Abstract
Tomato spotted wilt virus (TSWV) is one of the most devastating plant viruses and often causes severe crop losses worldwide. Generally, mature plants become more resistant to pathogens, known as adult plant resistance. In this study, we demonstrated a new phenomenon involving developmentally regulated susceptibility of Arabidopsis thaliana to TSWV. We found that Arabidopsis plants become more susceptible to TSWV as plants mature. Most young 3‐week‐old Arabidopsis were not infected by TSWV. Infection of TSWV in 4‐, 5‐, and 6‐week‐old Arabidopsis increased from 9%, 21%, and 25%, respectively, to 100% in 7‐ to 8‐week‐old Arabidopsis plants. Different isolates of TSWV and different tospoviruses show a low rate of infection in young Arabidopsis but a high rate in mature plants. When Arabidopsis dcl2/3/4 or rdr1/2/6 mutant plants were inoculated with TSWV, similar results as observed for the wild‐type Arabidopsis plants were obtained. A cell‐to‐cell movement assay showed that the intercellular movement efficiency of TSWV NSm:GFP fusion was significantly higher in 8‐week‐old Arabidopsis leaves compared with 4‐week‐old Arabidopsis leaves. Moreover, the expression levels of pectin methylesterase and β‐1,3‐glucanase, which play critical roles in macromolecule cell‐to‐cell trafficking, were significantly up‐regulated in 8‐week‐old Arabidopsis leaves compared with 4‐week‐old Arabidopsis leaves during TSWV infection. To date, this mature plant susceptibility to pathogen infections has rarely been investigated. Thus, the findings presented here should advance our knowledge on the developmentally regulated mature host susceptibility to plant virus infection.
Keywords: Arabidopsis thaliana, developmentally regulated susceptibility, mature‐dependent pathogen infection, Tomato spotted wilt virus
Generally, mature plants become more resistance to pathogens. In this study we demonstrated a developmentally regulated mature host susceptibility to tomato spotted wilt virus infection in Arabidopsis.

1. INTRODUCTION
Plant‐infecting tospovirus species often cause devastating losses to agricultural and horticultural crops worldwide (Pappu et al., 2000, 2009; Persley et al., 2006; Chiemsombat et al., 2008; Turina et al., 2012). These viruses belong to the genus Orthotospovirus, family Tospoviridae, order Bunyaviridae (Turina et al., 2016, Oliver & Whitfield, 2016, Adams et al., 2017). Tomato spotted wilt virus is the type species of Orthotospovirus and is transmitted by thrips in a circulative‐propagative manner (Oliver & Whitfield, 2016). To date, tomato spotted wilt virus (TSWV) is known to infect over 900 plant species belonging to 82 different families (Pappu et al., 2009; Oliver & Whitfield, 2016). TSWV is currently ranked as one of the top 10 plant viruses (Scholthof et al., 2011) and causes about $1 billion in losses each year worldwide (Prins & Goldbach, 1998; Pappu et al., 2009).
Tospovirus virions are spherical, enveloped, and 80–120 nm in diameter (Kormelink et al., 2011; Oliver & Whitfield, 2016). The virions contain three genomic RNA segments: large (L), medium (M), and small (S). The L RNA segment encodes an RNA‐dependent RNA polymerase (RdRp) from the viral complementary strand (Adkins et al., 1995; van Knippenberg et al., 2002). The M RNA segment encodes a movement protein (NSm) from the viral sense strand and a glycoprotein precursor, which is further processed into a Gn and a Gc protein, from the viral complementary strand (Kormelink et al., 1992; Ribeiro et al., 2009). The S RNA segment encodes a nucleocapsid (N) protein from the viral sense strand and an RNA silencing suppressor (NSs) from the viral complementary strand (Takeda et al., 2002; Bucher et al., 2003; Schnettler et al., 2010).
Mature plant resistance has been reported for bacteria, fungi, oomycetes, nematodes, and viruses (Kus et al., 2002; Gee et al., 2008; Kayani et al., 2017; Simamora et al., 2017). Mature host resistance was reported in response to southern rice black‐streaked dwarf virus (SRBSDV) infection in rice (Zhou et al., 2013), cauliflower mosaic virus (CaMV) infection in turnip (Leisner et al., 1992), cotton leaf curl virus (CLCuV) infection in cotton, cucumber mosaic virus (CMV) infection in bell pepper (Garcia‐Ruiz & Murphy, 2001), and tomato yellow leaf curl virus (TYLCV) infection in tomato (Levy & Lapidot, 2008).
Arabidopsis is an important model plant for studies on plant–virus interactions (Lellis et al., 2002; Garcia‐Ruiz et al., 2010, 2015; Wang et al., 2010, 2011b; Aregger et al., 2012; Uchiyama et al., 2014; Zhang et al., 2015; Gaguancela et al., 2016; Cheng et al., 2017; Hafren et al., 2017, 2018; Yuan et al., 2018). Arabidopsis has been shown to be susceptible to TSWV infection (German et al., 1995). In this study, we found that successful TSWV infection in Arabidopsis plants is linked to plant developmental stages. For example, at 3 weeks old, Arabidopsis plants are not susceptible to TSWV infection, but their susceptibility gradually increases as the plants mature. Inoculation of Arabidopsis dcl2/3/4 or rdr1/2/6 mutant plants with TSWV have shown that the resistance of young Arabidopsis plants to TSWV infection is not controlled by the genes involved in the RNA silencing pathway. Plasmids encoding the TSWV NSm:GFP fusion were delivered to leaves by particle bombardment and the fusion proteins moved efficiently between cells in 8‐week‐old Arabidopsis leaves compared with 4‐week‐old Arabidopsis leaves. Our results indicate that TSWV infection in Arabidopsis is controlled by the plant developmental stage, and that the age of the Arabidopsis plant should be considered when studying TSWV and possibly other viruses.
2. RESULTS
2.1. Effect of Arabidopsis developmental stages on TSWV infection
In our initial experiments, we inoculated 5‐week‐old Arabidopsis Col‐0 plants with TSWV lettuce isolate (TSWV‐LE). By 30 days post‐inoculation (dpi), the inoculated plants did not show symptoms, contradicting the commonly accepted knowledge that younger plants are more susceptible to virus infections. To determine whether this TSWV isolate has the ability to infect Arabidopsis, we inoculated 9‐week‐old Arabidopsis plants with TSWV‐LE and found that all the inoculated plants showed strong disease symptoms, including leaf curling and necrosis, and plant stunting followed by plant death at about 25–30 dpi (Figure S1). To rule out the possibility that the lack of TSWV infection in the 5‐week‐old plants was caused by an inefficient inoculation method, we inoculated the same amount of TSWV‐infected crude leaf sap (5 µl per leaf) to three leaves on each 5‐ or 9‐week‐old Arabidopsis plant. As expected, similar results as described above were obtained for the inoculated 5‐ and 9‐week‐old Arabidopsis plants, indicating that the susceptibility of Arabidopsis to TSWV infection is linked to the plant developmental stage.
The above observations prompted us to conduct more experiments by inoculating 3‐, 4‐, 5‐, 6‐, 7‐, and 8‐week‐old Arabidopsis plants with TSWV‐LE. The results showed that none of the 3‐week‐old inoculated Arabidopsis plants produced TSWV symptoms, and about 9% of the 4‐week‐old inoculated plants, 21% of the 4‐week‐old inoculated plants, and 25% of the 6‐week‐old inoculated plants developed TSWV symptoms by 15–30 dpi (Figure 1a and Table 1). Reverse transcription (RT)‐PCR showed that by 30 dpi, only plants without visible TSWV symptoms lacked virus (Figure 1b,c). All plants inoculated at 7 or 8 weeks old showed TSWV symptoms (Table 1), and died by 30 dpi (Figure 1a). The RT‐PCR results confirmed the accumulation of TSWV in the plants showing symptoms (Figure 1b,c).
FIGURE 1.
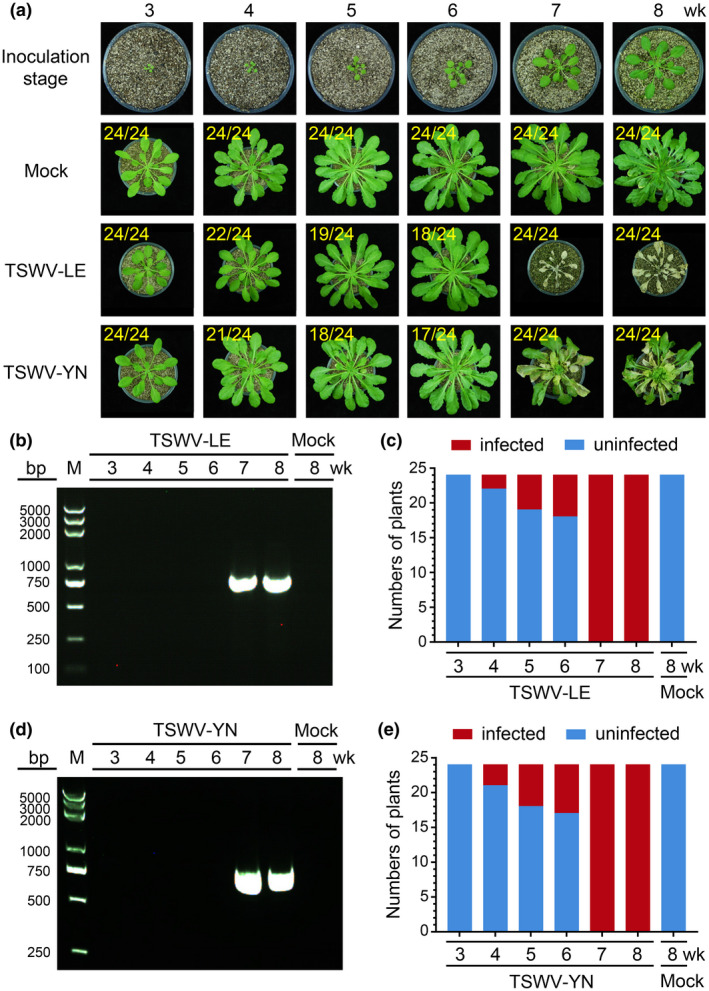
Infection of TSWV‐LE and TSWV‐YN in Arabidopsis thaliana Col‐0 plants at different growth stages. (a) Arabidopsis Col‐0 plants at different growth stages were rub‐inoculated with a TSWV‐LE‐ or TSWV‐YN‐infected crude leaf extract. A representative plant was selected from each treatment and photographed at 30 days post‐inoculation (dpi). Plants inoculated with phosphate‐buffered saline (PBS) were used as negative (Mock) controls. The numbers in yellow are the total number of inoculated plants versus noninfected (3‐, 4‐, 5‐, and 6‐week‐old) or infected (7‐ and 8‐week‐old) plants observed from various treatments. (b) and (d) Newly emerged leaves were harvested from the representative plants (a) and analysed for TSWV‐LE or TSWV‐YN infection through reverse transcription (RT)‐PCR. The 3‐, 4‐, 5‐, and 6‐week‐old inoculated plants were analysed at 30 dpi, and the 7‐ and 8‐week‐old inoculated plants were analysed at 15 dpi. Total RNA isolated from Arabidopsis Col‐0 plants inoculated with PBS at the 8‐week‐old stage was used as a negative control. (c) and (e) RT‐PCR results showing the numbers of TSWV‐LE‐ or TSWV‐YN‐infected (red) or uninfected (blue) Arabidopsis plants inoculated at different developmental stages. A total of 24 plants were used for each treatment
TABLE 1.
Infection of different viruses in Arabidopsis thaliana plants inoculated at different growth stages
| Inoculated age (week) | Col‐0 | Ws‐0 | ||||||
|---|---|---|---|---|---|---|---|---|
| Mock | TSW‐LE a | TSWV‐YN a | TZSV a | INSV a | CMV b | Mock | TSWV‐LE a | |
| 3 | 0/24 c | 0/24 | 0/24 | 0/18 | 0/18 | 2/18 | 0/18 | 0/18 |
| 4 | 0/24 | 2/24 | 3/24 | 1/18 | 2/18 | 12/18 | 0/18 | 0/18 |
| 5 | 0/24 | 5/24 | 6/24 | 3/18 | 5/18 | 15/18 | 0/18 | 7/18 |
| 6 | 0/24 | 6/24 | 7/24 | 5/18 | 6/18 | 16/18 | 0/18 | 8/18 |
| 7 | 0/24 | 24/24 | 24/24 | 18/18 | 18/18 | 18/18 | 0/18 | 14/18 |
| 8 | 0/24 | 24/24 | 24/24 | 18/18 | 18/18 | 18/18 | 0/18 | 18/18 |
The systemic presence of virus in uninoculated leaves of the inoculated plants was determined by both ELISA and reverse transcription (RT)‐PCR. The plants inoculated at the 3–6‐week‐old stage were assayed at 30 days post‐inoculation (dpi) and the plants inoculated at 7–8‐week‐old stage were assayed at 15 dpi.
The systemic presence of CMV in uninoculated leaves of the inoculated plants was determined by ELISA and RT‐PCR at 40 dpi.
The number of virus infected plants/the total number of inoculated plants.
To investigate whether this developmental stage‐controlled susceptibility is specific to this isolate of TSWV, we inoculated Arabidopsis plants ranging from 3 to 8 weeks old with TSWV Yunnan tomato isolate (TSWV‐YN). The 3‐week‐old Arabidopsis plants were not susceptible to TSWV‐YN infection, while the 7‐ or 8‐week‐old plants were highly susceptible to TSWV‐YN infection (Figure 1a,d,e and Table 1). The 4‐, 5‐ or 6‐week‐old inoculated plants gave similar percentages of virus infection as observed for plants inoculated with TSWV‐LE at the 4‐, 5‐ or 6‐week stage (Figure 1a, compare Figure 1c–e).
To determine whether this developmental stage‐controlled susceptibility is Arabidopsis ecotype‐specific, we inoculated Arabidopsis Wassilewskija (Ws‐0) ecotype plants as described for the Col‐0 plants. Our result showed that none of the 3‐ or 4‐week‐old inoculated Arabidopsis Ws‐0 plants developed virus symptoms, while 39% of the 5‐week‐old plants inoculated, 45% of the 6‐week‐old inoculated plants, 78% of the 7‐week‐old inoculated plants, and 100% of the 8‐week‐old inoculated plants showed typical TSWV symptoms after 15 dpi (Figure S2 and Table 1). Consequently, we conclude that the susceptibility of Arabidopsis to TSWV infection is linked to plant developmental stage.
2.2. Arabidopsis susceptibility to other tospoviruses
The genus Orthotospovirus contains more than 20 different species belonging to the Euro/Asian‐type tospovirus clade or the American‐type tospovirus clade (Oliver & Whitfield, 2016; Turina et al., 2016). To determine if Arabidopsis Col‐0 susceptibility to other tospoviruses is also controlled by their developmental stages, we inoculated 3‐ to 8‐week‐old plants with tomato zonate spot virus (TZSV, a Euro/Asian‐type tospovirus) or impatiens necrotic spot virus (INSV, an American‐type tospovirus). The results showed that none of the 3‐week‐old inoculated Arabidopsis plants developed virus‐like symptoms and none accumulated the virus by 15 dpi as determined by RT‐PCR (Table 1 and Figure 2a–c). Plants inoculated with TZSV at the 4‐, 5‐, or 6‐week stage showed infection rates of about 6%, 17%, and 28%, respectively. Plants inoculated with INSV at the 4‐, 5‐, or 6‐week stage showed infection rates of about 11%, 28%, and 33%, respectively. In contrast, plants inoculated with TZSV or INSV at the 7‐ or 8‐week stage all showed virus symptoms by 15 dpi, ranging from leaf chlorosis to necrosis (Table 1, Figure 2). RT‐PCR showed that TZSV or INSV did accumulate in the plants showing virus like symptoms (Figure 2b–e).
FIGURE 2.
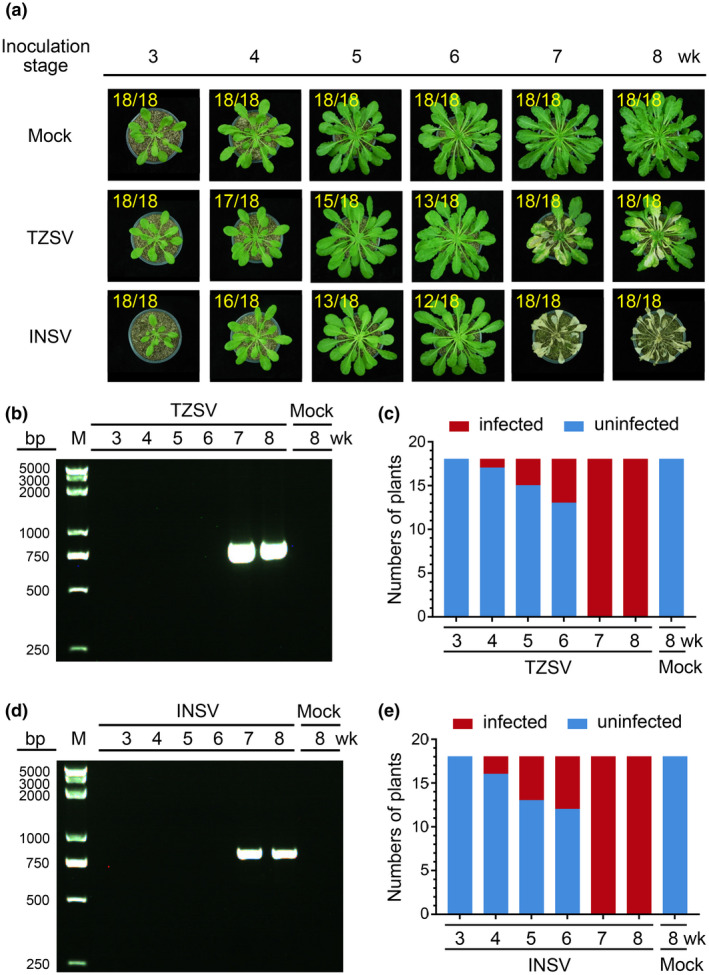
Infection of TZSV (a Euro/Asian type tospovirus) or INSV (an American type tospovirus) in Arabidopsis Col‐0 plants at various growth stages. (a) Arabidopsis Col‐0 plants at different growth stages were inoculated with a TZSV‐ or INSV‐infected crude leaf extract. A representative plant was selected from each treatment and photographed at 30 days post‐inoculation. Plants inoculated with phosphate‐buffered saline were used as negative (Mock) controls. The numbers in yellow are the total number of inoculated plants verses noninfected (3‐, 4‐, 5‐, and 6‐week‐old) or infected (7‐ and 8‐week‐old) plants observed from various treatments. (b) and (d) Newly emerged leaves were harvested from the representative plants from (a) and analysed for virus infection through reverse transcription (RT)‐PCR. (c) and (e) RT‐PCR results showing the numbers of TZSV‐ or INSV‐infected (red) or uninfected (blue) Arabidopsis plants inoculated at different growth stages. A total of 18 plants were used for each treatment. Samples harvested from the mock‐inoculated Arabidopsis plants were used as negative controls
2.3. Arabidopsis susceptibility to CMV infection
To determine if this developmental stage‐controlled susceptibility occurs for other plant viruses, we inoculated 3‐ to 8‐week‐old Arabidopsis plants with cucumber mosaic virus (CMV) isolate Fny as described for TSWV. The results showed that about 11% of the 3‐week‐old inoculated plants showed systemic CMV infection, and the 4‐, 5‐, and 6‐week‐inoculated plants showed infection rates of 67%, 83%, and 89%, respectively. All the 7‐ or 8‐week‐old inoculated plants showed CMV systemic infection (Figure 3a and Table 1). RT‐PCR confirmed that all the plants with visible CMV symptoms had accumulated the virus but not those without CMV symptoms (Figure 3b,c). In this study, none of the buffer (mock)‐inoculated plants showed virus‐like symptoms or accumulated the virus, suggesting that Arabidopsis developmental stage may have a partial influence on CMV infection in this host plant.
FIGURE 3.
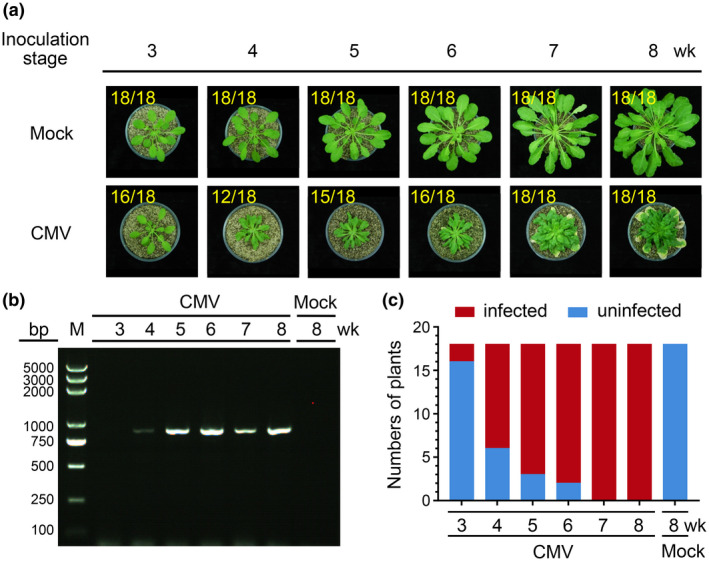
Infection of CMV isolate Fny in Arabidopsis Col‐0 plants at various growth stages. (a) Arabidopsis Col‐0 plants were inoculated at different growth stages with a CMV Fny‐infected crude leaf extract. A representative plant was selected from each treatment and photographed at 40 days post‐inoculation (dpi). Plants inoculated with phosphate‐buffered saline were used as negative (Mock) controls. The numbers in yellow are the total number of inoculated plants versus noninfected (3‐week‐old) or infected plants (4‐, 5‐, 6‐, 7‐, and 8‐week‐old) observed from various treatments. (b) A gel image showing CMV Fny infection in representative Arabidopsis plants (a), determined through reverse transcription (RT)‐PCR. (c) RT‐PCR showing the numbers of CMV Fny‐infected (red) and uninfected (blue) Arabidopsis plants at 40 dpi
2.4. Effects of tomato, pepper, and N. benthamiana developmental stages on TSWV infection
We inoculated tomato, pepper, and N. benthamiana plants with TSWV‐LE at the 2‐, 3‐, 4‐, and 5‐week‐old stages. The results showed that the susceptibility of these three plants to TSWV infection was not influenced by plant developmental stage (Figure 4 and Table 2). Symptoms in the TSWV‐infected plants were leaf chlorosis and plant stunting by 10 dpi. RT‐PCR showed that by 10 dpi, TSWV had accumulated in these three assayed host plants (Figure 4b,c,e,f,h,i), indicating that this developmental stage‐controlled plant susceptibility is not universal.
FIGURE 4.
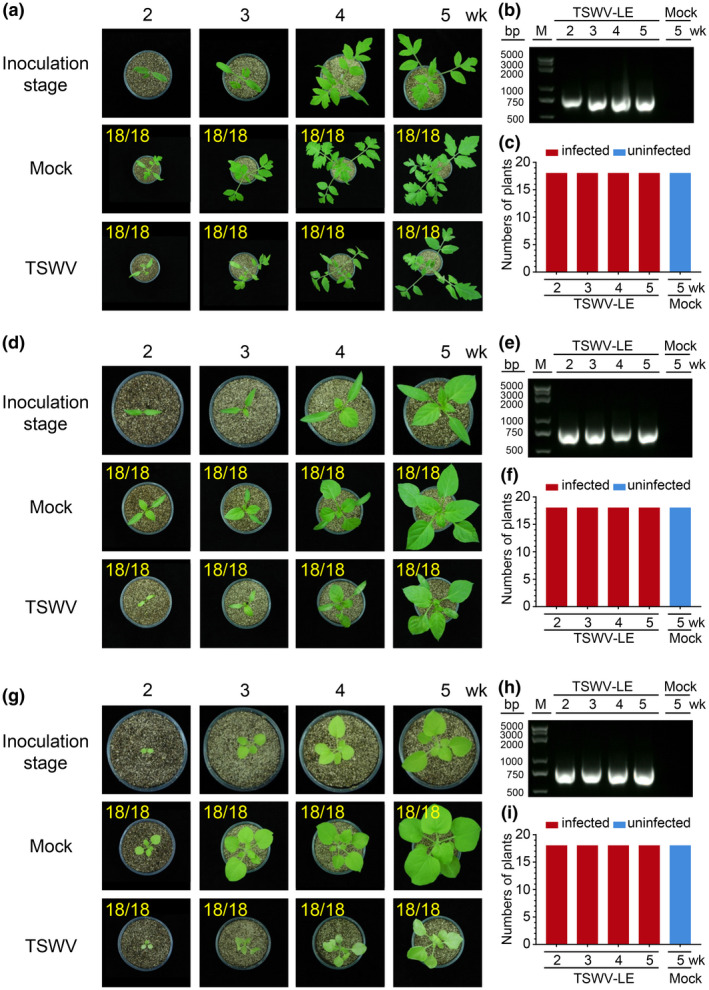
Infection of TSWV in tomato, pepper, and Nicotiana benthamiana plants at various growth stages. Tomato (a), pepper (d), and N. benthamiana (g) plants at different growth stages were inoculated with a TSWV‐LE‐infected crude leaf extract. A representative plant was selected from each treatment and photographed at 10 days post‐inoculation (dpi). Plants inoculated with phosphate‐buffered saline were used as negative (Mock) controls. The numbers in yellow are the total number of inoculated plants versus the number of infected plants observed from various treatments. Gel images showing systemic CMV Fny infection in the uninoculated leaves of the assayed tomato (b), pepper (e), and N. benthamiana (h) plants at 10 dpi. Virus infection in these samples was detected through reverse transcription (RT)‐PCR using TSWV‐specific primers. RT‐PCR results showing the numbers of TSWV‐infected (red) and uninfected (blue) tomato (c), pepper (g), and N. benthamiana (i) plants
TABLE 2.
Developmental stage of tomato, pepper, and Nicotiana benthamiana had no effect on TSWV‐LE systemic infection
| Inoculation stage (week) | Mock | Host plant | ||
|---|---|---|---|---|
| Tomato | Pepper | N. benthamiana | ||
| 2 | 0/18 a | 18/18 | 18/18 | 18/18 |
| 3 | 0/18 | 18/18 | 18/18 | 18/18 |
| 4 | 0/18 | 18/18 | 18/18 | 18/18 |
| 5 | 0/18 | 18/18 | 18/18 | 18/18 |
The number of virus‐infected plants/total number of inoculated plants. The systemic presence of TSWV in uninoculated leaves of these inoculated plants was determined by reverse transcription‐PCR or ELISA at 10 days post‐inoculation.
2.5. Developmental stage‐regulated Arabidopsis susceptibility to TSWV infection is not controlled by its RNA silencing pathway
To determine if the RNA silencing pathway is involved in this developmental stage‐controlled Arabidopsis susceptibility to TSWV infection, we inoculated 3‐ to 8‐week‐old Arabidopsis dcl2/3/4 or rdr1/2/6 mutant plants with TSWV‐LE as described above. The results showed that the TSWV‐LE‐inoculated dcl2/3/4 or rdr1/2/6 mutant Arabidopsis plants exhibited a similar developmental stage‐controlled susceptibility as described above for the wild‐type Arabidopsis plants (Figure 5a–c).
FIGURE 5.

Infection of TSWV in Arabidopsis dcl2/3/4 and rdr1/2/6 mutant lines at various growth stages. (a) Wild‐type Arabidopsis Col‐0 plants were inoculated with a TSWV‐LE‐infected crude leaf extract at different developmental stages. These plants were used as controls. (b) and (c) Arabidopsis dcl2/3/4 and rdr1/2/6 mutant lines were also inoculated with TSWV‐LE and analysed for TSWV infection. Newly emerged leaves were harvested from the assayed plants at 30 or 15 days post‐inoculation, as described in Figure 1, and analysed for TSWV infection through ELISA using a TSWV‐specific antibody. For each treatment, a total of 36 plants from three independent experiments were tested to determine the percentage infection of TSWV. The percentages of TSWV‐LE‐infected plants are shown in red and the percentages of uninfected plants are shown in blue
2.6. Intercellular movement of TSWV NSm:GFP is regulated by Arabidopsis developmental stages
The TSWV‐encoded NSm protein is critical for TSWV cell‐to‐cell and long‐distance movement in plants. We next investigated whether cell‐to‐cell movement of NSm:GFP fusion in Arabidopsis leaves is regulated by developmental stages through a particle bombardment assay. As a control, we bombarded leaves of the 4‐ or 8‐week‐old Arabidopsis plants with a control construct expressing green fluorescent protein (GFP):GFP fusion. The results showed that most loci expressing GFP:GFP fusion showed single cells with GFP green fluorescent signal (90.7% for the 8‐week‐old plant leaves and 85.3% for the 4‐week‐old plant leaves) (Table 3 and Figure 6a,c). In the 8‐week‐old plant leaves expressing NSm:GFP, a total of 296 loci were observed. Among these loci, 139 loci (47.0%) were single cell loci, 81 loci (27.4%) were clusters of 2–5 cells, 46 loci (15.5%) were clusters of 6–10 cells, and 30 loci (10.1%) were clusters of over 10 cells (Figure 6b and Table 3). In the 4‐week‐old plant leaves, a total of 244 loci showed NSm:GFP green fluorescent signal. Among these loci, 146 loci (59.8%) were single cell loci, 71 loci (29.1%) were clusters of 2–5 cells, 20 loci (8.2%) were clusters of 6–10 cells, and 7 loci (2.87%) were clusters of over 10 cells (Figure 6d and Table 3), indicating that intercellular movement of NSm:GFP is more efficient in the 8‐week‐old plant leaves than that in the 4‐week‐old plant leaves.
TABLE 3.
Cell‐to‐cell movement of TSWV NSm:GFP and GFP:GFP fusion in 4‐ or 8‐week‐old Arabidopsis plant leaves
| Bombarded plasmid | Plant age (week) | Total foci | Number of loci showing cell‐to‐cell movement | p value b | |||
|---|---|---|---|---|---|---|---|
| 1 cell/cluster a | 2–5 cells/cluster | 6–10 cells/cluster | ≥11 cells/cluster | ||||
| NSm:GFP | 8 | 296 | 139 (46.96%) | 81 (27.36%) | 46 (15.54%) | 30 (10.14%) | <.001 |
| 4 | 244 | 146 (59.84%)* | 71 (29.10%) | 20 (8.20%)* | 7 (2.87%)** | ||
| GFP:GFP | 8 | 118 | 107 (90.68%) | 11 (9.32%) | 0 | 0 | >.05 |
| 4 | 116 | 99 (85.34%) | 17 (14.66%) | 0 | 0 | ||
Number of loci showed 1, 2–5, 6–10 or over 10 cell movements. The number inside parentheses indicates the percentage of loci that showed cell‐to‐cell movement.
p values were calculated using unpaired two‐tailed Student t test.
p value < .05.
p value < .01.
FIGURE 6.
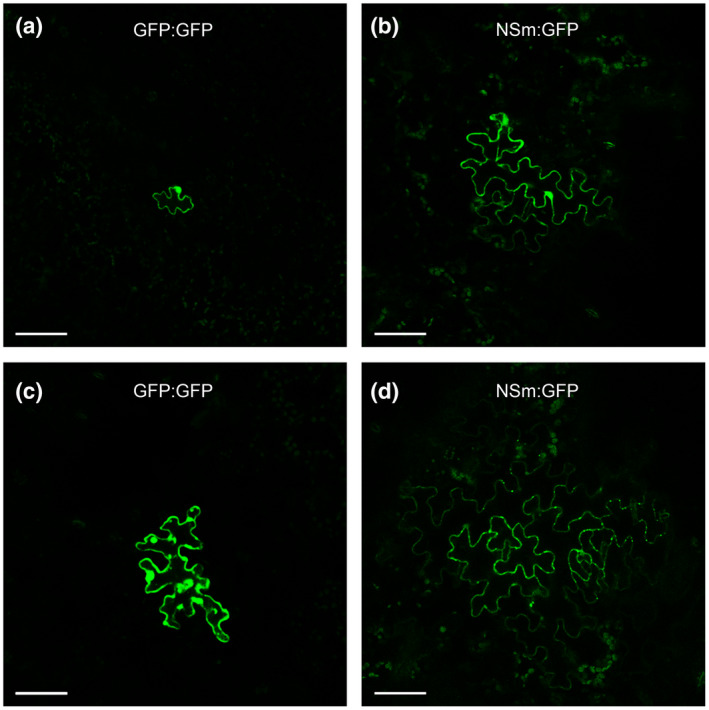
Cell‐to‐cell movement of TSWV NSm:GFP and GFP:GFP fusion in leaves of 4‐ or 8‐week old Arabidopsis plants. The leaves of the 4‐ and 8‐week‐old plants were particle bombarded. After 21 hr of incubation, the expression of GFP:GFP fusion (a and c) or TSWV NSm:GFP fusion (b and d) in the bombarded leaves was examined and imaged under a confocal microscope. Bar = 50 μm
2.7. Expression of PME and BGL genes was regulated by plant developmental stage and TSWV infection
Arabidopsis pectin methylesterase (PME) and β‐1,3‐glucanase (BGL) are known to regulate plasmodesmata functions during virus infection in plants (Dorokhov et al., 1999; Chen et al., 2000; Iglesias & Meins, 2000; Bucher et al., 2001). We analysed a previously published transcriptome data set obtained from TSWV‐infected Arabidopsis plants (Xu et al., 2020) and found that the expression of AtPME (AT2G45220) and AtBGL (AT3G57260) was strongly up‐regulated at 9, 12, and 15 days after TSWV inoculation (Figure 7a,b). To specifically examine expression of these genes, we analysed the expression of AtPME and AtBGL in the leaves of 4‐ or the 8‐week‐old Arabidopsis plants inoculated with TSWV or with buffer (mock) by quantitative RT‐PCR (RT‐qPCR). The results showed that the expression of AtBGL in the mock‐inoculated 8‐week‐old plants was significantly higher than that in the mock‐inoculated 4‐week‐old plants (Figure 7d). The results also showed that the expression of AtPME in the mock‐inoculated 8‐week‐old plants was somewhat higher in comparison to the mock‐inoculated 4‐week‐old plants, while the difference was not significant (Figure 7c). After TSWV inoculation, the expression of these two genes in both 4‐ and 8‐week‐old plants was significantly up‐regulated. Importantly, TSWV infection‐induced expression of AtPME and AtBGL in the 8‐week‐old plants was much stronger than in the 4‐week‐old plants. In a separate experiment, we silenced the expression of AtPME or AtBGL in Arabidopsis plants through apple latent spherical virus (ALSV)‐mediated virus‐induced gene silencing (VIGS) (Figures 7e and S3) and then inoculated these plants with TSWV. The result showed that silencing AtPME or AtBGL expression in Arabidopsis plants significantly reduced the accumulation of TSWV when compared with the nonsilenced Arabidopsis plants (Figure 7f).
FIGURE 7.
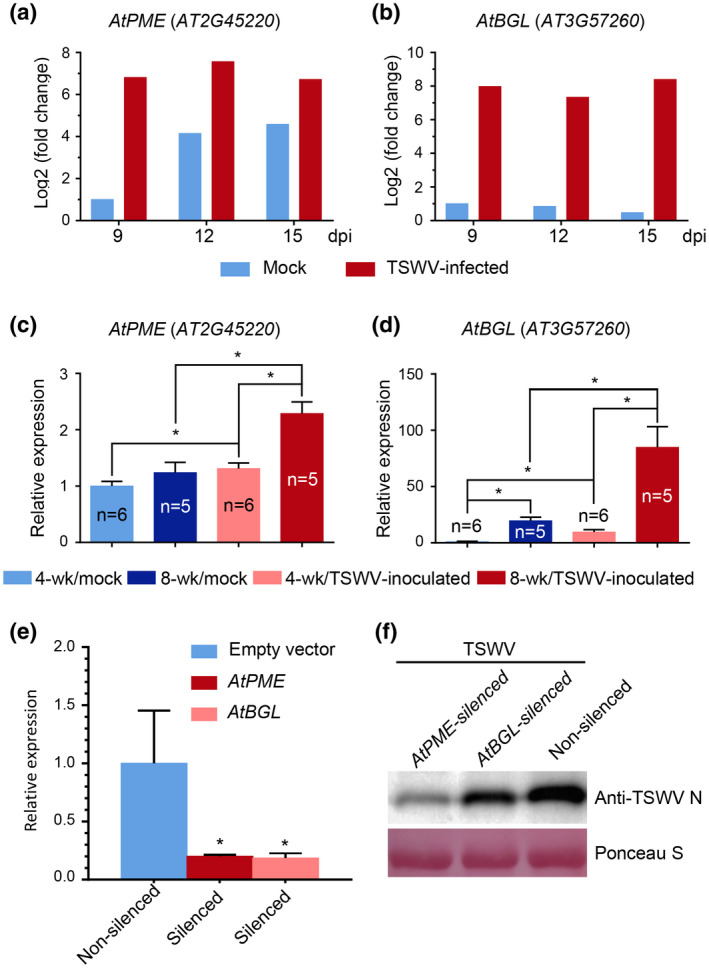
Expression of AtPME (AT2G45220) and AtBGL (AT3G57260) in 4‐ or 8‐week‐old Arabidopsis plants inoculated with TSWV or phosphate‐buffered saline (Mock). (a) and (b) Expression of AtPME and AtBGL in TSWV‐inoculated or mock‐inoculated Arabidopsis plants at 9, 12, and 15 days post‐inoculation (dpi). These data were summarized from the recent published transcriptome profile of TSWV‐infected or uninfected Arabidopsis plants. (c) and (d) The expression of AtPME and AtBGL was determined by quantitative reverse transcription PCR (RT‐qPCR) using gene‐specific primers. Leaves of 4‐ or 8‐week‐old Arabidopsis plants were harvested at 7 dpi and analysed through RT‐qPCR. The resulting data were calculated using the 2−ΔΔCt method. *p < .05, determined using the unpaired two‐tailed Student t test. (e) RT‐qPCR analysis of AtPME and AtBGL expression in the ALSV‐AtPME‐, ALSV‐AtGBL‐, or ALSV‐AtCH42‐inoculated Arabidopsis plants. Error bars represent SD (n = 4); *p < .05. (f) A western blot result showing the accumulation level of TSWV in the AtPME‐silenced + TSWV‐inoculated, AtBGL‐silenced + TSWV‐inoculated or ALSV‐empty (non‐silenced) + TSWV‐inoculated Arabidopsis plants. Five TSWV‐inoculated leaves were harvested from each treatment at 5 dpi, pooled, and analysed through a western blot assay using a TSWV N‐specific antibody. The Ponceau S stained gel is used to show the sample loadings
3. DISCUSSION
In general, younger plants are considered to be more susceptible to virus infection than older plants. For example, inoculation of potato spindle tuber viroid (PSTVd) to younger tobacco or tomato plants can result in a higher percentage of virus infection compared with older plants (Qi & Ding, 2003; Qi et al., 2004). In contrast, we found that 3‐week‐old Arabidopsis plants are not susceptible to TSWV infection. However, the susceptibility is gradually increased as the plant continues to grow and plants become highly susceptible to TSWV infection when they mature. For example, when the 3‐week‐old Arabidopsis plants were inoculated with TSWV‐LE isolate, TSWV‐YN isolate, INSV, or TZSV, none of the inoculated plants developed virus symptoms or accumulated the virus. When the 7‐ or 8‐week‐old plants were inoculated with these viruses, all the inoculated plants became infected. Interestingly, this development stage‐controlled susceptibility to TSWV infection was not observed when tomato, pepper, or N. benthamiana plants were inoculated with TSWV. Therefore, although Arabidopsis is used as a model plant for studies on plant development, growth, and abiotic and biotic stress responses, its developmental stage‐regulated susceptibility should be considered when studying the infection of TSWV.
To investigate the reason why younger Arabidopsis plants are more resistant to TSWV infection, we examined the functions of several RNA silencing machineries through inoculation of Arabidopsis dcl 2/3/4 or rdr 1/2/6 mutant plants (Garcia‐Ruiz et al., 2010; Wang et al., 2010; Aregger et al., 2012) with TSWV. Our results showed that these two Arabidopsis mutant lines acted similarly to wild‐type Arabidopsis plants. Based on this finding, we conclude that the resistance of young Arabidopsis plants to TSWV infection is not controlled by the host RNA silencing pathway.
Leisner and others reported that growth conditions important for Arabidopsis development can drastically affect cauliflower mosaic virus infection in Arabidopsis (Leisner et al., 1993). Tennant and others reported that older hemizygous transgenic papaya plants are more resistant to papaya ringspot virus Hawaiian isolate infection than younger papaya plants (Tennant et al., 2001). This developmental stage‐controlled resistance has also been found in Arabidopsis plants to Pseudomonas syringae pv. tomato DC3000 or P. syringae pv. maculicola 4326 infection (Kus et al., 2002). In this study, after we transiently expressed TSWV NSm:GFP fusion in the leaves of the 4‐ or the 8‐week‐old Arabidopsis plants through particle bombardment, we found that the NSm:GFP fusion moved faster between cells in 8‐week‐old Arabidopsis leaves compared with 4‐week‐old Arabidopsis leaves, suggesting a possible barrier between cells in the younger Arabidopsis plant leaves. PME and Class I GBL of Nicotiana tabacum are known to regulate the size exclusion limit of plasmodesmata in cell walls and facilitate the cell‐to‐cell movement of many plant viruses (Dorokhov et al., 1999; Chen et al., 2000; Iglesias & Meins, 2000; Bucher et al., 2001). Our RT‐qPCR results showed that the expression level of AtGBL in 8‐week‐old Arabidopsis plants is significantly higher than that in 4‐week‐old Arabidopsis plants. Although the average expression level of AtPME in 8‐week‐old Arabidopsis plants has no significant difference in comparison to 4‐week‐old Arabidopsis plants without TSWV infection, the TSWV infection‐induced expression of both AtGBL and AtPME genes in 8‐week‐old plants was significantly higher than in 4‐week‐old plants. We hypothesize that the developmental stage‐regulated susceptibility in 8‐week‐old Arabidopsis plants to TSWV infection is caused by the much higher expression of AtPME and AtGBL on TSWV infection. As expected, after the expression of AtPME or AtGBL was silenced through VIGS, the accumulation level of TSWV in the TSWV‐inoculated Arabidopsis plants was significantly reduced, further supporting the above hypothesis that the expression of AtPME and AtGBL is crucial for the developmental stage‐controlled susceptibility to TSWV infection in Arabidopsis plants. It is noteworthy that this developmental stage‐controlled susceptibility was not observed in tomato, pepper, or N. benthamiana. One possible explanation for this difference is that the basal levels of PME and GBL in young tomato, pepper, and N. benthamiana plants are sufficient for successful TSWV infection. The other possibility is that the expression of PME and GBL is readily induced in both young and mature tomato, pepper, and N. benthamiana plants on TSWV infection.
It has been shown that a developmentally regulated plasma membrane protein of N. benthamiana (NbDREPP) can interact with two different movement proteins (P3N‐PIPO and CI) of tobacco vein banding mosaic virus (TVBMV, a potyvirus) and facilitate the virus to move in infected leaves (Geng et al., 2015). Developmentally regulated intercellular trafficking has also been shown for CMV MP:GFP fusion movement in tobacco leaf cells (Itaya et al., 1998). In addition, these authors have shown that the walls of young tobacco leaf cells contain only primary plasmodesmata while the walls of mature leaf cells contain mostly secondary plasmodesmata that are more permeable to macromolecule trafficking. Iglesias and Meins have demonstrated that silencing of BGL expression in tobacco leaves can delay the cell‐to‐cell trafficking of CMV movement protein (Iglesias & Meins, 2000). In a different study, the authors found that the plasmodesmata between vascular bundle sheath cells and vein cells are structurally different from that between mesophyll cells, and have suggested that this difference may restrict brome mosaic virus infection predominantly in the mesophyll cells and the cells associated with vasculature in barley leaves at 24/20 °C (Ding et al., 1996, 1999). We speculate that similar barrier(s) may also exist in young Arabidopsis plants to impede TSWV systemic infection through a phloem‐dependent movement. Further investigations are needed to decipher the molecular mechanism(s) controlling TSWV infection in Arabidopsis plants.
Taken together, our results have shown that TSWV infection in Arabidopsis plants is regulated by its developmental stages. This finding further expands our knowledge on host developmentally controlled resistance/susceptibility to virus infections.
4. EXPERIMENTAL PROCEDURES
4.1. Plant growth
Seeds of A. thaliana ecotype Columbia (Col‐0), Arabidopsis ecotype Wassilewskija (Ws‐0), N. benthamiana, Solanum lycopersicum “Jiangshu 14” (tomato), and Capsicum annuum “Sujiao 5” (pepper) were propagated and maintained in this laboratory. Three‐, 4‐, 5‐, 6‐, 7‐, and 8‐week‐old A. thaliana plants or 2‐, 3‐, 4‐, and 5‐week‐old tomato, pepper, and N. benthamiana plants were used for TSWV inoculations. Seeds of Arabidopsis rdr1/2/6 and dcl2/3/4 mutant lines were from Dr Hongwei Zhao and Dr Donglei Yang (Nanjing Agricultural University, Nanjing, China). All plants were grown inside a growth chamber (Model GXZ500D, Jiangnan Motor Factory) maintained at 22 °C and a 8‐hr light/16‐hr dark photoperiod.
4.2. Virus source and inoculation
TSWV lettuce isolate (TSWV‐LE), TSWV Yunnan tomato isolate (TSWV‐YN), impatiens necrotic spot virus (INSV), and tomato zonate spot virus (TZSV) were collected from the Yunnan Province, China. These four viruses were propagated individually in N. benthamiana plants and stored at –80 °C. CMV Fny was initially obtained from Professor Marilyn J. Roossinck (Center for Infectious Disease Dynamics, Pennsylvania State University, PA, USA) and maintained in N. benthamiana in the laboratory. For virus inoculation, infected N. benthamiana leaf tissues were ground (1:10, wt/vol) in a 0.01 M phosphate buffer solution, pH 7.0, and the resulting crude leaf extracts were rub‐inoculated to leaves of Arabidopsis, tomato, pepper, or N. benthamiana plants. The inoculated plants were grown inside the growth chamber as described above.
4.3. Total RNA extraction, RT‐PCR, and RT‐qPCR
To detect virus infection in the inoculated plants, total RNA was extracted from individual harvested leaf samples (100 mg of tissue/sample) using TRIzol reagent (Invitrogen). The isolated total RNA (2 μg/sample) was used for a 25‐μl first‐strand cDNA synthesis using a M‐MLV reverse transcriptase (Promega) and a specific reverse primer for TSWV, TZSV, INSV, or CMV (Table S1). PCR amplifications were performed using the TaqPLUS DNA polymerase (Sangong Biotech) and primers specific for TSWV, TZSV, INSV, or CMV (Table S1). To determine host gene expression, total RNA was extracted from leaf samples harvested from various assayed Arabidopsis plants. First‐strand cDNA was synthesized using the PrimeScript RT reagent kit supplemented with a gDNA Eraser (Takara) and an oligo‐dT primer. qPCR amplifications were performed on a CFX Connect Real‐Time System (Bio‐Rad) using a PowerUp SYBR Green Master Mix (Applied Biosystems) as reported previously (Li et al., 2014). Primers specific for Arabidopsis pectin methylesterase (AtPME, AT2G45220) and Arabidopsis β‐1,3‐glucanase gene (AtBGL, AT3G57260) are listed in Table S1. The expression of Arabidopsis Actin 2 gene (AtACTIN 2, AT3G18780) was used as an internal control, and the relative expressions of AtPME and AtBGL were calculated using the 2−∆∆ C t method (Livak & Schmittgen, 2001).
4.4. Dot enzyme‐linked immunosorbent assay
Leaf samples were individually ground (1:10, wt/vol) in 0.05 M carbonate buffer (15 mM Na2CO3 and 35 mM NaHCO3 in distilled water, pH 9.6). The leaf extracts were centrifuged at 6,500 rpm for 3 min and blotted (2 μl/sample) individually onto a nitrocellulose membrane (Amersham Protran 0.45 NC, Whatman). After air‐drying, the membrane was incubated in a PBS‐M solution (137 mM NaCl, 3 mM KCl, 0.01 M Na2HPO4, 2 mM KH2PO4, pH 7.4, and 1% nonfat milk) for 30 min, rinsed three times with a phosphate‐buffered saline (PBS) solution, and then probed with a TSWV‐specific antibody diluted 1:10,000 (vol/vol) in the PBS‐M solution for 2 hr. The probed membrane was rinsed three times with a PBS‐T solution (PBS with 0.1% Tween‐20), incubated in an alkaline phosphatase (AP)‐conjugated goat anti‐rabbit or anti‐mouse IgG diluted 1:10,000 (vol/vol) (Sigma) for 1.5 hr, rinsed three times with PBS‐T, and developed by incubating the membrane in a 5‐bromo‐4‐chloro‐3‐indolyl phosphate/nitroblue tetrazolium (NBT/BCIP) solution as instructed (Sangon Biotech).
4.5. Particle bombardment
Plasmid DNA pRTL2‐NSm:GFP and pRTL2‐GFP:GFP were made previously (Feng et al., 2016). Particle bombardment was conducted as described (Sanford et al., 1993) with specific modifications. Briefly, 60 mg of tungsten M‐10 microcarrier (Bio‐Rad) was mixed with 1 ml of freshly prepared 70% ethanol in a 1.5‐ml Eppendorf tube, vortexed for 3 min, incubated at room temperature for 15 min, and then pelleted by 5 s centrifugation in a microfuge. The supernatant was removed from the microfuge tube and the pellet was rinsed three times with 70% ethanol. The pellet was resuspended in a 50% sterile glycerol solution to achieve a 60‐mg tungsten particle/ml stock solution. Fifty microlitres of tungsten particle stock solution was mixed with 5 µg of pRTL2‐NSm:GFP or pRTL2‐GFP:GFP plasmid DNA, 50 µl of 2.5 M CaCl2, and 20 µl of 0.1 M spermidine. The mixed tungsten particle:plasmid DNA complexes were pelleted, resuspended in 140 µl of 70% ethanol, pelleted again, and then resuspended in 48 µl of 100% ethanol. An aliquot (15 µl) of tungsten particle:plasmid DNA complex solution was loaded onto the centre of a macrocarrier (Bio‐Rad), air‐dried, and bombarded onto the abaxial side of leaves harvested from the 4‐ or the 8‐week‐old Arabidopsis Col‐0 plants using the He/1000 particle delivery system (Bio‐Rad). The bombarded leaves were incubated on wet filter papers inside Petri dishes for 21 hr at 22 °C in the dark.
4.6. Confocal laser scanning microscopy
The bombarded Arabidopsis leaves were harvested and examined under an LSM 710 confocal laser scanning microscope equipped with a plan‐apochromat 40×/0.95 Korr M27 lens (Carl Zeiss). The excitation wavelength was set at 488 nm and the emission wavelength was set at 528 nm as previously described (Wang et al., 2011a). The captured images were processed using the Zeiss 710 CLSM and Adobe Photoshop software.
4.7. Virus‐induced gene silencing in Arabidopsis
Silencing of AtPME or AtGBL expression in Arabidopsis was performed using ALSV‐based vector as described previously (Igarashi et al., 2009). Full‐length ALSV RNA1 (GenBank accession no. AB030940) and RNA2 (AB030941) sequences were synthesized by the GenScript (Nanjing, China) and cloned individually into the pCB301‐2 × 35S‐RZ‐NOS vector (Feng et al., 2020) to produce pALSV1 and pALSV2, respectively. A partial sequence (300 bp) of AtPME or AtGBL was RT‐PCR amplified from a total RNA sample using specific primers and cloned into the pALSV2 vector to produce pALSV2‐AtPME or pALSV2‐AtGBL. pALSV2 vector without an insert or with a 300 bp insert from AtCH42 (pALSV2‐AtCH42) were used as control vectors. Agrobacterium tumefaciens cultures carrying pALSV‐RNA1 and pALSV2, pALSV‐RNA1 and pALSV2‐AtPME, pALSV‐RNA1 and pALSV2‐AtGBL, or pALSV‐RNA1 and pALSV2‐AtCH42 were individually mixed with an equal volume of A. tumefaciens culture carrying a vector expressing an RNA silencing suppressor (tomato bushy stunt virus P19), and the mixed cultures were individually infiltrated into leaves of N. benthamiana plants. Eighteen days later, three newly emerged leaves were collected from each N. benthamiana plant and virus (i.e., ALSV, ALSV‐AtPME, ALSV‐AtGBL, or ALSV‐AtCH42) in each leaf sample was isolated as described previously (Zhu et al., 2014). The isolated virus was inoculated to six leaves of each 6‐week‐old Arabidopsis plants. After 5 days, the three newly merged leaves on each ALSV‐, ALSV‐AtPME‐, ALSV‐AtGBL‐, or ALSV‐AtCH42‐inoculated Arabidopsis plant were inoculated with a crude sap prepared from TSWV‐infected leaf tissues. Five days later, leaves of five plants representing a specific treatment were harvested, pooled, and analysed for TSWV accumulation using western blot assay.
Supporting information
FIGURE S1 Infection of TSWV in 5‐ and the 9‐week‐old Arabidopsis Col‐0 plants. Arabidopsis plants were rub‐inoculated with a TSWV‐LE‐infected crude leaf extract at 5 or 9 weeks after planting. The representative plants of different treatments were photographed at 0, 15, and 30 days post‐inoculation
FIGURE S2 Infection of TSWV in Arabidopsis Ws‐0 plants at different growth stages. (a) Arabidopsis Ws‐0 plants were rub‐inoculated with a TSWV‐LE‐infected crude leaf extract at different developmental stages. Plants inoculated with phosphate‐buffered saline were used as controls (Mock). The representative plants from different treatments were photographed at 30 days post‐inoculation (dpi). The numbers in yellow are the total number of inoculated plants versus the noninfected (3‐, 4‐, 5‐, and 6‐week‐old) or infected (7‐ and 8‐week‐old) plants observed from various treatments. (b) The representative plants in (a) were sampled and analysed for TSWV‐LE infection through reverse transcription‐PCR. A total of 18 plants were used for each treatment. (c) The number of TSWV‐LE‐infected (red) and uninfected (blue) Arabidopsis Ws‐0 plants at different growth stages are shown
FIGURE S3 Silencing of AtPME, AtGBL, and AtCH42 expressions in Arabidopsis plants through virus‐induced gene silencing (VIGS) using ALSV‐based vectors. Partially purified ALSV empty, ALSV‐AtPME, ALSV‐AtGBL, or ALSV‐AtCH42 virions were rub‐inoculated to six leaves of each assayed 6‐week‐old Arabidopsis plant. A representative plant of each treatment was photographed at 35 days after ALSV vector inoculation
TABLE S1 Primers used in this study
ACKNOWLEDGMENTS
We thank Professor Baoping Li (Nanjing Agricultural University) for his helpful suggestions and comments on statistical analysis. This work was supported by the National Natural Science Foundation of China (31925032, 31630062, and 31870143), Fundamental Research Funds for the Central Universities (JCQY201904), and the National Special Support Program for Outstanding Scientist to X.T. All authors declared no financial or other potential conflict of interest.
Huang Y, Hong H, Xu M, et al. Developmentally regulated Arabidopsis thaliana susceptibility to tomato spotted wilt virus infection. Molecular Plant Pathology. 2020;21:985–998. 10.1111/mpp.12944
Ying Huang and Hao Hong contributed equally.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Adams, M.J. , Lefkowitz, E.J. , King, A.M. , Harrach, B. , Harrison, R.L. , Knowles, N.J. et al (2017) Changes to taxonomy and the international code of virus classification and nomenclature ratified by the international committee on taxonomy of viruses (2017). Archives of Virology, 162, 2505–2538. [DOI] [PubMed] [Google Scholar]
- Adkins, S. , Quadt, R. , Choi, T.‐J. , Ahlquist, P. and German, T. (1995) An RNA‐dependent RNA polymerase activity associated with virions of tomato spotted wilt virus, a plant‐and insect‐infecting bunyavirus. Virology, 207, 308–311. [DOI] [PubMed] [Google Scholar]
- Aregger, M. , Borah, B.K. , Seguin, J. , Rajeswaran, R. , Gubaeva, E.G. , Zvereva, A.S. et al (2012) Primary and secondary siRNAs in geminivirus‐induced gene silencing. PLoS Pathogens, 8, e1002941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher, E. , Sijen, T. , de Haan, P. , Goldbach, R. and Prins, M. (2003) Negative‐strand tospoviruses and tenuiviruses carry a gene for a suppressor of gene silencing at analogous genomic positions. Journal of Virology, 77, 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher, G.L. , Tarina, C. , Heinlein, M. , Di Serio, F. , Meins, F. and Iglesias, V.A. (2001) Local expression of enzymatically active class I β‐1,3‐glucanase enhances symptoms of TMV infection in tobacco. The Plant Journal, 28, 361–369. [DOI] [PubMed] [Google Scholar]
- Chen, M.H. , Sheng, J. , Hind, G. , Handa, A.K. and Citovsky, V. (2000) Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell‐to‐cell movement. The EMBO Journal, 19, 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, X. , Xiong, R. , Li, Y. , Li, F. , Zhou, X. and Wang, A. (2017) Sumoylation of turnip mosaic virus RNA polymerase promotes viral infection by counteracting the host NPR1‐mediated immune response. The Plant Cell, 29, 508–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiemsombat, P. , Gajanandana, O. , Warin, N. , Hongprayoon, R. , Bhunchoth, A. and Pongsapich, P. (2008) Biological and molecular characterization of tospoviruses in Thailand. Archives of Virology, 153, 571–577. [DOI] [PubMed] [Google Scholar]
- Ding, X. , Shintaku, M.H. , Carter, S.A. and Nelson, R.S. (1996) Invasion of minor veins of tobacco leaves inoculated with tobacco mosaic virus mutants defective in phloem‐dependent movement. Proceedings of the National Academy of Sciences of the United States of America, 93, 11155–11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X.S. , Flasinski, S. and Nelson, R.S. (1999) Infection of barley by brome mosaic virus is restricted predominantly to cells in and associated with veins through a temperature‐dependent mechanism. Molecular Plant‐Microbe Interactions, 12, 615–623. [Google Scholar]
- Dorokhov, Y.L. , Mäkinen, K. , Frolova, O.Y. , Merits, A. , Saarinen, J. , Kalkkinen, N. et al (1999) A novel function for a ubiquitous plant enzyme pectin methylesterase: the host‐cell receptor for the tobacco mosaic virus movement protein. FEBS Letters, 461, 223–228. [DOI] [PubMed] [Google Scholar]
- Feng, M. , Cheng, R. , Chen, M. , Guo, R. , Li, L. , Feng, Z. et al (2020) Rescue of tomato spotted wilt virus entirely from complementary DNA clones. Proceedings of the National Academy of Sciences of the United States of America, 117, 1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Z. , Xue, F. , Xu, M. , Chen, X. , Zhao, W. , Garcia‐Murria, M.J. et al (2016) The ER‐membrane transport system is critical for intercellular trafficking of the NSm movement protein and tomato spotted wilt tospovirus. PLoS Pathogens, 12, e1005443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaguancela, O.A. , Zúñiga, L.P. , Arias, A.V. , Halterman, D. , Flores, F.J. , Johansen, I.E. et al (2016) The IRE1/bZIP60 pathway and bax inhibitor 1 suppress systemic accumulation of potyviruses and potexviruses in Arabidopsis and Nicotiana benthamiana plants. Molecular Plant‐Microbe Interactions, 29, 750–766. [DOI] [PubMed] [Google Scholar]
- Garcia‐Ruiz, H. , Carbonell, A. , Hoyer, J.S. , Fahlgren, N. , Gilbert, K.B. , Takeda, A. et al (2015) Roles and programming of Arabidopsis ARGONAUTE proteins during turnip mosaic virus infection. PLoS Pathogens, 11, e1004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Ruiz, H. and Murphy, J.F. (2001) Age‐related resistance in bell pepper to cucumber mosaic virus. Annals of Applied Biology, 139, 307–317. [Google Scholar]
- Garcia‐Ruiz, H. , Takeda, A. , Chapman, E.J. , Sullivan, C.M. , Fahlgren, N. , Brempelis, K.J. , et al (2010) Arabidopsis RNA‐dependent RNA polymerases and dicer‐like proteins in antiviral defense and small interfering RNA biogenesis during turnip mosaic virus infection. The Plant Cell, 22, 481–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee, C.T. , Gadoury, D.M. and Cadle‐Davidson, L. (2008) Ontogenic resistance to uncinula necator varies by genotype and tissue type in a diverse collection of Vitis spp. Plant Disease, 92, 1067–1073. [DOI] [PubMed] [Google Scholar]
- Geng, C. , Cong, Q.‐Q. , Li, X.‐D. , Mou, A.‐L. , Gao, R. , Liu, J.‐L. , et al (2015) Developmentally regulated plasma membrane protein of Nicotiana benthamiana contributes to potyvirus movement and transports to plasmodesmata via the early secretory pathway and the actomyosin system. Plant Physiology, 167, 394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German, T.L. , Adkins, S. , Witherell, A. , Richmond, K.E. , Knaack, W.R. and Willis, D.K. (1995) Infection of Arabidopsis thaliana ecotype Columbia by tomato spotted wilt virus. Plant Molecular Biology Reporter, 13, 110–117. [Google Scholar]
- Hafren, A. , Macia, J.L. , Love, A.J. , Milner, J.J. , Drucker, M. and Hofius, D. (2017) Selective autophagy limits cauliflower mosaic virus infection by NBR1‐mediated targeting of viral capsid protein and particles. Proceedings of the National Academy of Sciences of the United States of America, 114, E2026–E2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafren, A. , Ustun, S. , Hochmuth, A. , Svenning, S. , Johansen, T. and Hofius, D. (2018) Turnip mosaic virus counteracts selective autophagy of the viral silencing suppressor HCpro. Plant Physiology, 176, 649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi, A. , Yamagata, K. , Sugai, T. , Takahashi, Y. , Sugawara, E. , Tamura, A. et al (2009) Apple latent spherical virus vectors for reliable and effective virus‐induced gene silencing among a broad range of plants including tobacco, tomato, Arabidopsis thaliana, cucurbits, and legumes. Virology, 386, 407–416. [DOI] [PubMed] [Google Scholar]
- Iglesias, V.A. and Meins, F. (2000) Movement of plant viruses is delayed in a β‐1,3‐glucanase‐deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. The Plant Journal, 21, 157–166. [DOI] [PubMed] [Google Scholar]
- Itaya, A. , Woo, Y.‐M. , Masuta, C. , Bao, Y. , Nelson, R.S. and Ding, B. (1998) Developmental regulation of intercellular protein trafficking through plasmodesmata in tobacco leaf epidermis. Plant Physiology, 118, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayani, M.Z. , Mukhtar, T. and Hussain, M.A. (2017) Effects of southern root knot nematode population densities and plant age on growth and yield parameters of cucumber. Crop Protection, 92, 207–212. [Google Scholar]
- van Knippenberg, I. , Goldbach, R. and Kormelink, R. (2002) Purified tomato spotted wilt virus particles support both genome replication and transcription in vitro. Virology, 303, 278–286. [DOI] [PubMed] [Google Scholar]
- Kormelink, R. , De Haan, P. , Meurs, C. , Peters, D. and Goldbach, R. (1992) The nucleotide sequence of the M RNA segment of tomato spotted wilt virus, a bunyavirus with two ambisense RNA segments. Journal of General Virology, 73, 2795–2804. [DOI] [PubMed] [Google Scholar]
- Kormelink, R. , Garcia, M.L. , Goodin, M. , Sasaya, T. and Haenni, A.‐L. (2011) Negative‐strand RNA viruses: the plant‐infecting counterparts. Virus Research, 162, 184–202. [DOI] [PubMed] [Google Scholar]
- Kus, J.V. , Zaton, K. , Sarkar, R. and Cameron, R.K. (2002) Age‐related resistance in Arabidopsis is a developmentally regulated defense response to Pseudomonas syringae . The Plant Cell, 14, 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisner, S.M. , Turgeon, R. and Howell, S.H. (1992) Long distance movement of cauliflower mosaic virus in infected turnip plants. Molecular Plant‐Microbe Interactions, 5, 41–47. [Google Scholar]
- Leisner, S.M. , Turgeon, R. and Howell, S.H. (1993) Effects of host plant development and genetic determinants on the long‐distance movement of cauliflower mosaic virus in Arabidopsis . The Plant Cell, 5, 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lellis, A.D. , Kasschau, K.D. , Whitham, S.A. and Carrington, J.C. (2002) Loss‐of‐susceptibility mutants of Arabidopsis thaliana reveal an essential role for eIF (iso) 4E during potyvirus infection. Current Biology, 12, 1046–1051. [DOI] [PubMed] [Google Scholar]
- Levy, D. and Lapidot, M. (2008) Effect of plant age at inoculation on expression of genetic resistance to Tomato yellow leaf curl virus. Archives of Virology, 153, 171–179. [DOI] [PubMed] [Google Scholar]
- Li, R. , Weldegergis, B.T. , Li, J. , Jung, C. , Qu, J. , Sun, Y. et al (2014) Virulence factors of geminivirus interact with MYC2 to subvert plant resistance and promote vector performance. The Plant Cell, 26, 4991–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCT method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Oliver, J. and Whitfield, A. (2016) The genus Tospovirus: emerging bunyaviruses that threaten food security. Annual Review of Virology, 3, 101–124. [DOI] [PubMed] [Google Scholar]
- Pappu, H. , Jones, R. and Jain, R. (2009) Global status of Tospovirus epidemics in diverse cropping systems: successes achieved and challenges ahead. Virus Research, 141, 219–236. [DOI] [PubMed] [Google Scholar]
- Pappu, S. , Bhat, A. , Pappu, H. , Deom, C. and Culbreath, A. (2000) Phylogenetic studies of tospoviruses (family: Bunyaviridae) based on intergenic region sequences of small and medium genomic RNAs. Archives of Virology, 145, 1035–1045. [DOI] [PubMed] [Google Scholar]
- Persley, D. , Thomas, J. and Sharman, M. (2006) Tospoviruses—an Australian perspective. Australasian Plant Pathology, 35, 161–180. [Google Scholar]
- Prins, M. and Goldbach, R. (1998) The emerging problem of tospovirus infection and nonconventional methods of control. Trends in Microbiology, 6, 31–35. [DOI] [PubMed] [Google Scholar]
- Qi, Y. and Ding, B. (2003) Inhibition of cell growth and shoot development by a specific nucleotide sequence in a noncoding viroid RNA. The Plant Cell, 15, 1360–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, Y. , Pélissier, T. , Itaya, A. , Hunt, E. , Wassenegger, M. and Ding, B. (2004) Direct role of a viroid RNA motif in mediating directional RNA trafficking across a specific cellular boundary. The Plant Cell, 16, 1741–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, D. , Borst, J.W. , Goldbach, R. and Kormelink, R. (2009) Tomato spotted wilt virus nucleocapsid protein interacts with both viral glycoproteins Gn and Gc in planta. Virology, 383, 121–130. [DOI] [PubMed] [Google Scholar]
- Sanford, J.C. , Smith, F.D. and Russell, J.A. (1993) Optimizing the biolistic process for different biological applications. Methods in Enzymology, 217, 483–509. [DOI] [PubMed] [Google Scholar]
- Schnettler, E. , Hemmes, H. , Huismann, R. , Goldbach, R. , Prins, M. and Kormelink, R. (2010) Diverging affinity of tospovirus RNA silencing suppressor proteins, NSs, for various RNA duplex molecules. Journal of Virology, 84, 11542–11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof, K.B.G. , Adkins, S. , Czosnek, H. , Palukaitis, P. , Jacquot, E. , Hohn, T. et al (2011) Top 10 plant viruses in molecular plant pathology. Molecular Plant Pathology, 12, 938–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simamora, A. , Stukely, M. , Barber, P. , Hardy, G. and Burgess, T. (2017) Age‐related susceptibility of Eucalyptus species to Phytophthora boodjera . Plant Pathology, 66, 501–512. [Google Scholar]
- Tennant, P. , Fermin, G. , Fitch, M. , Manshardt, R. , Slightom, J. and Gonsalves, D. (2001) Papaya ringspot virus resistance of transgenic rainbow and SunUp is affected by gene dosage, plant development, and coat protein homology. European Journal of Plant Pathology, 107, 645–653. [Google Scholar]
- Takeda, A. , Sugiyama, K. , Nagano, H. , Mori, M. , Kaido, M. , Mise, K. et al (2002) Identification of a novel RNA silencing suppressor, NSs protein of Tomato spotted wilt virus. FEBS Letters, 532, 75–79. [DOI] [PubMed] [Google Scholar]
- Turina, M. , Kormelink, R. and Resende, R.O. (2016) Resistance to tospoviruses in vegetable crops: epidemiological and molecular aspects. Annual Review of Phytopathology, 54, 347–371. [DOI] [PubMed] [Google Scholar]
- Turina, M. , Tavella, L. and Ciuffo, M. (2012) Tospoviruses in the Mediterranean area. Advances in Virus Research, 84, 403–437. [DOI] [PubMed] [Google Scholar]
- Uchiyama, A. , Shimada‐Beltran, H. , Levy, A. , Zheng, J.Y. , Javia, P.A. and Lazarowitz, S.G. (2014) The Arabidopsis synaptotagmin SYTA regulates the cell‐to‐cell movement of diverse plant viruses. Frontiers in Plant Science, 5, 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Han, C. , Ferreira, A.O. , Yu, X. , Ye, W. , Tripathy, S. et al (2011a) Transcriptional programming and functional interactions within the Phytophthora sojae RXLR effector repertoire. The Plant Cell, 23, 2064–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.B. , Jovel, J. , Udomporn, P. , Wang, Y. , Wu, Q. , Li, W.X. et al (2011b) The 21‐nucleotide, but not 22‐nucleotide, viral secondary small interfering RNAs direct potent antiviral defense by two cooperative argonautes in Arabidopsis thaliana . The Plant Cell, 23, 1625–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.B. , Wu, Q. , Ito, T. , Cillo, F. , Li, W.X. , Chen, X. et al (2010) RNAi‐mediated viral immunity requires amplification of virus‐derived siRNAs in Arabidopsis thaliana . Proceedings of the National Academy of Sciences of the United States of America, 107, 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. , Chen, J. , Huang, Y. , Shen, D. , Sun, P. , Xu, Y. and et al (2020) Dynamic transcriptional profiles of Arabidopsis thaliana infected by tomato spotted wilt virus. Phytopathology, 110, 153–163. [DOI] [PubMed] [Google Scholar]
- Yuan, C. , Lazarowitz, S.G. and Citovsky, V. (2018) The plasmodesmal localization signal of TMV MP is recognized by plant synaptotagmin SYTA. mBio, 9, e01314–01318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Chen, H. , Brandizzi, F. , Verchot, J. and Wang, A. (2015) The UPR branch IRE1‐bZIP60 in plants plays an essential role in viral infection and is complementary to the only UPR pathway in yeast. PLoS Genetics, 11, e1005164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, G. , Xu, D. , Xu, D. and Zhang, M. (2013) Southern rice black‐streaked dwarf virus: a white‐backed planthopper‐transmitted fijivirus threatening rice production in Asia. Frontiers in Microbiology, 4, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, M. , Chen, Y. , Ding, X. , Webb, S.L. , Zhou, T. , Nelson, R.S. , et al (2014) Maize Elongin C interacts with the viral genome‐linked protein, VPg, of Sugarcane mosaic virus and facilitates virus infection. New Phytologist, 203, 1291–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Infection of TSWV in 5‐ and the 9‐week‐old Arabidopsis Col‐0 plants. Arabidopsis plants were rub‐inoculated with a TSWV‐LE‐infected crude leaf extract at 5 or 9 weeks after planting. The representative plants of different treatments were photographed at 0, 15, and 30 days post‐inoculation
FIGURE S2 Infection of TSWV in Arabidopsis Ws‐0 plants at different growth stages. (a) Arabidopsis Ws‐0 plants were rub‐inoculated with a TSWV‐LE‐infected crude leaf extract at different developmental stages. Plants inoculated with phosphate‐buffered saline were used as controls (Mock). The representative plants from different treatments were photographed at 30 days post‐inoculation (dpi). The numbers in yellow are the total number of inoculated plants versus the noninfected (3‐, 4‐, 5‐, and 6‐week‐old) or infected (7‐ and 8‐week‐old) plants observed from various treatments. (b) The representative plants in (a) were sampled and analysed for TSWV‐LE infection through reverse transcription‐PCR. A total of 18 plants were used for each treatment. (c) The number of TSWV‐LE‐infected (red) and uninfected (blue) Arabidopsis Ws‐0 plants at different growth stages are shown
FIGURE S3 Silencing of AtPME, AtGBL, and AtCH42 expressions in Arabidopsis plants through virus‐induced gene silencing (VIGS) using ALSV‐based vectors. Partially purified ALSV empty, ALSV‐AtPME, ALSV‐AtGBL, or ALSV‐AtCH42 virions were rub‐inoculated to six leaves of each assayed 6‐week‐old Arabidopsis plant. A representative plant of each treatment was photographed at 35 days after ALSV vector inoculation
TABLE S1 Primers used in this study
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


