cell, 175(7), 1731–1743e1713. 10.1016/j.cell.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Sharman, J. L. , et al. (2020). The IUPHAR/BPS Guide to PHARMACOLOGY in 2020: Extending immunopharmacology content and introducing the IUPHAR/MMV Guide to MALARIA PHARMACOLOGY. Nucleic Acids Research, 48(D1), D1006–D1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot, M. , Porcu, P. , Marie‐Cardine, A. , Battistella, M. , William, B. M. , Vermeer, M. , Kim, YH . (2019). IPH4102, a first‐in‐class anti‐KIR3DL2 monoclonal antibody, in patients with relapsed or refractory cutaneous T‐cell lymphoma: An international, first‐in‐human, open‐label, phase 1 trial. The Lancet Oncology, 20(8), 1160–1170. [DOI] [PubMed] [Google Scholar]
- Baitsch, L. , Baumgaertner, P. , Devevre, E. , Raghav, S. K. , Legat, A. , Barba, L. , Speiser, DE. . (2011). Exhaustion of tumor‐specific CD8+ T cells in metastases from melanoma patients. The Journal of Clinical Investigation, 121(6), 2350–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow, A. D. , Martin, C. J. , & Colonna, M. (2019). The natural cytotoxicity receptors in health and disease. Frontiers in Immunology, 10, 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldi‐Ferchiou, A. , Lambert, M. , Dogniaux, S. , Vely, F. , Vivier, E. , Olive, D. , Caillat‐Zucman, S. (2016). PD‐1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget, 7(45), 72961–72977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson, D. M. Jr. , Bakan, C. E. , Mishra, A. , Hofmeister, C. C. , Efebera, Y. , Becknell, B. , Caligiuri, MA. (2010). The PD‐1/PD‐L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT‐011, a novel monoclonal anti‐PD‐1 antibody. Blood, 116(13), 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertone, S. , Schiavetti, F. , Bellomo, R. , Vitale, C. , Ponte, M. , Moretta, L. , ‐ Mingari, MC. (1999). Transforming growth factor‐β‐induced expression of CD94/NKG2A inhibitory receptors in human T lymphocytes. European Journal of Immunology, 29(1), 23–29. [DOI] [PubMed] [Google Scholar]
- Borghaei, H. , Paz‐Ares, L. , Horn, L. , Spigel, D. R. , Steins, M. , Ready, N. E. , … Brahmer, J. R. (2015). Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. The New England Journal of Medicine, 373(17), 1627–1639. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger, M. C. , Zhang, C. , Harter, P. N. , Romanski, A. , Strassheimer, F. , Senft, C. , Wels, W.S. (2019). CAR‐engineered NK cells for the treatment of glioblastoma: Turning innate effectors into precision tools for cancer immunotherapy. Frontiers in Immunology, 10, 2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsten, M. , Korde, N. , Kotecha, R. , Reger, R. , Bor, S. , Kazandjian, D. , Childs, RW. (2016). Checkpoint inhibition of KIR2D with the monoclonal antibody IPH2101 induces contraction and hyporesponsiveness of NK cells in patients with myeloma. Clinical Cancer Research, 22(21), 5211–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carosella, E. D. , Rouas‐Freiss, N. , Tronik‐Le Roux, D. , Moreau, P. , & LeMaoult, J. (2015). HLA‐G: An immune checkpoint molecule. Advances in Immunology, 127, 33–144. 10.1016/bs.ai.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Castriconi, R. , Dondero, A. , Bellora, F. , Moretta, L. , Castellano, A. , Locatelli, F. , Bottino, C. (2013). Neuroblastoma‐derived TGF‐β1 modulates the chemokine receptor repertoire of human resting NK cells. Journal of Immunology, 190(10), 5321–5328. [DOI] [PubMed] [Google Scholar]
- Cekic, C. , Day, Y. J. , Sag, D. , & Linden, J. (2014). Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Research, 74(24), 7250–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerwenka, A. , & Lanier, L. L. (2018). Natural killers join the fight against cancer. Science, 359(6383), 1460–1461. [DOI] [PubMed] [Google Scholar]
- Chamoto, K. , Al‐Habsi, M. , & Honjo, T. (2017). Role of PD‐1 in immunity and diseases. Current Topics in Microbiology and Immunology, 410, 75–97. 10.1007/82_2017_67 [DOI] [PubMed] [Google Scholar]
- Chiossone, L. , & Vivier, E. (2017). Immune checkpoints on innate lymphoid cells. The Journal of Experimental Medicine, 214(6), 1561–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, J. , Deng, Y. , Benson, D. M. , He, S. , Hughes, T. , Zhang, J. , Yu, J. (2014). CS1‐specific chimeric antigen receptor (CAR)‐engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia, 28(4), 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone, E. , Pende, D. , Viale, O. , Than, A. , Di Donato, C. , Orengo, A. M. , et al. (1992). Involvement of HLA class I alleles in natural killer (NK) cell‐specific functions: Expression of HLA‐Cw3 confers selective protection from lysis by alloreactive NK clones displaying a defined specificity (specificity 2). The Journal of Experimental Medicine, 176(4), 963–971. 10.1084/jem.176.4.963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman, D. , Fanger, N. , Borges, L. , Kubin, M. , Chin, W. , Peterson, L. , & Hsu, ML. (1997). A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity, 7(2), 273–282. [DOI] [PubMed] [Google Scholar]
- Croxatto, D. , Martini, S. , Chiossone, L. , Scordamaglia, F. , Simonassi, C. F. , Moretta, L. , Vacca, P. (2017). IL15 induces a potent antitumor activity in NK cells isolated from malignant pleural effusions and overcomes the inhibitory effect of pleural fluid. Oncoimmunology, 6(4), e1293210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, A. , & Long, E. O. (2010). Lytic granule polarization, rather than degranulation, is the preferred target of inhibitory receptors in NK cells. Journal of Immunology, 185(8), 4698–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sousa, L. A. , Leitner, J. , Grabmeier‐Pfistershammer, K. , & Steinberger, P. (2018). Not all immune checkpoints are created equal. Frontiers in Immunology, 9, 1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Chiesa, M. , Carlomagno, S. , Frumento, G. , Balsamo, M. , Cantoni, C. , Conte, R. , Vitale, M. (2006). The tryptophan catabolite l‐kynurenine inhibits the surface expression of NKp46‐ and NKG2D‐activating receptors and regulates NK‐cell function. Blood, 108(13), 4118–4125. [DOI] [PubMed] [Google Scholar]
- Demaria, S. , Ng, B. , Devitt, M. L. , Babb, J. S. , Kawashima, N. , Liebes, L. , ‐ Formenti, SC. (2004). Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. International Journal of Radiation Oncology, Biology, Physics, 58(3), 862–870. [DOI] [PubMed] [Google Scholar]
- Deng, L. , Liang, H. , Burnette, B. , Beckett, M. , Darga, T. , Weichselbaum, R. R. , & Fu, Y. X. (2014). Irradiation and anti‐PD‐L1 treatment synergistically promote antitumor immunity in mice. The Journal of Clinical Investigation, 124(2), 687–695. 10.1172/JCI67313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depil, S. , Duchateau, P. , Grupp, S. A. , Mufti, G. , & Poirot, L. (2020). ‘Off‐the‐shelf’ allogeneic CAR T cells: Development and challenges. Nature Reviews Drug Discovery, 19(3), 185–199. [DOI] [PubMed] [Google Scholar]
- Elhag, O. A. , Hu, X. J. , Wen‐Ying, Z. , Li, X. , Yuan, Y. Z. , Deng, L. F. , Hui, G. (2012). Reconstructed adeno‐associated virus with the extracellular domain of murine PD‐1 induces antitumor immunity. Asian Pacific Journal of Cancer Prevention: APJCP, 13(8), 4031–4036. [DOI] [PubMed] [Google Scholar]
- Fenwick, C. , Loredo‐Varela, J. L. , Joo, V. , Pellaton, C. , Farina, A. , Rajah, N. , Pantaleo, G. . (2019). Tumor suppression of novel anti‐PD‐1 antibodies mediated through CD28 costimulatory pathway. The Journal of Experimental Medicine, 216(7), 1525–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife, B. T. , & Bluestone, J. A. (2008). Control of peripheral T‐cell tolerance and autoimmunity via the CTLA‐4 and PD‐1 pathways. Immunological Reviews, 224, 166–182. [DOI] [PubMed] [Google Scholar]
- Gaggero, S. , Bruschi, M. , Petretto, A. , Parodi, M. , Del Zotto, G. , Lavarello, C. , Cantoni, C. (2018). Nidogen‐1 is a novel extracellular ligand for the NKp44 activating receptor. Oncoimmunology, 7(9), e1470730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallois, A. , Silva, I. , Osman, I. , & Bhardwaj, N. (2014). Reversal of natural killer cell exhaustion by TIM‐3 blockade. Oncoimmunology, 3(12), e946365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galuppini, F. , Dal Pozzo, C. A. , Deckert, J. , Loupakis, F. , Fassan, M. , & Baffa, R. (2019). Tumor mutation burden: From comprehensive mutational screening to the clinic. Cancer Cell International, 19, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi, S. J. , Minn, A. J. , Vonderheide, R. H. , Wherry, E. J. , Hahn, S. M. , & Maity, A. (2015). Awakening the immune system with radiation: Optimal dose and fractionation. Cancer Letters, 368(2), 185–190. [DOI] [PubMed] [Google Scholar]
- Gao, Y. , Souza‐Fonseca‐Guimaraes, F. , Bald, T. , Ng, S. S. , Young, A. , Ngiow, S. F. , … Smyth, M. J. (2017). Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nature Immunology, 18(9), 1004–1015. 10.1038/ni.3800 [DOI] [PubMed] [Google Scholar]
- Garon, E. B. , Rizvi, N. A. , Hui, R. , Leighl, N. , Balmanoukian, A. S. , Eder, J. P. , Gandhi, L. KEYNOTE‐001 Investigators (2015). Pembrolizumab for the treatment of non‐small‐cell lung cancer. The New England Journal of Medicine, 372(21), 2018–2028. [DOI] [PubMed] [Google Scholar]
- Garrido, F. (2019). MHC/HLA class I loss in cancer cells. Advances in Experimental Medicine and Biology, 1151, 15–78. 10.1007/978-3-030-17864-2_2 [DOI] [PubMed] [Google Scholar]
- Gong, J. , Le, T. Q. , Massarelli, E. , Hendifar, A. E. , & Tuli, R. (2018). Radiation therapy and PD‐1/PD‐L1 blockade: The clinical development of an evolving anticancer combination. Journal for Immunotherapy of Cancer, 6(1), 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, C. , Wang, X. , Zhang, H. , Zhi, L. , Lv, T. , Li, M. , Zhu, W. (2019). Structure‐based rational design of a novel chimeric PD1–NKG2D receptor for natural killer cells. Molecular Immunology, 114, 108–113. [DOI] [PubMed] [Google Scholar]
- Hamid, O. , Robert, C. , Daud, A. , Hodi, F. S. , Hwu, W. J. , Kefford, R. , Ribas, A. . (2013). Safety and tumor responses with lambrolizumab (anti‐PD‐1) in melanoma. The New England Journal of Medicine, 369(2), 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. , Chu, J. , Keung Chan, W. , Zhang, J. , Wang, Y. , Cohen, J. B. , Yu, J. (2015). CAR‐engineered NK cells targeting wild‐type EGFR and EGFRvIII enhance killing of glioblastoma and patient‐derived glioblastoma stem cells. Scientific Reports, 5, 11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel, J. J. , Chowell, D. , & Chan, T. A. (2019). The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nature Reviews Cancer, 19(3), 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haymaker, C. L. , Kim, D. , Uemura, M. , Vence, L. M. , Phillip, A. , McQuail, N. , … Diab, A. (2017). Metastatic melanoma patient had a complete response with clonal expansion after whole brain radiation and PD‐1 blockade. Cancer Immunology Research, 5(2), 100–105. 10.1158/2326-6066.CIR-16-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi, F. S. , O'Day, S. J. , McDermott, D. F. , Weber, R. W. , Sosman, J. A. , Haanen, J. B. , Urba, W. J. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. The New England Journal of Medicine, 363(8), 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgaard, R. B. , Zamarin, D. , Munn, D. H. , Wolchok, J. D. , & Allison, J. P. (2013). Indoleamine 2,3‐dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA‐4. The Journal of Experimental Medicine, 210(7), 1389–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y. , Tian, Z. G. , & Zhang, C. (2018). Chimeric antigen receptor (CAR)‐transduced natural killer cells in tumor immunotherapy. Acta Pharmacologica Sinica, 39(2), 167–176. 10.1038/aps.2017.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, R. Y. , Francois, A. , McGray, A. R. , Miliotto, A. , & Odunsi, K. (2017). Compensatory upregulation of PD‐1, LAG‐3, and CTLA‐4 limits the efficacy of single‐agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology, 6(1), e1249561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, W. L. , Pike, L. R. G. , Royce, T. J. , Mahal, B. A. , & Loeffler, J. S. (2018). Safety of combining radiotherapy with immune‐checkpoint inhibition. Nature Reviews Clinical Oncology, 15(8), 477–494. [DOI] [PubMed] [Google Scholar]
- Ishida, Y. , Agata, Y. , Shibahara, K. , & Honjo, T. (1992). Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. The EMBO Journal, 11(11), 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, X. , Kim, J. , Deng, M. , John, S. , Chen, H. , Wu, G. , … Zhang, C. C. (2016). Inhibitory leukocyte immunoglobulin‐like receptors: Immune checkpoint proteins and tumor sustaining factors. Cell Cycle, 15(1), 25–40. 10.1080/15384101.2015.1121324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir, M. E. , Butte, M. J. , Freeman, G. J. , & Sharpe, A. H. (2008). PD‐1 and its ligands in tolerance and immunity. Annual Review of Immunology, 26, 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. Y. , Son, Y. O. , Park, S. W. , Bae, J. H. , Chung, J. S. , Kim, H. H. , Kang, C.D. (2006). Increase of NKG2D ligands and sensitivity to NK cell‐mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Experimental & Molecular Medicine, 38(5), 474–484. [DOI] [PubMed] [Google Scholar]
- Kim, N. , & Kim, H. S. (2018). Targeting checkpoint receptors and molecules for therapeutic modulation of natural killer cells. Frontiers in Immunology, 9, 2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson, K. M. , Hicks, K. C. , Luo, X. , Chen, J. Q. , Schlom, J. , & Gameiro, S. R. (2018). M7824, a novel bifunctional anti‐PD‐L1/TGFβ Trap fusion protein, promotes anti‐tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology, 7(5), e1426519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman, L. A. , Kopcow, H. D. , Rybalov, B. , Boyson, J. E. , Orange, J. S. , Schatz, F. , Strominger, J.L. (2003). Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. The Journal of Experimental Medicine, 198(8), 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger, S. , Legenstein, M. L. , Rosgen, V. , Haas, M. , Modest, D. P. , Westphalen, C. B. , et al. (2017). Serum levels of soluble programmed death protein 1 (sPD‐1) and soluble programmed death ligand 1 (sPD‐L1) in advanced pancreatic cancer. Oncoimmunology, 6(5), e1310358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtulus, S. , Sakuishi, K. , Ngiow, S. F. , Joller, N. , Tan, D. J. , Teng, M. W. , Anderson, A.C. . (2015). TIGIT predominantly regulates the immune response via regulatory T cells. The Journal of Clinical Investigation, 125(11), 4053–4062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lanier, L. L. (2015). NKG2D receptor and its ligands in host defense. Cancer Immunology Research, 3(6), 575–582. 10.1158/2326-6066.CIR-15-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier, L. L. , Corliss, B. C. , Wu, J. , Leong, C. , & Phillips, J. H. (1998). Immunoreceptor DAP12 bearing a tyrosine‐based activation motif is involved in activating NK cells. Nature, 391(6668), 703–707. [DOI] [PubMed] [Google Scholar]
- Li, J. B. , Ruan, Y. Y. , Hu, B. , Dong, S. S. , Bi, T. N. , Lin, A. ,& Yan, W.H. . (2017). Importance of the plasma soluble HLA‐G levels for prognostic stratification with traditional prognosticators in colorectal cancer. Oncotarget, 8(30), 48854–48862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Hermanson, D. L. , Moriarity, B. S. , & Kaufman, D. S. (2018). Human iPSC‐derived natural killer cells engineered with chimeric antigen receptors enhance anti‐tumor activity. Cell Stem Cell, 23(2), 181–192. e185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, A. , & Yan, W. H. (2018). Heterogeneity of HLA‐G expression in cancers: Facing the challenges. Frontiers in Immunology, 9, 2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, A. , & Yan, W. H. (2019). Intercellular transfer of HLA‐G: Its potential in cancer immunology. Clinical & Translational Immunology, 8(9), e1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Cheng, Y. , Xu, Y. , Wang, Z. , Du, X. , Li, C. , Ma, C. (2017). Increased expression of programmed cell death protein 1 on NK cells inhibits NK‐cell‐mediated anti‐tumor function and indicates poor prognosis in digestive cancers. Oncogene, 36(44), 6143–6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren, H. G. , & Karre, K. (1990). In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunology Today, 11(7), 237–244. 10.1016/0167-5699(90)90097-s [DOI] [PubMed] [Google Scholar]
- Long, G. V. , Dummer, R. , Hamid, O. , Gajewski, T. F. , Caglevic, C. , Dalle, S. , Mitchell, T. C. (2019). Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO‐301/KEYNOTE‐252): A phase 3, randomised, double‐blind study. The Lancet. Oncology, 20(8), 1083–1097. [DOI] [PubMed] [Google Scholar]
- Lopez‐Botet, M. , Perez‐Villar, J. J. , Carretero, M. , Rodriguez, A. , Melero, I. , Bellon, T. , Navarro, F. (1997). Structure and function of the CD94 C‐type lectin receptor complex involved in recognition of HLA class I molecules. Immunological Reviews, 155, 165–174. 10.1111/j.1600-065x.1997.tb00949.x [DOI] [PubMed] [Google Scholar]
- Mariathasan, S. , Turley, S. J. , Nickles, D. , Castiglioni, A. , Yuen, K. , Wang, Y. , … Powles, T. (2018). TGFβ attenuates tumour response to PD‐L1 blockade by contributing to exclusion of T cells. Nature, 554(7693), 544–548. 10.1038/nature25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti, F. R. , Petrini, S. , Ingegnere, T. , Tumino, N. , Besi, F. , Scordamaglia, F. , Moretta, L. (2019). PD‐1 in human NK cells: Evidence of cytoplasmic mRNA and protein expression. Oncoimmunology, 8(3), 1557030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti, F. R. , Quatrini, L. , Munari, E. , Vacca, P. , & Moretta, L. (2019). Innate lymphoid cells: Expression of PD‐1 and other checkpoints in normal and pathological conditions. Frontiers in Immunology, 10, 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta, J. , Baratin, M. , Chiche, L. , Forel, J. M. , Cognet, C. , Thomas, G. , … Vivier, E. (2013). Induction of B7‐H6, a ligand for the natural killer cell‐activating receptor NKp30, in inflammatory conditions. Blood, 122(3), 394–404. 10.1182/blood-2013-01-481705 [DOI] [PubMed] [Google Scholar]
- Menier, C. , Riteau, B. , Carosella, E. D. , & Rouas‐Freiss, N. (2002). MICA triggering signal for NK cell tumor lysis is counteracted by HLA‐G1‐mediated inhibitory signal. International Journal of Cancer, 100(1), 63–70. 10.1002/ijc.10460 [DOI] [PubMed] [Google Scholar]
- Mingari, M. C. , Moretta, A. , & Moretta, L. (1998). Regulation of KIR expression in human T cells: A safety mechanism that may impair protective T‐cell responses. Immunology Today, 19(4), 153–157. [DOI] [PubMed] [Google Scholar]
- Mingari, M. C. , Pietra, G. , & Moretta, L. (2019). Immune checkpoint inhibitors: Anti‐NKG2A antibodies on board. Trends in Immunology, 40(2), 83–85. [DOI] [PubMed] [Google Scholar]
- Mingari, M. C. , Ponte, M. , Bertone, S. , Schiavetti, F. , Vitale, C. , Bellomo, R. , Moretta, L. (1998). HLA class I‐specific inhibitory receptors in human T lymphocytes: Interleukin 15‐induced expression of CD94/NKG2A in superantigen‐ or alloantigen‐activated CD8+ T cells. Proceedings of the National Academy of Sciences of the United States of America, 95(3), 1172–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingari, M. C. , Vitale, C. , Cambiaggi, A. , Schiavetti, F. , Melioli, G. , Ferrini, S. ,& Poggi, A. (1995). Cytolytic T lymphocytes displaying natural killer (NK)‐like activity: Expression of NK‐related functional receptors for HLA class I molecules (p58 and CD94) and inhibitory effect on the TCR‐mediated target cell lysis or lymphokine production. International Immunology, 7(4), 697–703. [DOI] [PubMed] [Google Scholar]
- Mingari, M. C. , Vitale, C. , Schiavetti, F. , Cambiaggi, A. , Bertone, S. , Zunino, A. ,& Ponte, M. (1996). HLA‐class I‐specific inhibitory receptors of NK type on a subset of human T cells. Chemical Immunology, 64, 135–145. [PubMed] [Google Scholar]
- Mole, R. H. (1953). Whole body irradiation; radiobiology or medicine? The British Journal of Radiology, 26(305), 234–241. [DOI] [PubMed] [Google Scholar]
- Morandi, F. , Ferretti, E. , Castriconi, R. , Dondero, A. , Petretto, A. , Bottino, C. ,& Pistoia, V. (2011). Soluble HLA‐G dampens CD94/NKG2A expression and function and differentially modulates chemotaxis and cytokine and chemokine secretion in CD56bright and CD56dim NK cells. Blood, 118(22), 5840–5850. [DOI] [PubMed] [Google Scholar]
- Moretta, A. , Bottino, C. , Mingari, M. C. , Biassoni, R. , & Moretta, L. (2002). What is a natural killer cell? Nature Immunology, 3(1), 6–8. 10.1038/ni0102-6 [DOI] [PubMed] [Google Scholar]
- Moretta, A. , Bottino, C. , Vitale, M. , Pende, D. , Biassoni, R. , Mingari, M. C. , & Moretta, L. (1996). Receptors for HLA class‐I molecules in human natural killer cells. Annual Review of Immunology, 14, 619–648. 10.1146/annurev.immunol.14.1.619 [DOI] [PubMed] [Google Scholar]
- Moretta, A. , Bottino, C. , Vitale, M. , Pende, D. , Cantoni, C. , Mingari, M. C. , Moretta, L. (2001). Activating receptors and coreceptors involved in human natural killer cell‐mediated cytolysis. Annual Review of Immunology, 19, 197–223. [DOI] [PubMed] [Google Scholar]
- Moretta, A. , Tambussi, G. , Bottino, C. , Tripodi, G. , Merli, A. , Ciccone, E. , et al. (1990). A novel surface antigen expressed by a subset of human CD3− CD16+ natural killer cells. Role in cell activation and regulation of cytolytic function. The Journal of Experimental Medicine, 171(3), 695–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta, A. , Vitale, M. , Bottino, C. , Orengo, A. M. , Morelli, L. , Augugliaro, R. , … Moretta, L. (1993). P58 molecules as putative receptors for major histocompatibility complex (MHC) class I molecules in human natural killer (NK) cells. Anti‐p58 antibodies reconstitute lysis of MHC class I‐protected cells in NK clones displaying different specificities. The Journal of Experimental Medicine, 178(2), 597–604. 10.1084/jem.178.2.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta, L. , Bottino, C. , Pende, D. , Vitale, M. , Mingari, M. C. , & Moretta, A. (2004). Different checkpoints in human NK‐cell activation. Trends in Immunology, 25(12), 670–676. [DOI] [PubMed] [Google Scholar]
- Moretta, L. , Mingari, M. C. , Pende, D. , Bottino, C. , Biassoni, R. , & Moretta, A. (1996). The molecular basis of natural killer (NK) cell recognition and function. Journal of Clinical Immunology, 16(5), 243–253. 10.1007/bf01541388 [DOI] [PubMed] [Google Scholar]
- Moretta, L. , Montaldo, E. , Vacca, P. , Del Zotto, G. , Moretta, F. , Merli, P. , Mingari, M. C. . (2014). Human natural killer cells: Origin, receptors, function, and clinical applications. International Archives of Allergy and Immunology, 164(4), 253–264. 10.1159/000365632 [DOI] [PubMed] [Google Scholar]
- Munari, E. , Rossi, G. , Zamboni, G. , Lunardi, G. , Marconi, M. , Sommaggio, M. , Bogina, G. (2018). PD‐L1 assays 22C3 and SP263 are not interchangeable in non‐small cell lung cancer when considering clinically relevant cutoffs: An interclone evaluation by differently trained pathologists. The American Journal of Surgical Pathology, 42(10), 1384–1389. [DOI] [PubMed] [Google Scholar]
- Munari, E. , Zamboni, G. , Lunardi, G. , Marchionni, L. , Marconi, M. , Sommaggio, M. , … Moretta, F. (2018). PD‐L1 expression heterogeneity in non‐small cell lung cancer: Defining criteria for harmonization between biopsy specimens and whole sections. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer, 13(8), 1113–1120. 10.1016/j.jtho.2018.04.017 [DOI] [PubMed] [Google Scholar]
- Munari, E. , Zamboni, G. , Lunardi, G. , Marconi, M. , Sommaggio, M. , Brunelli, M. , et al. (2018). PD‐L1 expression comparison between primary and relapsed non‐small cell lung carcinoma using whole sections and clone SP263. Oncotarget, 9(54), 30465–30471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munari, E. , Zamboni, G. , Marconi, M. , Sommaggio, M. , Brunelli, M. , Martignoni, G. , Bogina, G. (2017). PD‐L1 expression heterogeneity in non‐small cell lung cancer: Evaluation of small biopsies reliability. Oncotarget, 8(52), 90123–90131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn, D. H. , & Mellor, A. L. (2007). Indoleamine 2,3‐dioxygenase and tumor‐induced tolerance. The Journal of Clinical Investigation, 117(5), 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narni‐Mancinelli, E. , Gauthier, L. , Baratin, M. , Guia, S. , Fenis, A. , Deghmane, A. E. , et al. (2017). Complement factor P is a ligand for the natural killer cell‐activating receptor NKp46. Science Immunology, 2(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelapu, S. S. , Locke, F. L. , Bartlett, N. L. , Lekakis, L. J. , Miklos, D. B. , Jacobson, C. A. , Go, W. Y. (2017). Axicabtagene ciloleucel CAR T‐cell therapy in refractory large B‐cell lymphoma. The New England Journal of Medicine, 377(26), 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwa, W. , Irabor, O. C. , Schoenfeld, J. D. , Hesser, J. , Demaria, S. , & Formenti, S. C. (2018). Using immunotherapy to boost the abscopal effect. Nature Reviews Cancer, 18(5), 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, C. , Ohm‐Laursen, L. , Barington, T. , Husby, S. , & Lillevang, S. T. (2005). Alternative splice variants of the human PD‐1 gene. Cellular Immunology, 235(2), 109–116. [DOI] [PubMed] [Google Scholar]
- Nirschl, C. J. , & Drake, C. G. (2013). Molecular pathways: Coexpression of immune checkpoint molecules: Signaling pathways and implications for cancer immunotherapy. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 19(18), 4917–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, H. , Nose, M. , Hiai, H. , Minato, N. , & Honjo, T. (1999). Development of lupus‐like autoimmune diseases by disruption of the PD‐1 gene encoding an ITIM motif‐carrying immunoreceptor. Immunity, 11(2), 141–151. [DOI] [PubMed] [Google Scholar]
- Okazaki, T. , & Honjo, T. (2007). PD‐1 and PD‐1 ligands: From discovery to clinical application. International Immunology, 19(7), 813–824. [DOI] [PubMed] [Google Scholar]
- Okazaki, T. , Maeda, A. , Nishimura, H. , Kurosaki, T. , & Honjo, T. (2001). PD‐1 immunoreceptor inhibits B cell receptor‐mediated signaling by recruiting src homology 2‐domain‐containing tyrosine phosphatase 2 to phosphotyrosine. Proceedings of the National Academy of Sciences of the United States of America, 98(24), 13866–13871. 10.1073/pnas.231486598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar, R. , Rivas, C. , Huynh, M. , Omer, B. , Lapteva, N. , Metelitsa, L. S. , Rooney, C. M. (2019). NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR‐T cell activity against solid tumors. Cancer Immunology Research, 7(3), 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. H. , Riviere, I. , Gonen, M. , Wang, X. , Senechal, B. , Curran, K. J. , et al. (2018). Long‐term follow‐up of CD19 CAR therapy in acute lymphoblastic leukemia. The New England Journal of Medicine, 378(5), 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, S. A. , & Minn, A. J. (2018). Combination cancer therapy with immune checkpoint blockade: Mechanisms and strategies. Immunity, 48(3), 417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende, D. , Parolini, S. , Pessino, A. , Sivori, S. , Augugliaro, R. , Morelli, L. , Moretta, A. (1999). Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. The Journal of Experimental Medicine, 190(10), 1505–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot, I. , Michaud, H. A. , Giraudon‐Paoli, M. , Augier, S. , Docquier, A. , Gros, L. , et al. (2019). Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Reports, 27(8), 2411–2425. e2419 [DOI] [PubMed] [Google Scholar]
- Pesce, S. , Greppi, M. , Grossi, F. , Del Zotto, G. , Moretta, L. , Sivori, S. ,& Marcenaro, E. (2019). PD/1‐PD‐Ls checkpoint: Insight on the potential role of NK cells. Frontiers in Immunology, 10, 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce, S. , Greppi, M. , Tabellini, G. , Rampinelli, F. , Parolini, S. , Olive, D. , Marcenaro, E. (2017). Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. The Journal of Allergy and Clinical Immunology, 139(1), 335–346. e333 [DOI] [PubMed] [Google Scholar]
- Pessino, A. , Sivori, S. , Bottino, C. , Malaspina, A. , Morelli, L. , Moretta, L. ,& Moretta, A. (1998). Molecular cloning of NKp46: A novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. The Journal of Experimental Medicine, 188(5), 953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitroda, S. P. , Chmura, S. J. , & Weichselbaum, R. R. (2019). Integration of radiotherapy and immunotherapy for treatment of oligometastases. Lancet Oncology, 20(8), E434–E442. [DOI] [PubMed] [Google Scholar]
- Ponte, M. , Cantoni, C. , Biassoni, R. , Tradori‐Cappai, A. , Bentivoglio, G. , Vitale, C. , Mingari, M. C. (1999). Inhibitory receptors sensing HLA‐G1 molecules in pregnancy: Decidua‐associated natural killer cells express LIR‐1 and CD94/NKG2A and acquire p49, an HLA‐G1‐specific receptor. Proceedings of the National Academy of Sciences of the United States of America, 96(10), 5674–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatrini, L. , Wieduwild, E. , Escaliere, B. , Filtjens, J. , Chasson, L. , Laprie, C. , Ugolini, S. (2018). Endogenous glucocorticoids control host resistance to viral infection through the tissue‐specific regulation of PD‐1 expression on NK cells. Nature Immunology, 19(9), 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintarelli, C. , Sivori, S. , Caruso, S. , Carlomagno, S. , Falco, M. , Boffa, I. , Locatelli, F. (2020). Efficacy of third‐party chimeric antigen receptor modified peripheral blood natural killer cells for adoptive cell therapy of B‐cell precursor acute lymphoblastic leukemia. Leukemia, 34(4), 1102–1115. [DOI] [PubMed] [Google Scholar]
- Rajagopalan, S. , & Long, E. O. (2012). KIR2DL4 (CD158d): An activation receptor for HLA‐G. Frontiers in Immunology, 3, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck, M. , Rodriguez‐Abreu, D. , Robinson, A. G. , Hui, R. , Csoszi, T. , Fulop, A. , KEYNOTE‐024 Investigators (2016). Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. The New England Journal of Medicine, 375(19), 1823–1833. [DOI] [PubMed] [Google Scholar]
- Rizvi, N. A. , Mazieres, J. , Planchard, D. , Stinchcombe, T. E. , Dy, G. K. , Antonia, S. J. , Ramalingam, S. S. (2015). Activity and safety of nivolumab, an anti‐PD‐1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non‐small‐cell lung cancer (CheckMate 063): A phase 2, single‐arm trial. The Lancet. Oncology, 16(3), 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert, C. , Thomas, L. , Bondarenko, I. , O'Day, S. , Weber, J. , Garbe, C. , Wolchok, J. D. (2011). Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England Journal of Medicine, 364(26), 2517–2526. [DOI] [PubMed] [Google Scholar]
- Shi, F. D. , Ljunggren, H. G. , La Cava, A. , & Van Kaer, L. (2011). Organ‐specific features of natural killer cells. Nature Reviews Immunology, 11(10), 658–671. 10.1038/nri3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl, L. M. , Aisner, D. L. , Allen, T. C. , Beasley, M. B. , Borczuk, A. C. , Cagle, P. T. , Members of Pulmonary Pathology Society (2016). Programmed death ligand‐1 immunohistochemistry—A new challenge for pathologists: A perspective from members of the Pulmonary Pathology Society. Archives of Pathology & Laboratory Medicine, 140(4), 341–344. [DOI] [PubMed] [Google Scholar]
- Singh, A. K. , & McGuirk, J. P. (2020). CAR T cells: Continuation in a revolution of immunotherapy. The Lancet. Oncology, 21(3), e168–e178. [DOI] [PubMed] [Google Scholar]
- Siu, L. L. , Burris, H. , Le, D. T. , Hollebecque, A. , Steeghs, N. , Delord, J. P. , Carvajal, R. D. (2018). Preliminary phase 1 profile of BMS‐986179, an anti‐CD73 antibody, in combination with nivolumab in patients with advanced solid tumors. Cancer Research, 78(13). [Google Scholar]
- Sivori, S. , Meazza, R. , Quintarelli, C. , Carlomagno, S. , Della Chiesa, M. , Falco, M. , et al. (2019). NK cell‐based immunotherapy for hematological malignancies. Journal of Clinical Medicine, 8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivori, S. , Parolini, S. , Marcenaro, E. , Castriconi, R. , Pende, D. , Millo, R. ,& Moretta, A. (2000). Involvement of natural cytotoxicity receptors in human natural killer cell‐mediated lysis of neuroblastoma and glioblastoma cell lines. Journal of Neuroimmunology, 107(2), 220–225. [DOI] [PubMed] [Google Scholar]
- Sivori, S. , Vacca, P. , Del Zotto, G. , Munari, E. , Mingari, M. C. , & Moretta, L. (2019). Human NK cells: Surface receptors, inhibitory checkpoints, and translational applications. Cellular & Molecular Immunology, 16(5), 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivori, S. , Vitale, M. , Morelli, L. , Sanseverino, L. , Augugliaro, R. , Bottino, C. , … Moretta, A. (1997). p46, a novel natural killer cell‐specific surface molecule that mediates cell activation. The Journal of Experimental Medicine, 186(7), 1129–1136. 10.1084/jem.186.7.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- So, T. , Takenoyama, M. , Mizukami, M. , Ichiki, Y. , Sugaya, M. , Hanagiri, T. ,& Yasumoto, K. (2005). Haplotype loss of HLA class I antigen as an escape mechanism from immune attack in lung cancer. Cancer Research, 65(13), 5945–5952. [DOI] [PubMed] [Google Scholar]
- Sorensen, S. F. , Demuth, C. , Weber, B. , Sorensen, B. S. , & Meldgaard, P. (2016). Increase in soluble PD‐1 is associated with prolonged survival in patients with advanced EGFR‐mutated non‐small cell lung cancer treated with erlotinib. Lung Cancer, 100, 77–84. [DOI] [PubMed] [Google Scholar]
- Stanietsky, N. , Simic, H. , Arapovic, J. , Toporik, A. , Levy, O. , Novik, A. , Mandelboim, O. (2009). The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America, 106(42), 17858–17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori, S. , Toyokawa, G. , Takada, K. , Shoji, F. , Okamoto, T. , & Maehara, Y. (2018). Combination therapy of radiotherapy and anti‐PD‐1/PD‐L1 treatment in non‐small‐cell lung cancer: A mini‐review. Clinical Lung Cancer, 19(1), 12–16. [DOI] [PubMed] [Google Scholar]
- Tauriello, D. V. F. , Palomo‐Ponce, S. , Stork, D. , Berenguer‐Llergo, A. , Badia‐Ramentol, J. , Iglesias, M. , … Batlle, E. (2018). TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature, 554(7693), 538–543. 10.1038/nature25492 [DOI] [PubMed] [Google Scholar]
- Topalian, S. L. , Hodi, F. S. , Brahmer, J. R. , Gettinger, S. N. , Smith, D. C. , McDermott, D. F. , Szenol, M. (2012). Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. The New England Journal of Medicine, 366(26), 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumino, N. , Martini, S. , Munari, E. , Scordamaglia, F. , Besi, F. , Mariotti, F. R. , Moretta, L. (2019). Presence of innate lymphoid cells in pleural effusions of primary and metastatic tumors: Functional analysis and expression of PD‐1 receptor. International Journal of Cancer, 145(6), 1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca, P. , Martini, S. , Mingari, M. C. , & Moretta, L. (2013). NK cells from malignant pleural effusions are potent antitumor effectors: A clue for adoptive immunotherapy? Oncoimmunology, 2(4), e23638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneman, M. , & Dranoff, G. (2012). Combining immunotherapy and targeted therapies in cancer treatment. Nature Reviews Cancer, 12(4), 237–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vey, N. , Karlin, L. , Sadot‐Lebouvier, S. , Broussais, F. , Berton‐Rigaud, D. , Rey, J. , Goncalves, A. (2018). A phase 1 study of lirilumab (antibody against killer immunoglobulin‐like receptor antibody KIR2D; IPH2102) in patients with solid tumors and hematologic malignancies. Oncotarget, 9(25), 17675–17688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viel, S. , Marcais, A. , Guimaraes, F. S. , Loftus, R. , Rabilloud, J. , Grau, M. , Walzer, T. (2016). TGF‐β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Science signaling, 9(415), ra19. [DOI] [PubMed] [Google Scholar]
- Vitale, M. , Bottino, C. , Sivori, S. , Sanseverino, L. , Castriconi, R. , Marcenaro, E. , Moretta, A. (1998). NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non‐major histocompatibility complex‐restricted tumor cell lysis. The Journal of Experimental Medicine, 187(12), 2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, M. , Castriconi, R. , Parolini, S. , Pende, D. , Hsu, M. L. , Moretta, L. , Moretta, A. (1999). The leukocyte Ig‐like receptor (LIR)‐1 for the cytomegalovirus UL18 protein displays a broad specificity for different HLA class I alleles: Analysis of LIR‐1+ NK cell clones. International Immunology, 11(1), 29–35. [DOI] [PubMed] [Google Scholar]
- Walunas, T. L. , Bakker, C. Y. , & Bluestone, J. A. (1996). CTLA‐4 ligation blocks CD28‐dependent T cell activation. The Journal of Experimental Medicine, 183(6), 2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas, T. L. , Lenschow, D. J. , Bakker, C. Y. , Linsley, P. S. , Freeman, G. J. , Green, J. M. , … Bluestone, J. A. (1994). CTLA‐4 can function as a negative regulator of T cell activation. Immunity, 1(5), 405–413. 10.1016/1074-7613(94)90071-x [DOI] [PubMed] [Google Scholar]
- Xie, G. , Gu, D. , Zhang, L. , Chen, S. , & Wu, D. (2017). A rapid and systemic complete response to stereotactic body radiation therapy and pembrolizumab in a patient with metastatic renal cell carcinoma. Cancer Biology & Therapy, 18(8), 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan, M. , Hopkins, A. , & Jaffee, E. M. (2017). Tumor mutational burden and response rate to PD‐1 inhibition. The New England Journal of Medicine, 377(25), 2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, Z. , Fromm, A. , Ahmed, K. A. , Grass, G. D. , Yang, G. Q. , Oliver, D. E. , Perez, B. A. . (2017). Radiotherapy rescue of a nivolumab‐refractory immune response in a patient with PD‐L1‐negative metastatic squamous cell carcinoma of the lung. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer, 12(9), e135–e136. [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Hu, Y. , & Shi, C. (2020). Targeting natural killer cells for tumor immunotherapy. Frontiers in Immunology, 11, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. , Bi, J. , Zheng, X. , Chen, Y. , Wang, H. , Wu, W. , … Tian, Z. (2018). Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti‐tumor immunity. Nature Immunology, 19(7), 723–732. 10.1038/s41590-018-0132-0 [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Schwartz, J. C. , Guo, X. , Bhatia, S. , Cao, E. , Lorenz, M. , Almo, S. C. (2004). Structural and functional analysis of the costimulatory receptor programmed death‐1. Immunity, 20(3), 337–347. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Zheng, Y. , Wang, Z. , Huang, S. , Chen, Y. , Jiang, W. , Qi, H. (2014). Methotrexate‐loaded PLGA nanobubbles for ultrasound imaging and synergistic targeted therapy of residual tumor during HIFU ablation. Biomaterials, 35(19), 5148–5161. [DOI] [PubMed] [Google Scholar]
- Zheng, W. , Skowron, K. B. , Namm, J. P. , Burnette, B. , Fernandez, C. , Arina, A. , Weichselbaum, R. R. . (2016). Combination of radiotherapy and vaccination overcomes checkpoint blockade resistance. Oncotarget, 7(28), 43039–43051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, X. , Tumang, J. R. , Gao, W. , Bai, C. , & Rothstein, T. L. (2007). PD‐L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for VH11/VH12 and phosphatidylcholine binding. European Journal of Immunology, 37(9), 2405–2410. [DOI] [PubMed] [Google Scholar]
- Zitvogel, L. , Tesniere, A. , & Kroemer, G. (2006). Cancer despite immunosurveillance: Immunoselection and immunosubversion. Nature Reviews Immunology, 6(10), 715–727. [DOI] [PubMed] [Google Scholar]


