Abstract
PURPOSE
Patients with B-cell acute lymphoblastic leukemia who experience relapse after or are resistant to CD19-targeted immunotherapies have limited treatment options. Targeting CD22, an alternative B-cell antigen, represents an alternate strategy. We report outcomes on the largest patient cohort treated with CD22 chimeric antigen receptor (CAR) T cells.
PATIENTS AND METHODS
We conducted a single-center, phase I, 3 + 3 dose-escalation trial with a large expansion cohort that tested CD22-targeted CAR T cells for children and young adults with relapsed/refractory CD22+ malignancies. Primary objectives were to assess the safety, toxicity, and feasibility. Secondary objectives included efficacy, CD22 CAR T-cell persistence, and cytokine profiling.
RESULTS
Fifty-eight participants were infused; 51 (87.9%) after prior CD19-targeted therapy. Cytokine release syndrome occurred in 50 participants (86.2%) and was grade 1-2 in 45 (90%). Symptoms of neurotoxicity were minimal and transient. Hemophagocytic lymphohistiocytosis–like manifestations were seen in 19/58 (32.8%) of subjects, prompting utilization of anakinra. CD4/CD8 T-cell selection of the apheresis product improved CAR T-cell manufacturing feasibility as well as heightened inflammatory toxicities, leading to dose de-escalation. The complete remission rate was 70%. The median overall survival was 13.4 months (95% CI, 7.7 to 20.3 months). Among those who achieved a complete response, the median relapse-free survival was 6.0 months (95% CI, 4.1 to 6.5 months). Thirteen participants proceeded to stem-cell transplantation.
CONCLUSION
In the largest experience of CD22 CAR T-cells to our knowledge, we provide novel information on the impact of manufacturing changes on clinical outcomes and report on unique CD22 CAR T-cell toxicities and toxicity mitigation strategies. The remission induction rate supports further development of CD22 CAR T cells as a therapeutic option in patients resistant to CD19-targeted immunotherapy.
INTRODUCTION
CD19-targeted chimeric antigen receptor (CAR) T cells and bispecific T-cell–engaging antibodies have transformed the treatment of relapsed or chemotherapy-refractory B-cell malignancies, and are now US Food and Drug Administration approved for B-cell leukemias and lymphomas.1-4 Despite a 70%-90% remission induction rate in acute lymphoblastic leukemia (ALL) after CD19-directed CAR T cells and potential for durable response, growing experience suggests that approximately 50% of patients may experience relapse within the first year,3,5-9 the majority with CD19 loss.1-3,8,10 In addition, second CD19 CAR T-cell infusions are frequently unsuccessful for CD19+ relapse, which further limits therapeutic options in these highly refractory patients.11
We developed a novel CD22-targeted/4-1BB CAR T cell12,13 and tested it in a phase I dose-escalation trial in children and young adults with relapsed/refractory CD22+ hematologic malignancies. In our initial report of the first 21 participants with ALL,14 we described a dose-dependent antileukemic response in patients with CD19-negative/dim or CD19+ relapsed ALL, with an acceptable toxicity profile consisting of limited cytokine release syndrome (CRS), minimal neurotoxicity,15 and an efficacy signal not affected by prior CD19 targeting.14
With ongoing enrollment, the remission induction rate remained high, validating CD22 CAR T cells as an effective salvage regimen, which is particularly important for patients in whom CD19 targeting fails. The expanded experience also revealed novel insights into distinct, not previously appreciated toxicities of CD22 CAR T cells, which demonstrates that toxicities of CAR T cells that target a different antigen, even if on the same malignancy, may be unique. These observations led to incorporating unique toxicity mitigation strategies that have provided insight into CAR T-cell therapy optimization. In addition, after the initial report, we modified selection procedures of the apheresis product to systematically improve the consistency and reduce inherent interpatient variability of the starting material. This minor modification enhanced manufacturing feasibility but led to a direct increase in inflammatory toxicities, which prompted dose de-escalation with preserved efficacy at a dose that we previously declared as suboptimal.14 Collectively, these observations broadly inform the field of CAR T-cell therapy and are particularly relevant as novel immunotherapies target alternative antigens in other refractory cancers.
PATIENTS AND METHODS
Participants and Study Design
This phase I dose-escalation study tested CD22 CAR T cells in patients with relapsed/refractory CD22+ B-cell malignancies. Dose levels (DLs), on the basis of transduced CAR T cells per kilogram, included DL1, 3 × 105/kg; DL2, 1 × 106/kg; and DL3, 3 × 106/kg. All patients received fludarabine 25 mg/m2/d on days −4, −3, and −2 and cyclophosphamide 900 mg/m2 on day −2, with CD22 CAR T-cell infusion on day 0. Primary objectives evaluated safety and toxicity amid dose finding and manufacturing feasibility. Secondary objectives included efficacy, CAR T-cell persistence, evaluation of reinfusion strategies, and cytokine profiling.
Eligibility criteria included CD22+ malignancy, age 3-30 years, and adequate performance status and organ function. Patients receiving prior CAR T cells were required to have < 5% circulating CAR T cells. Initial enrollment excluded patients with isolated CNS disease or CNS3 disease; however, with experience, enrollment of those with active CNS disease was in a separate cohort. All participants provided written informed consent or parental permission with minor assent when appropriate. All were treated in the National Institutes of Health (NIH) Clinical Center, and the protocol was approved by the National Cancer Institute institutional review board and the NIH Recombinant DNA Advisory Committee. This report incorporates data from all participants who received CD22 CAR T cells in the study before April 3, 2019, and through a minimum of 30 days postinfusion. Data were locked as of May 8, 2019.
Per protocol, participants who relapsed following an interval allogeneic hematopoietic stem-cell transplantation (HSCT) after a first infusion and had a new apheresis product collected could be re-enrolled and considered as a unique participant. Accordingly, participants 5 and 35 and participants 26 and 46 contributed only once to overall survival (OS) and twice for all other analyses.
CAR T-Cell Manufacturing
The initial dose escalation was previously described with DL2 (1 × 106/kg) expanded (n = 18).14 To enhance CAR T-cell manufacturing feasibility and reduce interpatient product variability, we incorporated CD4/CD8 T-cell selection (CD4/8-TCS) of all starting apheresis material as a single manufacturing change with no further downstream modifications. After this modification, participants experienced heightened inflammatory responses, and the dose was de-escalated for all subsequent patients to DL1-TCS (3 × 105/kg; n = 25).
Toxicity and Efficacy Evaluations
Adverse events were captured using Common Terminology Criteria for Adverse Events (version 4.0) through 30 days post-CAR infusion or resolution. CRS was prospectively graded using the Lee scale.16 American Society for Transplantation and Cellular Therapy CRS consensus grading was retrospectively incorporated.17 Augmented grading for hemophagocytic lymphohistiocytosis (HLH)/macrophage activation syndrome (MAS)–like manifestations was retrospectively performed and modified from definitions used by Neelapu et al.18 Specifically, this was defined by peak ferritin > 100,000 μg/L with at least two of the following criteria:
Hepatic aminotransferases or bilirubin grade ≥ 3
Creatinine grade ≥ 3
Pulmonary edema grade ≥ 3
Evidence of hemophagocytosis on bone marrow aspirate/biopsy.
Disease evaluation and neurotoxicity monitoring methodologies are provided in the Data Supplement (online only).
Statistical Analysis
Descriptive statistics were computed to summarize participant and disease characteristics. Mann-Whitney U test was used to compare unpaired data sets, using a two-tailed P value. Wilcoxon signed rank test was used to compared paired data sets. Fisher’s exact tests were used to compare binary outcomes between two groups. Kaplan-Meier survival curves were used to show event-free survival (EFS) and OS for all participants and relapse-free survival (RFS) limited to those who achieved complete remission (CR). EFS used the earliest of no response, relapse, or death as events, with patients considered to have experienced treatment failure on day 28 if they did not have a CR by that date. Patients who did not have one of these events were censored on their date of last follow-up. OS was calculated from the date of CAR infusion until date of death or last follow-up. RFS was calculated from the date of CAR infusion until the date of relapse or last follow-up among those who went into CR. The patients who died as a result of sepsis or transplant-related causes and were in remission at their death were censored with respect to RFS at their dates of death. Paired t tests compared cognitive test scores pre- to postinfusion. Additional methods are provided in the Data Supplement.
RESULTS
Participant and Disease Characteristics
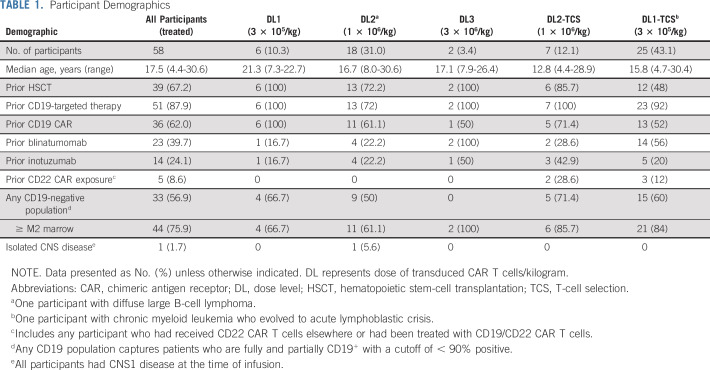
Sixty-four participants were enrolled; 58 received infusion and were evaluable for toxicity (Table 1). Reasons for noninfusion are provided in the Data Supplement. Outcomes for the first 22 participants have been previously described.14,19 All but 2 participants had ALL. One had diffuse large B-cell lymphoma19; another had chronic myelogenous leukemia with ALL blast crisis. The median age was 17.5 years (range, 4.4-30.6 years). Prior therapy included CD19-targeted therapy in 51 (87.9%), HSCT in 39 (67.2%), inotuzumab ozogamicin in 14 (24.1%), and prior CD22 CAR T-cell exposure in 5 (8.6%; incorporating 3 alternative constructs). Thirty-three participants (56.9%) were CD19-negative/partial/dim of whom 2 were partial CD19-expressing with no prior CD19-targeted immunotherapy. All participants had detectable disease: 44 (75.9%) had ≥ M2 marrow, and the median bone marrow involvement was 52%; 11 had extramedullary disease; 1 had isolated CNS disease (CNS2).
TABLE 1.
Participant Demographics
Toxicity
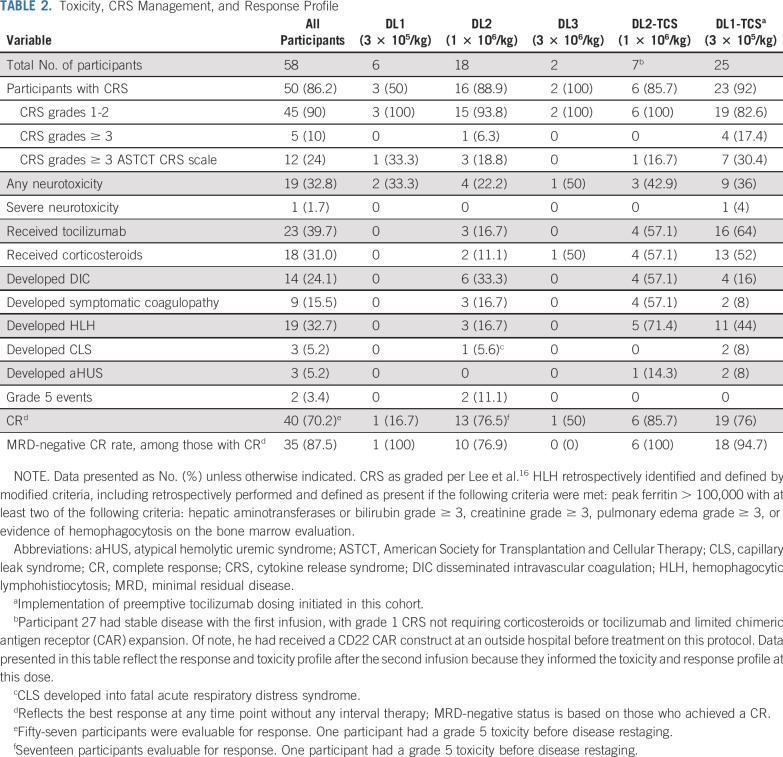
Fifty (86.2%) of 58 participants developed CRS, which was grade 1-2 in 45 (90%; Table 2). The average time to CRS onset was day 7 postinfusion (range, days 3-16); the median duration was 5 days. Two grade 5 events occurred at DL2, one in the setting of gram-negative sepsis and multiorgan dysfunction19 and the other from fulminant capillary leak syndrome (CLS) during CRS, which led to grade 5 acute respiratory distress syndrome. The protocol was transiently halted and modified to incorporate earlier use of tocilizumab and/or corticosteroids in patients with evidence of pulmonary toxicity, with no additional grade 5 events.
TABLE 2.
Toxicity, CRS Management, and Response Profile
Neurotoxicity in the first 22 participants was generally mild with no seizures, encephalopathy, or more severe toxicity.15 Among 58 participants, 19 (32.8%) had one or more reported neurologic manifestation, all of which were grade 1 and 2 toxicities except in one patient who had grade 4 intracranial hemorrhage (ICH). This participant was treated at DL1-TCS and was recovering from CRS without neurotoxicity when he developed a sudden-onset grade 4 ICH on day 17, which required emergent neurosurgical intervention. Laboratory findings at that time revealed normal prothrombin time/partial thromboplastin time and mild thrombocytopenia (platelet count ≥ 100,000/μL for the 5 days preceding the event and was 47,000/μL at the time of ICH). Of note, this participant had concurrent Bacillus cereus bacteremia during CRS—an established risk factor for ICH—and was found to have multifocal hemorrhage concerning for a potential infectious etiology. ICH was attributed to both CRS and infection.20-22 Other symptoms of neurotoxicity were of limited duration or resolved by day 28. We found no substantial change from pre- to postinfusion on tests of attention, executive function, working memory, or processing speed (Data Supplement).
Other toxicities included ocular manifestations (conjunctivitis, photophobia, blurred vision or dry eyes; n = 12); CLS (n = 3); and atypical hemolytic uremic syndrome (aHUS; n = 3), which manifested as hypertension and hemolysis with elevated terminal membrane attack complex requiring eculizumab therapy (2 of whom did not have a prior HSCT). No participant developed sinusoidal obstructive syndrome. Additional toxicities are listed in the Data Supplement.
HLH/MAS-Like Toxicity
After incorporation of CD4/8-TCS at DL2, more participants developed HLH/MAS-like manifestations (DL2, 3 of 18; DL2-TCS, 5 of 7; P = .017), despite a similar incidence and grade of CRS, with a higher frequency of participants at DL2-TCS developing coagulopathy. Thus, we electively de-escalated to DL1-TCS, which effectively decreased the incidence of HLH/MAS-like features and coagulopathy without reducing efficacy (Table 2).
HLH/MAS-like toxicities occurred only in participants who experienced CRS; 19 (38%) of 50 participants with CRS developed HLH/MAS-like manifestations. The average time to onset of HLH-like features was 14 days (range, 7-26 days) post-CAR, and CRS was generally resolved or resolving before the onset of HLH-like manifestations. The incidence of HLH/MAS-like toxicities was higher in those who underwent CD4/8-TCS (16 [55.2%] of 29 v 3 [14.3%] of 21; P = .0039). Peak ferritin was substantially higher in those at DL2 versus DL2-TCS (Fig 1A) and among all who received a product with CD4/8-TCS (Fig 1B), with a median ferritin of 163,200 μg/L (range, 5,769-565,510 μg/L) v 14,349 μg/L (range, 106-590,100 μg/L; P = .0007).
FIG 1.
Cytokine and inflammatory markers, chimeric antigen receptor (CAR) expansion, and toxicity profiling. (A) Comparison of peak ferritin across all 5 doses explored. (B) Comparison of peak values of ferritin between those who received CD4/CD8 T-cell selection (CD4/8-TCS) v CD3/CD28-enriched CAR T-cell products. (C and D) Comparison of peak values of interleukin 6 (IL-6) and IL-1B between those who received CD4/8-TCS v CD3/CD28-enriched CAR T-cell products. (E and F) CAR T-cell expansion shown for all patients in the first 30 days, separated by dose level (DL) and as assessed by absolute CAR T cells on the basis of percent absolute lymphocyte count that was CAR T-cell positive at the various time points, DL1-TCS had limited data at earlier time points. (E) Peak CAR T-cell expansion for all participants by DL in the first 30 days as determined by quantitative polymerase chain reaction (PCR). P not significant. (F) DL3 did not have any samples available for PCR analysis.
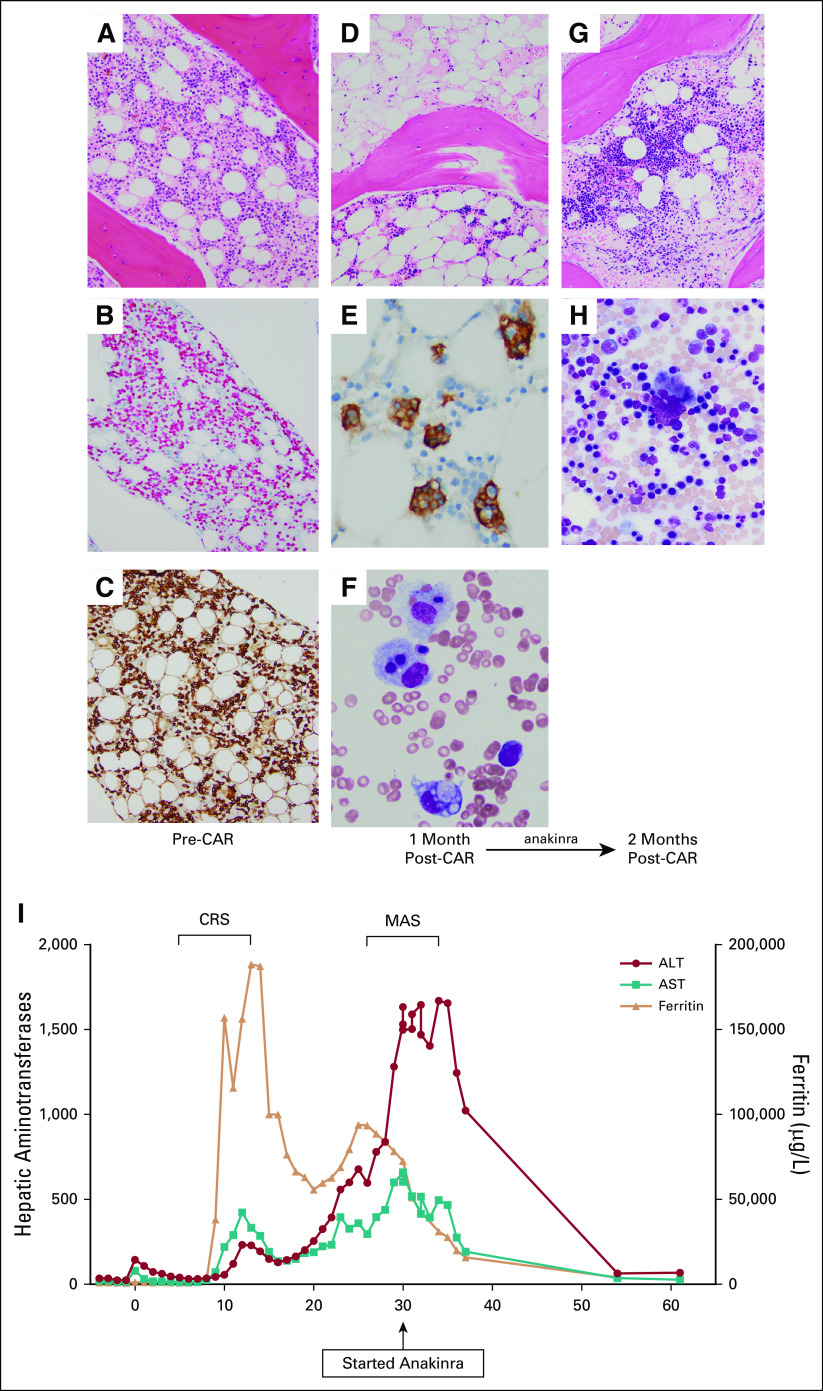
HLH/MAS-like toxicities included laboratory abnormalities (eg, hepatic transaminitis; n = 19) and hemophagocytosis on the day 28 bone marrow evaluation (n = 9). HLH/MAS self-resolved in 5 participants. HLH/MAS-directed treatment was initiated in 14 participants because of worsening laboratory parameters or clinical symptoms (eg, pulmonary edema; renal dysfunction; worsening coagulopathy; steadily increasing inflammatory markers concerning for a worsening trajectory or symptomatic global inflammation, such as noninfectious cholecystitis). Systematic use of anti-interleukin-1 (IL-1) receptor antagonist (anakinra) at starting doses of 5-8 mg/kg/d subcutaneously was incorporated to treat or prevent worsening of HLH/MAS-like manifestations in participants with clinically relevant findings on the basis of data in treatment of secondary HLH/MAS.23,24 Treatment was initiated with anakinra alone (n = 3), corticosteroids plus anakinra (n = 5), or corticosteroids alone (n = 6). All treated participants had resolution of HLH/MAS-like toxicities without any apparent negative impact on response or CAR T-cell expansion (Data Supplement). In one participant, HLH-like manifestations developed at day 28 after bone marrow restaging revealed a minimal residual disease (MRD)–positive CR. With 1 month of anakinra monotherapy, all laboratory abnormities normalized, and subsequent restaging demonstrated ongoing CAR activity with eradication of MRD (Fig 2).
FIG 2.
Manifestations of hemophagotcytic lymphohistiocytosis (HLH)–like toxicities and use of anakinra in participant 33. (A) Bone marrow (BM) biopsy stained with hematoxylin and eosin (HE; original magnification ×200) that shows normocellular marrow with increased blasts. (B) CD79a and (C) CD10 immunohistochemical stains highlight increased B lymphoblasts. (D) BM biopsy (HE original magnification ×200) shows hypocellular marrow with decreased trilineage hematopoiesis and increased macrophages. (E) CD163 immunohistochemical stain highlights hemophagocytic macrophages. (F) BM aspirate stained with modified Giemsa shows hemophagocytic macrophages. (G) BM biopsy (HE original magnification ×200) shows normocellular marrow with trilineage hematopoiesis with no evidence of leukemia or hemophagocytosis. (H) BM aspirate stained with Giemsa (original magnification ×500) shows progressive trilineage hematopoiesis. (I) Clinical course demonstrates separation in time from onset of cytokine release syndrome (CRS) to HLH/macrophage activation syndrome (MAS)–like manifestations. CAR, chimeric antigen receptor.
Cytokine profiling revealed that IL-6, interferon gamma, IL-8, IL-15, IL-10, tumor necrosis factor-α, and IL-1B were all higher in those with CD4/8-TCS than in those with CD3/CD28 enrichment (each P < .05, two-tailed; Figs 1C and 1D; Data Supplement). This included IL-1B, which supports the use of anakinra in these patients.
CAR Expansion and Persistence
Peak CAR expansion occurred between days 14 and 21 postinfusion. The median percentage CAR-positive T cells at peak expansion was 77%, with a median absolute CAR T cells/μL of 480.5 (range, 39.7-11,346/μL; Figs 1E and 1F) and generally higher in those who underwent CD4/8-TCS. In participants with residual lymphomatous disease, bimodal CAR T-cell expansion was occasionally seen; one such case was associated with clonal expansion.25
Response
Fifty-seven participants who underwent infusion were evaluable for response; one participant with grade 5 CLS died before disease restaging. Forty (70.2%) of 57 participants achieved a CR of whom 35 (87.5%) were MRD-negative by flow cytometry (Fig 3A). This includes one participant whose best response (CR) was with a second successive infusion. Response was unaffected by prior CD19-targeted therapy (P = .24) or HSCT (P = .76). Limited to those with ALL, the overall CR and MRD-negative CR rates were 40 (72.7%) of 55 and 35 (63.6%) of 55, respectively. Two CD19+ nonresponders to prior CD19-targeted therapies each achieved an MRD-negative CR with CD22 CAR T cells, which demonstrates that nonresponse of CD19-based immunotherapy did not preclude response to CD22 targeting. CR rates > 70% were seen at DL2, DL2-TCS, and DL1-TCS. Of note, before incorporation of CD4/8-TCS, DL1 (3 × 105/kg), was previously deemed biologically ineffective yet was ultimately chosen as the expansion dose.14
FIG 3.
Response and outcomes after CD22 chimeric antigen receptor (CAR) T-cell infusion. (A) Waterfall plot of best response after CD22 CAR T cell. Participants were stratified by dose level (DL) and cytokine release syndrome (CRS) grade. Note that participants 26 and 46 represent the same patient. The participant was initially treated at DL2, achieved an MRD-negative complete remission (CR) and proceeded to hematopoietic stem-cell transplantation (HSCT), but subsequently experienced relapsed approximately 1 year post-HSCT. After relapse, the participant was re-enrolled as a new participant and had a new apheresis and new product manufactured and was treated at DL1 T-cell selection (TCS), achieved an MRD-negative CR, and proceeded to a second HSCT. Participants 5 and 35 similarly also represent the same patient. Participant 5 was treated at DL1 and was a nonresponder. The participant subsequently underwent allogeneic HSCT with a new donor after additional alternative therapy, with subsequent relapse. This participant underwent a new apheresis and had a new product manufactured at DL2 and achieved a CR. (B) CD22 site density stratified by those who attained MRD-negative CR v those who did not. (C) CD22 site density compared pretreatment with the CD22 site density in those with residual disease or at the time of relapse. (D) Duration in continuous remission among those who achieved CR. Shown are the duration of remission and time of relapse stratified by antigen negative/dim relapse or antigen-positive relapse. Two participants were re-enrolled as new participants as clarified in (A). (E) Relapse-free survival (RFS) from time of infusion for those who achieved remission. (F) Overall survival (OS) stratified by those who proceeded to HSCT (red line) v those who did not (blue line), using a landmark analysis of 126 days after CAR infusion. P = .045. (*) Participant 26 experienced relapsed with antigen-positive disease and was re-enrolled as a new participant with a new product infused and went directly to a second HSCT. (^) Participant 37 received a second infusion for antigen-positive relapse and remains in an ongoing remission at approximately 9 months postinfusion (data not shown). TRM, treatment-related mortality; subject 54 died from complications of transplant; subject 16 died from sepsis following CD22 CAR T cells and did not proceed to transplant.
Participants with prior CD22-targeted therapy (either inotuzumab [n = 14] or CD22 CAR [n = 5]) had decreased MRD-negative CR rates (P = .039), were more likely to have residual CD22-dim/partial disease at restaging (6 of 17 v 2 of 40; P = .006), and had shorter remission durability (3 months [range, 2-6 months] v 6 months [range, 2-14 months]) than those who did not receive prior CD22-targeted therapy, with approximately one half experiencing relapse with CD22-dim/negative disease. The median baseline CD22 antigen density was higher among those who achieved MRD-negative CR than those who did not (P = .02; Fig 3B). CD22 expression was lower in those with residual disease or at the time of relapse (P ≤ .001), consistent with our observation that CD22 modulation is an important mechanism of immune escape14 (Fig 3C). Approach and response to second infusions are provided in the Data Supplement.
Fourteen participants (13 individual patients) proceeded to HSCT, including all who achieved an MRD-negative CR and not had a prior HSCT, except for the participant who developed ICH (Fig 3D). Nine participants had CD19-negative/partial expression. In 10 participants, this represented a first HSCT; decisions with regard to a second HSCT were based on individual patient and provider preferences. The median time from CAR T-cell infusion to HSCT was 72 days (range, 49-126 days). All but 1 participant proceeded to HSCT while in MRD-negative CR. Six participants experienced post-HSCT relapse, including 2 for whom this represented a second transplant.
With a median potential follow-up of 24 months, the median OS and RFS (restricted to those in CR) were 13.4 months (95% CI, 7.7 to 20.3 months) and 6.0 months (95% CI, 4.1 to 6.5 months), respectively (Fig 3E). Median EFS for all participants, including nonresponders and deaths before day 28, was 3.2 months (95% CI, 1.4 to 5.5 months). Thirty participants (75%) experienced relapse, the majority with CD22-negative/dim disease. Using a time-varying covariate analysis, receipt of HSCT was somewhat favorably associated with OS (P = .09) and very favorably associated with RFS (P = .0083) and EFS (P = .016; Fig 3F). Twenty-one participants remain alive, with a median follow-up of 9.7 months (range, 1.1-43.9 months), and 11 remain in remission of whom 3 received additional therapy for relapsed disease. One participant is in an ongoing CR ≥ 3.5 years postinfusion without any interval therapy.
Dose Escalation and Product Manufacturing
Product manufacturing was successful in 63 of 64 participants. High peripheral leukemia burden and/or high monocyte frequencies negatively affected CAR T-cell manufacturing by inhibiting transduction and expansion of CAR T cells (Fig 4A). Incorporation of CD4/8-TCS effectively salvaged apheresis material unable to be used for CAR T-cell manufacturing using previously described selection methods26 (Fig 4B). Transduction efficiency, fold expansion, and CD3 percent consistency and recovery were all improved after TCS (Figs 4C-4G). Details of product characteristics are listed in the Data Supplement.
FIG 4.
Impact of CD4/CD8 T-cell selection (TCS) on the starting apheresis product. (A) Shown are three examples of products manufactured using the CD4/CD8 selection method. Flow cytometry plots show that participants 32 and 40 had elevated frequencies of CD19/CD22 B cells in their starting apheresis. Participant 43 had elevated frequencies of CD14 monocytes and CD56 natural killer (NK) cells, which precluded generation of a CD19 chimeric antigen receptor (CAR) T-cell product elsewhere. Upon selection, all these products showed high T-cell purities (> 85%). In participants 32 and 40, ≥ 45% of the cells in the starting apheresis product were CD22-expressing B cells, and approximately 35% of the cells were T cells. The final product displayed high transduction efficiencies as measured by protein L, and all showed high fold expansion (FE). (B) Shown is a direct comparison between two different manufacturing methods, CD3/CD28 enrichment v CD4/CD8 selection. A single apheresis product was cryopreserved into multiple aliquots and used to start the manufacturing processes. Participant 25 exhibited very high frequencies of monocytes and NK cells in the apheresis material. Upon CD3/CD28 enrichment, the final product did not transduce or expand (upper right). CD4/CD8 selection, however, showed that the product could be recovered to a high level of T-cell purity postselection (bottom left) and exhibited both high transduction efficiencies and FE of the final product (bottom right). FE was calculated by dividing the final total cell number by the starting cell number on the day of transduction (day 2). (C-G) Comparison of the CD22 CAR T-cell product across manufacturing strategies. (C) Transduction efficiency and (D) FE were assessed in patient samples that had undergone manufacturing using elutriation (n = 6) or CD3/CD28 enrichment (n = 19) and compared with CD4/CD8 selection (n = 26). The CD3 percentage was evaluated in the (E) postapheresis starting material and in the post-CD3/CD28 enrichment or (F) CD4/CD8 TCS product. (G) CD3 T-cell recovery was calculated for available samples postenrichment or postselection.
DISCUSSION
With growing use of CD19-targeted therapies,3,7,11,27 CD19-negative relapse is increasingly recognized as a cause of therapeutic failure,19,28-31 and treatment options are limited. In this expanded experience with the first, to our knowledge, successful CAR to target an alternative antigen on ALL, we establish CD22 CAR T cells as an effective salvage therapy for patients who have experienced relapse after or are refractory to CD19-targeted therapies. The ability to render this highly refractory population into MRD-negative remission effectively enabled patients to proceed to a consolidative HSCT, an established treatment paradigm for patients with relapse/refractory ALL.32,33 Remission durability was adversely affected by prior CD22-directed therapies and antigen downregulation, which suggests that durability might be improved by avoidance of prior CD22 targeting and antigen upregulation.34
CRS rates after CD22 CAR T cells was comparable to reports with CD19 CAR T cells. However, toxicities distinct from CD19 CAR T cells included aHUS, severe CLS (out of proportion to CRS), and ocular manifestations, reminiscent of other CD22-targeted approaches.35,36 Despite these associations with endothelial injury, neurotoxicity seemed to be less severe than with CD19 CAR T cells, which warrants additional study, particularly with endothelial activation postulated as a mechanism for CAR T-cell–mediated neurotoxicity.37-39 Nonetheless, these results suggest that CD22 CAR T cells may represent an alternative option in those at higher risk for neurotoxicity but will require a larger experience to conclusively establish.
HLH/MAS toxicities were seen at a relatively high frequency and heightened after a manufacturing modification that improved expansion and transduction efficiency. There are multiple CAR T-cell manufacturing processes currently being used, with a suggestion that the platform can affect product potency. This is the first clear demonstration that a single minor manufacturing change in the context of one trial was clinically effective, which illustrates the importance of manufacturing modifications in outcomes.19
HLH/MAS-like manifestations have typically been considered in the spectrum of severe CRS. In this study, HLH/MAS generally developed outside the temporal context of CRS, which suggests a unique pathophysiology. Additional efforts, both clinically and preclinically, are ongoing to further explore this toxicity. Use of anakinra for treatment of HLH-like manifestations, independent of neurotoxicity, has been incorporated into our toxicity management, and on the basis of preliminary experience, additional study of the role of anakinra is warranted. Collectively, the toxicity profile that emerged from this relatively large experience with CD22 CAR T cells in the same disease for which there is extensive experience with CD19 CAR T cells indicates that target and/or construct differences will affect outcomes.
In summary, this report confirms CD22 CAR T cells as a highly effective salvage option while providing novel insights into CAR T-cell therapy broadly. These results strongly support further development of CD22 CAR T cells in a pivotal phase II trial and provide a foundation for the first combinations of targeted immunotherapy using clinically validated CAR constructs with the potential to improve upon response and remission durability after targeted immunotherapy for B-cell malignancies.
ACKNOWLEDGMENT
We gratefully acknowledge the study participants and their families, referring medical care teams, the faculty and staff of the NIH Clinical Center who provide their expertise in the management of the study participants, and the data managers Showri Kakumanu and Ekaterina Nikitina involved with this work.
SUPPORT
Supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and National Institutes of Health Clinical Center; a Stand Up to Cancer–St Baldrick’s Pediatric Dream Team translational research grant (SU2C-AACR-DT113); and a Cooperative Research and Development Agreement with Juno Therapeutics. Stand Up to Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
AUTHOR CONTRIBUTIONS
Conception and design: Nirali N. Shah, Steven L. Highfill, Pamela L. Wolters, Amanda Ombrello, Seth M. Steinberg, Staci Martin, Crystal L. Mackall, David F. Stroncek, Terry J. Fry
Administrative support: Bonnie Yates, Cindy Delbrook, Leah Hoffman, Lauren Little
Provision of study material or patients: Nirali N. Shah, Steve L. Highfill, Haneen Shalabi, Cindy Delbrook, Minh Tran, David F. Stroncek, Terry J. Fry
Collection and assembly of data: Nirali N. Shah, Steven L. Highfill, Haneen Shalabi, Bonnie Yates, Jianjian Jin, Pamela L. Wolters, Staci Martin, Cindy Delbrook, Leah Hoffman, Lauren Little, Anusha Ponduri, Haiying Qin, Haris Qureshi, Dalia Salem, Hao-Wei Wang, Constance Yuan, Maryalice Stetler-Stevenson, Sandhya Panch, Minh Tran, Terry J. Fry
Data analysis and interpretation: Nirali N. Shah, Steven L. Highfill, Haneen Shalabi, Bonnie Yates, Pamela L. Wolters, Seth M. Steinberg, Staci Martin, Anusha Ponduri, Haiying Qin, Haris Qureshi, Alina Dulau-Florea, Dalia Salem, Hao-Wei Wang, Constance Yuan, Maryalice Stetler-Stevenson, Sandhya Panch, Crystal L. Mackall, David F. Stroncek, Terry J. Fry
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jianjian Jin
Stock and Other Ownership Interests: Gilead Sciences, Gilead Sciences (I)
Patents, Royalties, Other Intellectual Property: Patent: The Use of Gas Permeable Culture Vessels for the Growth of Tumor-Infiltrating Lymphocytes
Pamela L. Wolters
Stock and Other Ownership Interests: Bristol-Myers Squibb
Crystal L. Mackall
Stock and Other Ownership Interests: Lyell Immunopharma, Allogene, Vor Pharmaceuticals, Apricity Health
Consulting or Advisory Role: Bryology, Vor Biopharma, Apricity Health, TPG, Allogene, PACT Pharma, Nektar, Lyell Immunopharma, Neoimmune Tech
Patents, Royalties, Other Intellectual Property: An inventor on numerous patents related to CAR therapeutics and royalties from NIH for the CD22 CAR patent licensed to Juno Therapeutics.
Travel, Accommodations, Expenses: Neoimmune Tech, Roche, Nektar
Other Relationship: Lyell Immunopharma
Terry J. Fry
Research Funding: Elevate Bio (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. doi: 10.1158/1078-0432.CCR-18-2337. Jen EY, Xu Q, Schetter A, et al: FDA approval: Blinatumomab for patients with B-cell precursor acute lymphoblastic leukemia in morphologic remission with minimal residual disease. Clin Cancer Res, 25:473-477, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Przepiorka D, Ko CW, Deisseroth A, et al. FDA approval: Blinatumomab. Clin Cancer Res. 2015;21:4035–4039. doi: 10.1158/1078-0432.CCR-15-0612. [DOI] [PubMed] [Google Scholar]
- 3.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34:4381–4389. doi: 10.1200/JCO.2016.67.3301. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulte ED, Vallejo J, Przepiorka D, et al. FDA supplemental approval: Blinatumomab for treatment of relapsed and refractory precursor B-cell acute lymphoblastic leukemia. Oncologist. 2018;23:1366–1371. doi: 10.1634/theoncologist.2018-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haso W, Lee DW, Shah NN, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haso W, Qin H, Zhang L, et al: CD22-targeted chimeric antigen receptor (CAR) T cells containing the 4-1BB costimulatory domain demonstrate enhanced persistence and superior efficacy against B-cell precursor acute lymphoblastic leukemia (ALL) compared to those containing CD28. Blood 122:1431, 2013. [Google Scholar]
- 14.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shalabi H, Wolters PL, Martin S, et al. Systematic evaluation of neurotoxicity in children and young adults undergoing CD22 chimeric antigen receptor T-cell therapy. J Immunother. 2018;41:350–358. doi: 10.1097/CJI.0000000000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. doi: 10.1182/blood-2014-05-552729. Lee DW, Gardner R, Porter DL, et al: Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188-195, 2014 [Errata: Blood 124:188-195, 2014; Blood 126:1048, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. doi: 10.1016/j.bbmt.2018.12.758. Lee DW, Santomasso BD, Locke FL, et al: ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant, 25:624-638, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shalabi H, Kraft IL, Wang HW, et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica. 2018;103:e215–e218. doi: 10.3324/haematol.2017.183459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hori YS, Kodera S, Nagai Y, et al: Fulminant Bacillus cereus septicaemia with multiple organ ischaemic/haemorrhagic complications in a patient undergoing chemotherapy for acute myelogenous leukaemia. BMJ Case Rep 2017:bcr-2017-219996, 2017. [DOI] [PMC free article] [PubMed]
- 21.Vodopivec I, Rinehart EM, Griffin GK, et al. A cluster of CNS infections due to B. cereus in the setting of acute myeloid leukemia: Neuropathology in 5 patients. J Neuropathol Exp Neurol. 2015;74:1000–1011. doi: 10.1097/NEN.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 22.Tusgul S, Prod’hom G, Senn L, et al. Bacillus cereus bacteraemia: Comparison between haematologic and nonhaematologic patients. New Microbes New Infect. 2016;15:65–71. doi: 10.1016/j.nmni.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15:401–408. doi: 10.1097/PCC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 24.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: Reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah NN, Qin H, Yates B, et al. Clonal expansion of CAR T cells harboring lentivector integration in the CBL gene following anti-CD22 CAR T-cell therapy. Blood Adv. 2019;3:2317–2322. doi: 10.1182/bloodadvances.2019000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stroncek DF, Lee DW, Ren J, et al. Elutriated lymphocytes for manufacturing chimeric antigen receptor T cells. J Transl Med. 2017;15:59. doi: 10.1186/s12967-017-1160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40:187–195. doi: 10.1097/CJI.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–2410. doi: 10.1182/blood-2015-08-665547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacoby E, Nguyen SM, Fountaine TJ, et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun. 2016;7:12320. doi: 10.1038/ncomms12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merli P, Algeri M, Del Bufalo F, et al. Hematopoietic stem cell transplantation in pediatric acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2019;14:94–105. doi: 10.1007/s11899-019-00502-2. [DOI] [PubMed] [Google Scholar]
- 33.Bader P, Salzmann-Manrique E, Balduzzi A, et al. More precisely defining risk peri-HCT in pediatric ALL: Pre- vs post-MRD measures, serial positivity, and risk modeling. Blood Adv. 2019;3:3393–3405. doi: 10.1182/bloodadvances.2019000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramakrishna S, Highfill SL, Walsh Z, et al. Modulation of target antigen density improves CAR T-cell functionality and persistence. Clin Cancer Res. 2019;25:5329–5341. doi: 10.1158/1078-0432.CCR-18-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreitman RJ, Dearden C, Zinzani PL, et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia. 2018;32:1768–1777. doi: 10.1038/s41375-018-0210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wayne AS, Shah NN, Bhojwani D, et al. Phase 1 study of the anti-CD22 immunotoxin moxetumomab pasudotox for childhood acute lymphoblastic leukemia. Blood. 2017;130:1620–1627. doi: 10.1182/blood-2017-02-749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackall CL, Miklos DB. CNS endothelial cell activation emerges as a driver of CAR T cell-associated neurotoxicity. Cancer Discov. 2017;7:1371–1373. doi: 10.1158/2159-8290.CD-17-1084. [DOI] [PubMed] [Google Scholar]