Abstract
Primary central nervous system lymphoma (PCNSL) patients have a poorer prognosis than systemic lymphoma. Gain-of-function MYD88 c.794T>C (p. L265P) mutation and programmed cell death-1 (PD-1) pathway alterations are potential targetable pathways. Our study objective was to determine the clinicopathologic correlates of MYD88 mutation and PD-1 alterations in PCNSL and the impact of Epstein-Barr virus (EBV) infection. We studied 53 cases including 13 EBV-associated (EBVpos) PCNSL, 49% harbored MYD88 mutation, none seen in EBVpos PCNSL. MYD88 protein expression did not correlate with MYD88 mutation. T-cell and macrophage infiltration was common. All PD-L1 positive tumors were EBVpos. Two PD-L1 positive tumors showed 9p24.1/PD-L1 locus alterations by Fluorescence In Situ Hybridization. T cells and macrophages expressed PD-1 and/or PD-L1 in 98% and 83% cases, respectively. MYD88 mutation or protein expression and PD-1 or PD-L1 expression did not predict outcome. We hypothesize that EBVpos PCNSL has a distinct activation mechanism, independent of genetic alterations.
Keywords: PD-1, Checkpoint blockade, MYD88, Epstein-Barr virus, PCNSL
Introduction:
Primary central nervous system lymphoma (PCNSL) is an aggressive lymphoma with a unique pathobiology, which can be leveraged to develop novel therapeutic approaches. Most cases have diffuse large B-cell lymphoma (DLBCL) histology and a worse prognosis than systemic DLBCL [1]. More than 95% PCNSL cases are of activated B-cell like (ABC) subtype [2]. Epstein-Barr virus (EBV) infection is present in a subset,, particularly associated with an immunocompromised state. The impact of EBV infection on cellular pathways and the tumor microenvironment in EBV-associated (EBVpos) lymphomas has been described [3, 4]. Moreover, recent advances have identified key pathogenic abnormalities in PCNSL, including mutations in the B-cell receptor pathway (CD79B) [5], toll-like receptor (TLR) pathway (MYD88) [6] and the BCL10-CARD11-MALT1 (BCM) complex (CARD11) [7]. These molecular alterations converge on the NF-kB signaling axis, an anti-apoptotic pathway involved in lymphoma pathogenesis, especially ABC-type DLBCL [8, 9, 10, 11].
MYD88 is an adaptor protein for TLRs and several cytokine-signaling pathways. MYD88 mediates the formation of a protein complex containing IRAK1 and IRAK4 kinases. This results in the activation of IRAK4 kinase and phosphorylation of IRAK1, which further mediates downstream activation of NF-κB. Gain-of-function mutations in MYD88 were first described in ABC-type DLBCL by Ngo et al and were found to be driver mutations in up to 29% of cases [12]. The most commonly identified mutation results in an amino acid substitution, L265P, altering a residue in the MYD88 Toll/IL-1 receptor (TIR) domain. This MYD88 c.794T>C (p.L265P) mutation was reported to occur at an even higher frequency (>80%) in lymphoplasmacytic lymphoma (LPL) [13] and recent studies have shown a high frequency of the MYD88 L265P mutation in sanctuary site DLBCLs, including PCNSL and testicular lymphomas [11].
The role of the tumor microenvironment (TME) in promoting tumor progression has been recognized across several malignancies. Immune checkpoints including programmed death 1 (PD-1) and its ligand, programmed death ligand-1 (PD-L1) play an important role in mediating tumor immune escape [14, 15, 16, 17, 18]. Genetic evaluation of PCNSL cases has revealed copy number abnormalities (CNA) of the 9p24.1/PD-L1 locus [11]. In addition, the presence of PD-L1 protein expression in PCNSL [19, 20] has led to studies incorporating immunotherapeutic strategies for PCNSL t with early evidence of clinical activity of nivolumab in relapsed/refractory PCNSL [21].
The presence of MYD88 L265P mutation has been associated with decreased progression-free survival (PFS) and overall survival (OS) in systemic DLBCL [22], however, the clinical impact of this mutation in PCNSL is not well described. Moreover, the relationship between MYD88 protein expression and the MYD88 L265P mutation in PCNSL has not been reported. Similarly the impact of PD-1 pathway markers on systemic DLBCL outcome has been evaluated, however, the corresponding data for PCNSL are sparse [23]. Finally, the frequency of co-occurrence of MYD88 mutation and PD-1 pathway markers in PCNSL and the impact of EBV infection on these markers has not been reported.
The present study evaluates the prognostic significance of therapeutically relevant alterations in MYD88 and PD-1 pathways. The primary objective of this study was to examine the clinicopathologic correlates and co-occurrence of MYD88 mutation and PD-1 pathways in PCNSL and their impact on clinical outcome. The secondary objectives were to determine the relationship between MYD88 mutation and MYD88 protein expression in tumor cells and to study the differences in prevalence of MYD88 mutation and PD-1 pathway markers in EBVpos and EBV negative (EBVneg) PCNSL. Expanding knowledge of these pathways can help support clinical trials of agents targeting these pathways in PCNSL and also inform the use of rational combinations and targeted correlative studies.
Materials and Methods:
Patient Selection
After obtaining institutional review board (IRB) approval with waiver of consent, clinical data and archived tumor biopsy specimens from 53 PCNSLs [DLBCL histology including post-transplant lymphoproliferative disease (PTLD) involving the CNS] were analyzed. Patients were eligible for the study if they were at least 18 years of age and diagnosed with PCNSL between 2000 and 2016 at two academic medical centers, Vanderbilt University Medical Center (VUMC) and the University of North Carolina Medical Center (UNCMC) at Chapel Hill. In addition, patients were required to have adequate archived tissue specimens. Archived formalin-fixed paraffin-embedded (FFPE) tissue biopsy material was identified from the files of the Department of Pathology, Microbiology & Immunology (PMI) at VUMC and the Department of Pathology and Laboratory Medicine at UNCMC. Biopsies of secondary CNS involvement in cases of systemic lymphoma were excluded.
Pathologic Review
Studies and analyses were performed at VUMC. Two pathologists (A.E.K and M.A.T) reviewed the cases and reported results are based on consensus findings. Pathology reports and hematoxylin-and-eosin (H&E)-stained sections were reviewed. World Health Organization (WHO) histologic subtype was assigned to each case [24]. If previously documented, cell-of-origin classified by the Hans algorithm (CD10, BCL6, and/or MUM1 immunohistochemistry) [25] and EBV-encoded RNA (EBER) in situ hybridization results were recorded. Tumor content on the single tissue block subsequently used for additional studies was estimated to the nearest decile. In tumor-rich areas, tumor density (estimated to the nearest decile) and growth pattern (diffuse, scattered, perivascular) were recorded. Unstained 5-µm tissue sections on glass slides were prepared for immunohistochemistry and in situ hybridization, and 3–5 tissue curls (10–50µm) in Eppendorf tubes were prepared for DNA extraction and subsequent polymerase-chain reaction (PCR) experiments.
Immunohistochemistry and In Situ Hybridization for EBER
The following immunohistochemical stains were applied to unstained sections of each case after deparaffinization (1-hour incubation, Leica Bond Max IHC stainer, Buffalo Grove, IL): anti-Pax-5 (PA0552, Ready-To-Use, Leica, Buffalo Grove, IL); anti-CD3 (NCL-CD3-PS1, 1:100, Leica, Newcastle, UK); anti-CD68 (PA0191, Ready-To-Use, Leica, Buffalo Grove, IL); anti-PD-L1 (CD274, 13684, 1:100, Cell Signaling, Danvers, MA); anti-PD-1 (PDCD1, HPA035981, 1:75, Sigma-Aldrich Co., St. Louis, MO); and anti-MYD88 (ab133739, 1:500, Abcam, Cambridge, MA). Heat-induced antigen retrieval was performed on the Bond Max with Epitope Retrieval 2 solution for Pax-5, CD3, and PD-1 (20 minutes); CD68 and PD-L1 (5 minutes); and MYD88 (10 minutes). For PD-L1, slides were placed in Protein Block (x0909, Dako, Carpenteria, CA). For EBER in situ hybridization, enzyme retrieval was performed using Proteinase K (Ref# S3020, Dako, Santa Clara, CA) for 5 minutes. Slides underwent ISH with the Ready-To-Use EBER probe (ISH5687-A, Leica) for two hours followed by incubation with an anti-fluorescein antibody (AR0222, Leica) for 15 minutes.
The Leica Bond Polymer Refine system was used for visualization of all stains. Slides were dehydrated, cleared, and coverslipped. Positive control tissues included benign tonsil (all immunohistochemical stains), placenta (anti-PD-L1), kidney (anti-MYD88), and EBV-infected tonsil (EBER). Positive RNA control staining for EBER could not be performed due to insufficient material in a majority of cases.
Pax-5 expression was used to confirm tumor content, density, and growth pattern estimated by H&E stain. T-cell content within tumor rich-areas was estimated by CD3 expression. Macrophage content within tumor rich-areas was estimated by CD68 expression. PD-L1 expression on macrophages and tumor cells and PD-1 expression on T cells and tumor cells was recorded as positive or negative. MYD88 expression was graded on a semiquantitative 0-to-3-point scale for each density and intensity of expression as previously described [26, 27]. Low expression was defined as a score of 2–4 and high expression as a score of 5–6. EBER was recorded as positive or negative in tumor cells. These methods are summarized in Supplemental Table S1. Method details for fluorescence in situ hybridization for 9p24.1 locus and allele-specific PCR for MYD88 c.794T>C p.L265P mutation analysis are included in the supplement.
Disease Variables, Treatment, and Response Assessment
Clinical, laboratory, and outcome data were obtained from the electronic medical record. We collected baseline data for prognostic factors including age, performance status (PS), elevated serum lactate dehydrogenase (LDH), high cerebrospinal fluid (CSF) protein concentration, and tumor involvement of deep brain regions (periventricular regions, basal ganglia, brainstem, and/or cerebellum) [28]. Performance status was assessed using the Eastern Cooperative Oncology Group (ECOG) scale. Data regarding the use of high-dose methotrexate (HDMTX) and radiation were collected. Response was assessed based on International Primary CNS Lymphoma Collaborative Group (IPCG) criteria, including brain imaging, eye examination, and CSF cytology [29]. Patient data were collected from the date of diagnosis to the date of last follow-up or death. We included patients diagnosed at least 12 months prior to data collection start date to achieve a stable cohort and minimize attrition bias. To minimize information bias, the personnel collecting clinical data were initially blinded to pathology results and vice versa.
Statistical Plan
Outcome Definitions
PFS was defined as the time between date of diagnosis and date of progression or last clinical contact. OS was defined as the time between the date of diagnosis and date of death or last clinical contact.
Sample Size Justification
PCNSL is rare, and sample size was limited by specimen availability in this clinicopathologic correlative study. Tissue availability is often limited due to small stereotactic biopsies. Our cohort of 53 patients is relatively large compared with the majority of reported clinicopathologic studies in PCNSL. The sample size allowed inclusion of 2–3 co-variates for multivariable analysis of PFS (failure n= 29) and OS (failure n= 34). This was based on the rule of thumb of one covariate per 10–15 failure events (for survival) to provide tighter confidence intervals for our estimates of hazard ratios [30]. The covariate selection was pre-specified, determined by clinical significance and included MSKCC prognostic score[31] and HDMTX treatment.
Analysis Plan
Continuous variables were described using median and range, and categorical variables using number and percentage. Univariate analyses were performed using chi-square or Fisher’s exact tests for categorical variables and Mann-Whitney U tests for continuous variables. PFS and OS (months) were estimated by the Kaplan-Meier method and compared using a log-rank test. A Cox proportional-hazards regression model determined the effect of disease-related variables on PFS and OS. Two-sided 95% confidence intervals (95% CI) were reported for all estimates. A two-sided test with p-value < 0.05 was considered statistically significant. Statistical analyses were performed using Stata/IC 14.2 software.
Results:
Patient, Disease Characteristics, and Treatment
Fifty-three patients were included in our analysis. Median age at diagnosis was 59 years (range 21–84 yrs.). Twenty-seven patients (51%) were male. Four patients (7.5%) succumbed to disease prior to receiving treatment. The patient and disease characteristics of the complete cohort are summarized in Table I. Baseline characteristics and pathologic feature comparison by immune status are included in supplemental tables S2 and S3, respectively. Comparison by MYD88 L265P mutation status is outlined in table S4.
Table I:
Patient Characteristics
| N* | All Patients# | |
|---|---|---|
| Median age, years (range) | 53 | 59 (21–84) |
| Male | 53 | 51% (27) |
| ECOG performance status ≥ 2 | 53 | 58% (31) |
| MSKCC Class 1 2 3 |
53 |
34%(18) 25%(13) 41% (22) |
| LDH elevated | 46 | 50% (23) |
| Median ALC at Diagnosis (range) | 50 | 110–4080 |
| Elevated CSF Protein | 35 | 80% (28) |
| Deep Brain Involvement | 53 | 55% (29) |
| Multiple Foci of Involvement | 53 | 47% (25) |
| CSF involvement | 51 | 12% (6) |
| Ocular Involvement | 53 | 8% (4) |
| HIV positive | 53 | 23% (12) |
| Post-organ transplant | 53 | 6% (3) |
| High-dose methotrexate | 53 | 72% (38) |
| Whole-brain radiation therapy | 53 | 42% (22) |
N is number of non-missing values.
Numbers after proportions are frequencies unless otherwise specified
HIV: human immunodeficiency virus; ECOG: Eastern Cooperative Oncology Group; MSKCC: Memorial Sloan Kettering Cancer Center; LDH: lactate dehydrogenase; ALC: absolute lymphocyte count; CSF: cerebrospinal fluid
Cell of Origin and EBER
Data for cell-of-origin d by immunohistochemistry were available for 50 patients, of which 42 showed an ABC-like immunophenotype (84%). EBER-ISH was positive in 25% (13/52) of cases. Ten of the EBVpos cases were associated with human immunodeficiency virus (HIV). The remaining 3 EBVpos cases were associated with PTLD in the CNS. EBV status was unknown for one patient.
Tumor Content, T-Cell and Macrophage Infiltration, and PD-L1/PD-1 Expression
Tumor content of the tissue sections was estimated from H&E and PAX-5 stains and showed 5–100% tumor (only 3 cases had <20% tumor content). Fifteen cases (28%) showed PD-L1 and/or PD-1 expression by lymphoma cells: 8 cases (15%) that were PD-L1 positive and 8 cases (15%) that were PD-1 positive (including one case that was positive for both PD-L1 and PD-1). In 52 cases (98%), T cells (identified by CD3 positivity) expressed PD1, and in 44 cases (83%), tumor-associated macrophages (TAMs) (identified by CD68 positivity) showed PD-L1 expression (Table II). Figure 1 shows representative photomicrographs from a 28-year-old male patient with HIV.
Table II:
Pathologic Characteristics
| N* | All Patients# | |
|---|---|---|
| EBER-ISH positive | 52 | 25% (13) |
| Tumor content (PAX-5) ≤20% >20% |
51 | 6% (3) 94% (48) |
| T cell infiltration (CD3) Low (<5%) Moderate (5–20%) High (>20%) |
53 | 30% (16) 32% (17) 38% (20) |
| Macrophage infiltration (CD68) Low (<5%) Moderate (5–20%) High (>20%) |
52 | 2% (1) 44% (23) 54% (28) |
| Tumor PD-L1 expression | 52 | 15% (8) |
| Tumor PD-1 expression | 53 | 15% (8) |
| Tumor PD-L1 and PD-1 expression | 53 | 28% (15) |
| PD-L1 expression on macrophages | 53 | 83% (44) |
| PD-1 on T cells | 53 | 98% (52) |
N is number of non-missing values.
Numbers after proportions are frequencies unless otherwise specified
HIV: human immunodeficiency virus, EBER-ISH: Epstein-Barr virus-encoded ribonucleic acid in situ hybridization
Figure 1.
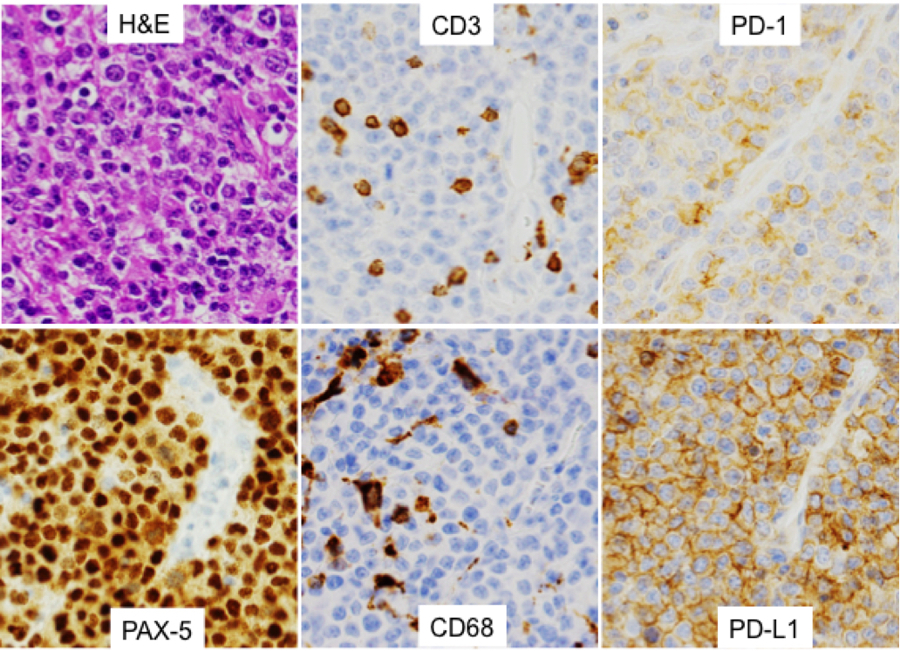
Representative photomicrographs. A) Hematoxylin-and-eosin (H&E)-stained section of a left frontal lobe mass from a 28-year-old male with HIV. The lymphoma cells are large with round to oval nuclei, coarse vesicular chromatin, distinct nucleoli, and scant cytoplasm. B) PAX5 immunostain highlights the lymphoma cells, confirming their B-cell origin. C) CD3 immunostain highlights tumor-associated small T lymphocytes. In this case, T cells represent approximately 15% of the overall specimen cellularity. D) CD68 highlights tumor-associated macrophages, in this case also representing approximately 30% of the cellularity. In this case, the lymphoma cells express both PD-L1 (E) and PD-1 (F). All images were obtained at 400x magnification.
EBER and PD-1/PD-L1 Expression
All 8 cases with tumor cell PD-L1 expression were associated with EBER positivity by ISH. Conversely, 8/12 of EBVpos cases (67%) had tumor cell PD-L1 expression (PD-L1 not tested in one EBVpos case. None of the EBVneg cases showed tumor cell PD-L1 expression (P=0.001). One of the 13 EBVpos cases (7.7%) showed tumor cell PD-1 expression, while 7/39 EBVneg cases (18%) showed tumor cell PD-1 expression (P= 0.66).
9p24.1/PD-L1 Locus Abnormalities by FISH
9p24.1/PD-L1 FISH was performed on 48 cases; for the remaining cases, testing was limited by the tissue availability. One case revealed CNA,and another case revealed amplification of the PD-L1 gene locus. The corresponding IHC slides also showed tumor PD-L1 expression. These two cases were negative for MYD88 L265P mutation, both were EBVpos, and one also had tumor PD-1 expression.
MYD88 Protein Expression and MYD88 c.794T>C (p.L265P) Mutation Analysis
MYD88 protein expression was seen in 50/52 cases (94%) and varied widely by intensity and density of expression. Thirty-six patients (69%) showed high-level MYD88 expression. MYD88 mutation test was performed in 33 of these patients and a total of 13/33 (39%) harbored the MYD88 L265P mutation. There was no correlation between high MYD88 expression and presence of MYD88 L265P mutation (P= 0.12). MYD88 L265P mutation results were available for 47 of 53 cases; for remaining cases, testing was limited by exhaustion of the tissue within the available tissue block(s). Forty-nine percent (49%, or 23/47) of cases were positive for the MYD88 L265P mutation. Figure 2 shows the relationship between MYD88 protein expression score and mutation status. Figure 3 depicts photomicrograph examples of variable MYD88 expression scores.
Figure 2:
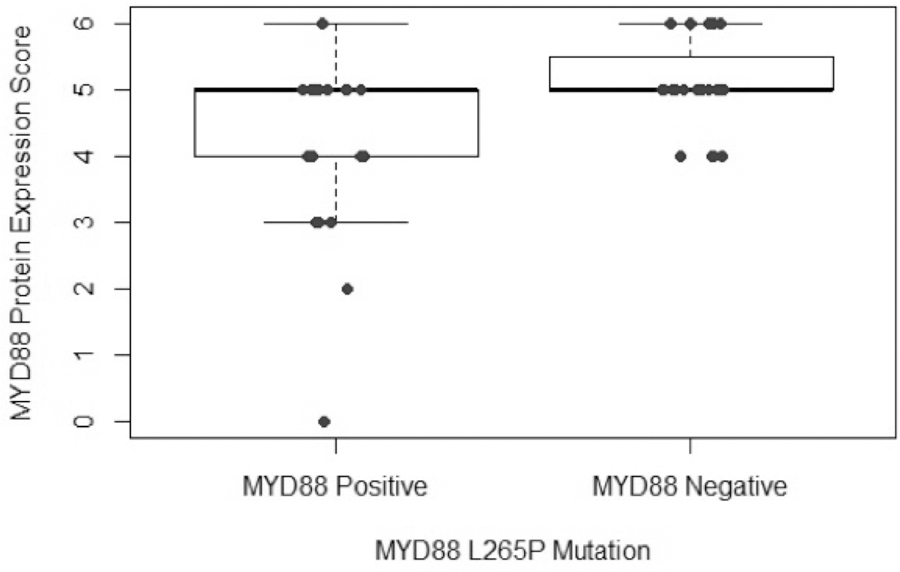
Plot representing the MYD88 protein expression score (by IHC) vs. MYD88 L265P mutation by PCR
Figure 3:
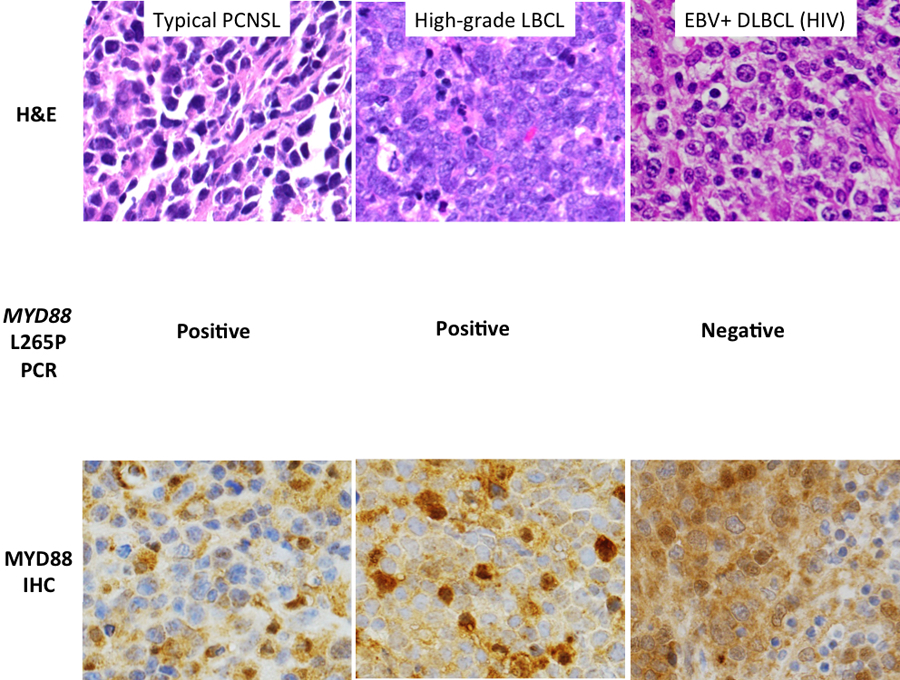
MYD88 immunostain results of representative cases (each photomicrograph 400x magnification): A) Low overall MYD88 expression score of 3 (2/3 for density of tumor-cell expression and 1/3 for level of staining intensity) in a typical case of PCNSL; B) moderate overall expression score (1/3 for density, 3/3 for intensity) in a PCNSL case with a germinal center immunophenotype; and C) high overall expression score (2/3 for density, 3/3/ for intensity) in a case of EBVpos DLBCL isolated to the CNS.
EBER, Tumor PD-1/PD-L1 Expression and MYD88 L265P Mutation:
All 12 EBVpos cases lacked MYD88 L265P mutation, whereas 22 out of 34 (65%) EBVneg cases harbored MYD88 L265P mutation (P=0.001). Figures S2 and S3 show the overlap between MYD88 L265P, EBV status and Tumor PD-1/PD-L1 expression. In our study cohort all tumor PD-L1 positive cases were also EBVpos and lacked MYD88 L265P mutation.
Treatment Response, Progression-Free Survival, and Overall Survival
Thirty-eight (72%) patients received high-dose methotrexate based therapy. The complete response rate in 42 patients was 29%. At a median follow-up of 10.3 months, median PFS for all patients was 11.47 months (95% CI: 7.77 to 15.16). The median OS was 12.27 months (95% CI: 4.43 to 20.11). The median PFS for HIV-negative and HIV-positive cohorts was 11.23 and 11.47 months, respectively (P=0.86); the corresponding median OS was 12.4 and 3.8 months, respectively (P=0.89). The median PFS for MYD88 L265P mutation-positive and mutation-negative cases was 8.83 months versus 12.4 months, respectively (P=0.55); the corresponding median OS was 12.27 months versus 12.4 months, respectively (P=0.99). The median PFS for patients with tumor cell PD-L1 expression by IHC was not reached versus 11.47 months in negative cases (P=0.29); the corresponding median OS was 12.76 months versus 12.27 months, respectively (P=0.65). The median PFS for patients with tumor cell PD-1 expression was 12.4 months versus 10.17 months in negative cases (P=0.94); the corresponding median OS for these patients was 12.4 months versus 12.27 months, respectively (P=0.92). The median PFS for patients with PD-L1 expression in macrophages was 11.47 months compared with 8.83 months (P=0.34) for patients lacking PD-L1 expression; the corresponding median OS for these patients was 12.27 months versus 24.77 months, respectively (P=0.49). In multivariate analysis using Cox proportional hazards model with MSKCC class, HD MTX, MYD88 L265P mutation and tumor cell PD-L1 expression, only higher MSKCC class [HR 4.03 (95% CI 1.42 to 11.44; P=0.008), and lack of HD MTX treatment [HR 4.89 (95% CI 1.73 to 13.83; P=0.002) were predictive of OS. None of these co-variates impacted PFS. In the corresponding models with tumor cell PD-1 and microenvironment PD-L1 expression, MSKCC class and HDMTX treatment remained predictive of OS with no effect on PFS. Figure 4 outlines the impact of MYD88 L265P mutation, tumor PD-1 and PD-L1 expression on survival. Supplemental figures S5 and S6 show additional survival curves.
Figure 4:
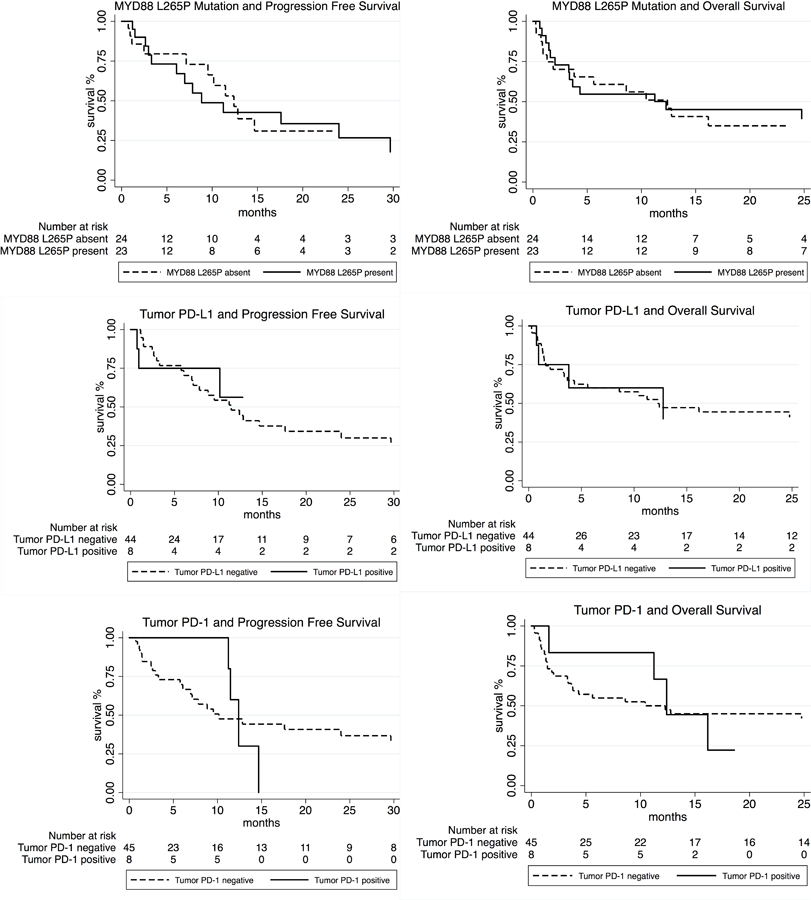
Survival curves outlining the impact of MYD88 L265P mutation, tumor cell PD-L1 and PD-1 expression on PFS and OS.
Discussion:
This study extends our knowledge of the clinical, pathologic, and immune correlates of PCNSL focusing on MYD88 L265P mutations and PD-1 checkpoint pathway. We report for the first time how MYD88 protein expression in PCNSL relates to an underlying MYD88 L265P mutation. In addition, like prior studies of EBVpos lymphoma, these results support the hypothesis that EBV infection has a distinct effect on tumor microenvironment and oncogenic landscape mediated through viral components rather than underlying genetic mutations.
A majority of the patients in our PCNSL cohort had an ABC-like immunophenotype, as has been previously reported [2]. Previous studies have reported a varying incidence of PD-L1 expression by IHC on PCNSL tumor cells. Berghoff et al reported the presence of PD-L1 expression in 2/20 (10%) EBVneg cases of PCNSL [19]. Other studies have since reported a wide variation of positivity in 4–37% EBV unselected cases [32, 33]. We found no tumor cell PD-L1 expression in our 39 EBVneg cases, whereas 8/12 EBVpos cases (66.7%) showed PD-L1 expression by lymphoma cells. The latter finding is corroborated by several studies of EBVpos lymphoproliferative disorders showing expression of PD-L1 by lymphoma cells [34, 35, 36]. It has been reported that EBV latent membrane protein 1 (LMP1) enhances PD-L1 promoter activity [35], thereby increasing protein expression. This increase often occurs in the absence of underlying 9p24.1 amplification [35], as seen in our study where only 1 of 8 PD-L1-positive EBVpos cases (12.5%) had 9p24.1 amplification.
Tumor cells expressed PD-1 in 7/40 (17%) EBVneg cases, which is comparable to previous reports [19]. Other studies have also shown PD-1 expression by lymphoma cells, however, the pathobiologic significance of this expression remains unknown and may reflect the presence of PD-1 surface marker on the lymphoma clone [37, 38]. Almost all cases (98%) showed PD-1 expression by intratumor T-cells. Similarly, a majority of tumor-associated macrophages (83%) were PD-L1 positive. These findings show that a high proportion of cells in the tumor microenvironment express immune checkpoints and point to a “protective” environment conducive to immune evasion. We also noted an overall higher density of macrophage infiltration compared with T-cell infiltration in the tumor microenvironment.
We also studied the relationship between MYD88 protein expression and MYD88 L265P mutation to determine whether it can be used as a surrogate for the latter. MYD88 protein expression did not positively correlate with MYD88 L265P mutation. Our findings are consistent with the results seen in systemic DLBCL and testicular DLBCL [26, 27]. MYD88 protein expression was seen in 94% cases while MYD88 L265P mutation was detected in one-half of cases, similar to previous reports in PCNSL [6, 10, 11, 39, 40, 41, 42, 43]. This pathway is likely active in most cases of PCNSL, regardless of the presence of an underlying activating MYD88 mutation. None of the EBVpos cases had an underlying MYD88 mutation, compared with 64.7% of the EBVneg cases. While this has not been widely studied in PCNSL, our study is in agreement with the findings in Chapuy et al where MYD88 L265P was absent in an 8-patient cohort of EBVpos PCNSL cases [11] and more recent data by Gandhi et al showing a higher mutation rate in EBVneg cases [44]. Similar findings have been reported for EBVpos DLBCL [45]. Extrapolating from other EBVpos lymphoma studies, most cases involve activation of NF-kB [46, 47, 48], likely mediated by LMP1 [49], rather than an underlying gene mutation [45].
HIV status, MYD88 L265P mutation, MYD88 protein expression, tumor cell PD-L1 or PD-1 expression, or tumor microenvironment PD-L1 expression (primarily macrophages) were not predictive of PFS or OS. Very few studies have evaluated the effect of these factors on outcome. In a 42-patient cohort of HIV-negative PCNSL patients, Hattori et al reported that MYD88 L265P mutation was associated with a trend towards a negative impact on survival, highlighting that larger pooled cohorts would be better suited to revealing any significant impact of these biomarkers on patient outcome [42]. Examining immune markers in PCNSL, Cho et al reported PD-1 expression (PD-1 ≥70 cells/HPF) to be associated with worse PFS and OS. This study used a different cut off PD-1 expression and also did not distinguish between tumor cell and tumor microenvironment expression; thus, it is difficult to compare these results with those of our study [50].
Limitations of our study include a relatively small size to examine the effect of all clinically relevant covariates on survival. The inclusion of patients based on tissue availability can introduce a selection bias, but is difficult to avoid in a clinicopathologic study of a relatively rare tumor. We collected baseline prognostic factors and additional variables such as HIV infection, EBV infection and treatment variables that can potentially affect the outcome to minimize selection bias. An additional limitation of our study is the lack of precise quantitation of PD-1 and PD-L1 expression on cell types. A number of technical factors inherent to the tissue of interest in this study are associated with staining heterogeneity, both among slides (different cases) and within a given slide (single tissue fragment) for each immunohistochemical stain. Technical factors that we have identified include adjacent paucicellular protein-rich brain tissue, partial tumor necrosis (common), edge artefact related to variable penetration of formalin fixation, the archival nature of the material and variable tissue ages, and differences in tissue fixation between the two institutions from which we are drawing cases. Attempts at comparative image analysis on successive tissue sections in this setting are proving challenging.
Strengths of our study include a detailed evaluation of pathways with translational relevance in PCNSL treatment, this is especially important in informing correlative studies with limited tissue availability. Furthermore, the inclusion of immunocompromised patients expands knowledge regarding this cohort that is often excluded from other studies.
In conclusion, our results support and can potentially inform correlative studies in novel drug combination trials in PCNSL, specifically those with checkpoint inhibitors and NF-κB pathway directed agents like ibrutinib and lenalidomide. For the latter, MYD88 protein expression should be evaluated as a potential predictive marker along with MYD88 L265P mutational status since a larger proportion of cases show MYD88 protein expression. In these cases, MYD88 may be upregulated by a mechanism other than mutation. Another important consideration for correlative studies is the difference in EBVpos and EBVneg PCNSL. Furthermore, future studies that pool data from several centers for this rare disease and the use of uniform criteria for defining the abnormalities in pathogenic pathways will help to better determine the prognostic significance of these markers while controlling for clinical prognostic markers.
Supplementary Material
Acknowledgments:
Tissue Processing Support
Vanderbilt Translational Pathology Shared Resource
Supported by NCI/NIH Cancer Center Support Grant 2P30 CA068485–14 and the Vanderbilt Mouse Metabolic Phenotyping Center Grant 5U24DK059637–13
Cytogenetic Studies: Amibeth Hollis helped with FISH studies
Funding
This study was supported by the National Center for Advancing Translational Sciences under CTSA award (no. UL1TR000445 and UL1 TR002243) and Vanderbilt University Medical Center, Department of Pathology, Microbiology & Immunology under the Translational Research Enhancement Award.
Footnotes
Conflict of Interest Disclosures:
NSG: Sangamo Biosciences (stock)
SIP: BMS, Rafael Pharma, G1 therapeutics, TEVA (consultant); Gilead, Seattle Genetics (Speaker’s Bureau); Takada, Teva, Seattle Genetics, BMS (research funding)
NMR: KITE Pharma, BMS, AbbVie, Celgene (consultant); BMS, Genentech (research funding)
References:
- 1.Miller DC, Hochberg FH, Harris NL, et al. Pathology with clinical correlations of primary central nervous system non-Hodgkin’s lymphoma. The massachusetts general hospital experience 1958-1989. Cancer. 1994;74(4):1383–1397. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri-Broët S, Criniere E, Broët P, et al. A uniform activated B-cell–like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107(1):190–196. [DOI] [PubMed] [Google Scholar]
- 3.Dolcetti R, Dal Col J, Martorelli D, et al. Interplay among viral antigens, cellular pathways and tumor microenvironment in the pathogenesis of EBV-driven lymphomas. Seminars in cancer biology. 2013;23(6, Part A):441–456. [DOI] [PubMed] [Google Scholar]
- 4.Chetaille B, Bertucci F, Finetti P, et al. Molecular profiling of classical Hodgkin lymphoma tissues uncovers variations in the tumor microenvironment and correlations with EBV infection and outcome. Blood. 2009;113(12):2765–3775. [DOI] [PubMed] [Google Scholar]
- 5.Montesinos-Rongen M, Schafer E, Siebert R, et al. Genes regulating the B cell receptor pathway are recurrently mutated in primary central nervous system lymphoma. Acta neuropathologica. 2012;124(6):905–6. [DOI] [PubMed] [Google Scholar]
- 6.Montesinos-Rongen M, Godlewska E, Brunn A, et al. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta neuropathologica. 2011;122(6):791–2. [DOI] [PubMed] [Google Scholar]
- 7.Montesinos-Rongen M, Schmitz R, Brunn A, et al. Mutations of CARD11 but not TNFAIP3 may activate the NF-κB pathway in primary CNS lymphoma. Acta neuropathologica. 2010;120(4):529–535. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Aguilar A, Idbaih A, Boisselier B, et al. Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(19):5203–11. [DOI] [PubMed] [Google Scholar]
- 9.Yamada S, Ishida Y, Matsuno A, et al. Primary diffuse large B-cell lymphomas of central nervous system exhibit remarkably high prevalence of oncogenic MYD88 and CD79B mutations. Leukemia & lymphoma. 2015;56(7):2141–5. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Tateishi K, Niwa T, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathology and applied neurobiology. 2016;42(3):279–90. [DOI] [PubMed] [Google Scholar]
- 11.Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulain S, Roumier C, Decambron A, et al. MYD88 L265P mutation in Waldenstrom macroglobulinemia. Blood. 2013;121(22):4504–11. [DOI] [PubMed] [Google Scholar]
- 14.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 SYSTEM OF T CELL COSTIMULATION. Annual Review of Immunology. 1996;14(1):233–258. [DOI] [PubMed] [Google Scholar]
- 15.Okazaki T, Chikuma S, Iwai Y, et al. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nature immunology. 2013;14(12):1212–1218. [DOI] [PubMed] [Google Scholar]
- 16.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192(7):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(19):12293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zha Y, Blank C, Gajewski TF. Negative regulation of T-cell function by PD-1. Critical reviews in immunology. 2004;24(4):229–37. [DOI] [PubMed] [Google Scholar]
- 19.Berghoff AS, Ricken G, Widhalm G, et al. PD1 (CD279) and PD-L1 (CD274, B7H1) expression in primary central nervous system lymphomas (PCNSL). Clinical neuropathology. 2014;33(1):42–9. [DOI] [PubMed] [Google Scholar]
- 20.Saad N, Anand A, Naous R, et al. Co-Expression of MYC, BCL2 and PD-L1 in Primary Central Nervous System Diffuse Large B Cell Lymphomas (PCNSL). Am Soc Hematology; 2016.
- 21.Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidorova AA, Zvonkov EE, Sudarikov AB, et al. MYD88 L265P Mutation Is a Possible Unfavorable Prognostic Factor in Patients with Diffuse B-Cell Lymphoma. Blood. 2015;126(23):5051. [Google Scholar]
- 23.Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126(19):2193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms [ 10.1182/blood-2016-01-643569]. Blood. 2016;127(20):2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray [ 10.1182/blood-2003-05-1545]. Blood. 2003;103(1):275. [DOI] [PubMed] [Google Scholar]
- 26.Choi JW, Kim Y, Lee JH, et al. MYD88 expression and L265P mutation in diffuse large B-cell lymphoma. Human pathology. 2013;44(7):1375–81. [DOI] [PubMed] [Google Scholar]
- 27.Oishi N, Kondo T, Nakazawa T, et al. High prevalence of the MYD88 mutation in testicular lymphoma: Immunohistochemical and genetic analyses. Pathology international. 2015;65(10):528–35. [DOI] [PubMed] [Google Scholar]
- 28.Ferreri AJM, Blay J-Y, Reni M, et al. Prognostic Scoring System for Primary CNS Lymphomas: The International Extranodal Lymphoma Study Group Experience. Journal of Clinical Oncology. 2003;21(2):266–272. [DOI] [PubMed] [Google Scholar]
- 29.Abrey LE, Batchelor TT, Ferreri AJM, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. Journal of clinical oncology. 2005;23(22):5034–5043. [DOI] [PubMed] [Google Scholar]
- 30.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. American journal of epidemiology. 2007;165(6):710–718. [DOI] [PubMed] [Google Scholar]
- 31.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary Central Nervous System Lymphoma: The Memorial Sloan-Kettering Cancer Center Prognostic Model. Journal of Clinical Oncology. 2006;24(36):5711–5715. [DOI] [PubMed] [Google Scholar]
- 32.Four M, Cacheux V, Tempier A, et al. PD1 and PDL1 expression in primary central nervous system diffuse large B-cell lymphoma are frequent and expression of PD1 predicts poor survival. Hematological oncology. 2017;35(4):487–496. [DOI] [PubMed] [Google Scholar]
- 33.Hayano A, Komohara Y, Takashima Y, et al. Programmed Cell Death Ligand 1 Expression in Primary Central Nervous System Lymphomas: A Clinicopathological Study. Anticancer research. 2017;37(10):5655–5666. [DOI] [PubMed] [Google Scholar]
- 34.Nicolae A, Pittaluga S, Abdullah S, et al. EBV-positive large B-cell lymphomas in young patients: a nodal lymphoma with evidence for a tolerogenic immune environment. Blood. 2015;126(7):863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 Activity and EBV Infection Induce PD-L1 in Hodgkin Lymphomas and Posttransplant Lymphoproliferative Disorders: Implications for Targeted Therapy [ 10.1158/1078-0432.CCR-11-1942]. Clinical Cancer Research. 2012;18(6):1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 Expression Is Characteristic of a Subset of Aggressive B-cell Lymphomas and Virus-Associated Malignancies [ 10.1158/1078-0432.CCR-13-0855]. Clinical Cancer Research. 2013;19(13):3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurent C, Charmpi K, Gravelle P, et al. Several immune escape patterns in non-Hodgkin’s lymphomas. Oncoimmunology. 2015;4(8):e1026530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia Y, Jeffrey Medeiros L, Young KH. Signaling pathway and dysregulation of PD1 and its ligands in lymphoid malignancies. Biochimica et biophysica acta. 2016;1865(1):58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akhter A, Masir N, Elyamany G, et al. Differential expression of Toll-like receptor (TLR) and B cell receptor (BCR) signaling molecules in primary diffuse large B-cell lymphoma of the central nervous system. Journal of neuro-oncology. 2015;121(2):289–96. [DOI] [PubMed] [Google Scholar]
- 40.Bruno A, Boisselier B, Labreche K, et al. Mutational analysis of primary central nervous system lymphoma. Oncotarget. 2014;5(13):5065–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukumura K, Kawazu M, Kojima S, et al. Genomic characterization of primary central nervous system lymphoma. Acta neuropathologica. 2016;131(6):865–75. [DOI] [PubMed] [Google Scholar]
- 42.Hattori K, Sakata-Yanagimoto M, Okoshi Y, et al. MYD88 (L265P) mutation is associated with an unfavourable outcome of primary central nervous system lymphoma. British journal of haematology. 2017;177(3):492–494. [DOI] [PubMed] [Google Scholar]
- 43.Poulain S, Boyle EM, Tricot S, et al. Absence of CXCR4 mutations but high incidence of double mutant in CD79A/B and MYD88 in primary central nervous system lymphoma. British journal of haematology. 2015;170(2):285–7. [DOI] [PubMed] [Google Scholar]
- 44.Gandhi MK, Keane C, Tobin JWD, et al. The Impact of EBV upon the Tumor Microenvironment and Mutational Profile of Primary CNS Lymphoma in PTLD. Blood. 2017;130(Suppl 1):2731. [Google Scholar]
- 45.Gebauer N, Gebauer J, Hardel TT, et al. Prevalence of targetable oncogenic mutations and genomic alterations in Epstein-Barr virus-associated diffuse large B-cell lymphoma of the elderly. Leukemia & lymphoma. 2015;56(4):1100–6. [DOI] [PubMed] [Google Scholar]
- 46.Montes-Moreno S, Odqvist L, Diaz-Perez JA, et al. EBV-positive diffuse large B-cell lymphoma of the elderly is an aggressive post-germinal center B-cell neoplasm characterized by prominent nuclear factor-kB activation. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25(7):968–82. [DOI] [PubMed] [Google Scholar]
- 47.Kato H, Karube K, Yamamoto K, et al. Gene expression profiling of Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly reveals alterations of characteristic oncogenetic pathways. Cancer science. 2014;105(5):537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ok CY, Papathomas TG, Medeiros LJ, et al. EBV-positive diffuse large B-cell lymphoma of the elderly. Blood. 2013;122(3):328–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuppers R B cells under influence: transformation of B cells by Epstein-Barr virus. Nature reviews Immunology. 2003;3(10):801–12. [DOI] [PubMed] [Google Scholar]
- 50.Cho H, Kim SH, Kim S-J, et al. Programmed cell death 1 expression is associated with inferior survival in patients with primary central nervous system lymphoma. Oncotarget. 2017;8(50):87317–87328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


