Abstract
PURPOSE
Trastuzumab deruxtecan (T-DXd, formerly DS-8201a) is a novel human epidermal growth factor receptor 2 (HER2)-targeted antibody drug conjugate (ADC) with a topoisomerase I inhibitor payload. A dose escalation and expansion phase I study evaluated the safety and activity of T-DXd in patients with advanced HER2-expressing/mutated solid tumors. Here, results for T-DXd at the recommended doses for expansion (RDE) in patients with HER2-low (immunohistochemistry [IHC] 1+ or IHC 2+/in situ hybridization−) breast cancer (ClinicalTrials.gov identifier: NCT02564900) are reported.
PATIENTS AND METHODS
Eligible patients had advanced/metastatic HER2-low–expressing breast cancer refractory to standard therapies. The RDE of 5.4 or 6.4 mg/kg T-DXd were administered intravenously once every 3 weeks until withdrawal of consent, unacceptable toxicity, or progressive disease. Antitumor activity and safety were assessed.
RESULTS
Between August 2016 and August 2018, 54 patients were enrolled and received ≥ 1 dose of T-DXd at the RDE. Patients were extensively pretreated (median, 7.5 prior therapies). The confirmed objective response rate by independent central review was 20/54 (37.0%; 95% CI, 24.3% to 51.3%) with median duration of response of 10.4 months (95% CI, 8.8 month to not evaluable). Most patients (53/54; 98.1%) experienced ≥ 1 treatment-emergent adverse event (TEAE; grade ≥ 3; 34/54; 63.0%). Common (≥ 5%) grade ≥ 3 TEAEs included decreases in neutrophil, platelet, and WBC counts; anemia; hypokalemia; AST increase; decreased appetite; and diarrhea. Three patients treated at 6.4 mg/kg suffered fatal events associated with T-DXd–induced interstitial lung disease (ILD)/pneumonitis as determined by an independent adjudication committee.
CONCLUSION
The novel HER2-targeted ADC, T-DXd, demonstrated promising preliminary antitumor activity in patients with HER2-low breast cancer. Most toxicities were GI or hematologic in nature. ILD is an important identified risk and should be monitored closely and proactively managed.
INTRODUCTION
Over the past 2 decades, the availability of human epidermal growth factor receptor 2 (HER2)-targeted therapies has significantly improved clinical outcomes for patients with HER2-positive breast cancer,1,2 defined as tumors with high levels of HER2 protein expression as assessed by immunohistochemistry (IHC) and/or are HER2 amplified as assessed by in situ hybridization (ISH).3,4 However, between 40% and 50% of patients with breast cancer have tumors with low HER2 expression (defined as IHC 1+ or IHC 2+ and ISH−), which is a heterogeneous population including both luminal-type hormone receptor (HR)-positive and triple-negative breast cancers.5-7 According to prevailing HER2 testing guidelines, these patients are considered to have HER2-negative breast cancer, for whom currently available HER2-targeted treatments have not proven effective and, therefore, are not recommended.8-10
Trastuzumab deruxtecan (T-DXd; formerly DS-8201a) is a novel HER2-targeted antibody drug conjugate (ADC) that was designed to effectively deliver a potent topoisomerase I inhibitor payload (an exatecan derivative) to HER2-expressing cancer cells and thereby limit potential systemic toxicity.11-13 The payload is linked to a humanized anti-HER2 antibody by a unique cleavable peptide-based linker that is stable in plasma and cleaved by lysosomal cathepsins, which are upregulated in cancer cells.12 After cleavage, the released drug is cell membrane permeable by design, allowing for a bystander effect in which the released payload can affect tumor cells in close proximity regardless of their HER2 expression status.13 In preclinical studies, T-DXd demonstrated antitumor activity in a variety of tumor types, including those with low HER2 expression.12 Antitumor activity in heterogeneous or HER2-low–expressing tumors may be related to the bystander effect and the combination of the high drug-to-antibody ratio of T-DXd with the high potency payload.11-13 This combination may lead to higher levels of cytotoxicity at the tumor site in spite of relatively low levels of T-DXd binding expected in cells with low HER2 expression.
A phase I, 2-part, first-in-human study of T-DXd was initiated to determine the recommended dose for expansion (RDE) and evaluate the safety, tolerability, and clinical activity in patients with advanced HER2-expressing (low or high) or HER2-mutated solid tumors. This report presents the clinical activity and safety of T-DXd at the RDE in patients with HER2-low–expressing breast cancer from the phase I trial.
PATIENTS AND METHODS
Study Design and Patients
Detailed methods have been previously published.14-16 This was a first-in-human, phase I, nonrandomized, open-label, multiple-dose study conducted in 2 parts (Fig 1) at 8 sites in the United States and 6 in Japan (ClinicalTrials.gov identifier: NCT02564900; ClinicalTrials.jp: JapicCTI-152978). Enrollment began on August 28, 2015.
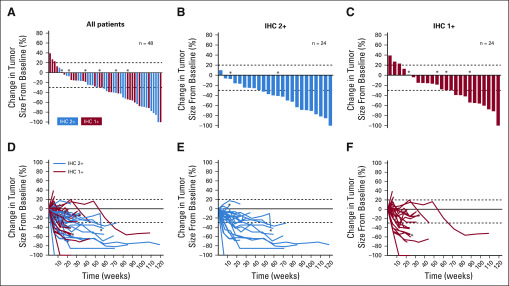
FIG 1.
Best percent change in tumor size and percent change in tumor size, respectively, over time for individual patients in (A, D) the entire human epidermal growth factor receptor 2 (HER2)-low population, (B, E) the HER2 immunihistochemistry (IHC) 2+ group, and (C, F) the HER2 IHC 1+ group. Data cutoff was February 1, 2019. Dotted lines denote 30% decrease and 20% increase in tumor size cutoffs for partial response and progressive disease, respectively. Tumor responses shown are per independent central review. The IHC status subgroups represent the IHC status as determined by local assessment. (*) HR negative. HR, hormone receptor.
Part 1 was a dose escalation (0.8 to 8.0 mg/kg intravenous infusion every 3 weeks) guided by the modified continuous reassessment method and escalation with overdose control principle to determine the safety (including dose-limiting toxicities), maximum tolerated dose, and RDE for T-DXd. Part 2 was a dose expansion to further assess the safety, tolerability, and clinical activity at the RDE of 5.4 and 6.4 mg/kg every 3 weeks. Patients were enrolled in 1 of 5 cohorts based on cancer type and HER2 status (Data Supplement); those with HER2-low breast cancer were enrolled in cohorts 2c and 2e. For the purpose of this analysis, patients with HER2-low advanced/unresectable or metastatic breast cancer who received the 5.4- or 6.4-mg/kg dose of T-DXd from parts 1, 2c, and 2e were pooled.
Eligible patients were ≥ 18 years of age in the United States or ≥ 20 years in Japan and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, left ventricular ejection fraction ≥ 50% within the 28 days before study registration, and measurable disease based on RECIST version 1.1 (see the Data Supplement for full eligibility criteria). For HER2-low breast cancer, patients who could not tolerate standard treatment or with advanced/unresectable or metastatic disease that was refractory to it, with no standard treatment available, or for which clinically meaningful treatments were exhausted were included. HER2 status was evaluated locally using archival tissue; however, a retrospective central assessment of HER2 status was also implemented later in the trial. HER2-low breast cancer was defined as IHC 2+/ISH−, IHC 1+/ISH−, or IHC 1+/ISH untested.
All patients provided written informed consent, and independent ethics committees or institutional review boards at each site approved the study protocol. The study was conducted in accordance with the protocol, the International Conference on Harmonization guidelines for Good Clinical Practice, the Declaration of Helsinki, and country-specific regulations.
Treatment and Assessments
T-DXd was administered by intravenous infusion once every 3 weeks until withdrawal of consent, unacceptable toxicity, or progressive disease (PD). Tumors were assessed by magnetic resonance imaging or computed tomography every 6 weeks for the first 24 weeks after administration of the first dose. Subsequently, assessments were made every 12 weeks. Tumor response was evaluated per RECIST version 1.1 by the investigators and retrospectively by a blinded, independent central imaging facility.
Safety assessments were performed at each study visit to evaluate serious adverse events, treatment-emergent adverse events (TEAEs), physical examination findings, vital sign measurements, and standard clinical laboratory parameters. TEAEs were graded per the Common Terminology Criteria for Adverse Events version 4.0. Assessment of left ventricular ejection fraction and management of interstitial lung disease (ILD) were previously reported.16 An international, independent adjudication committee reviewed all patients with potential ILD. ILD may have included 44 Medical Dictionary for Regulatory Activities Terminology (MedDRA) preferred terms—42 that comprised the standardized MedDRA query for ILD and 2 that were requested by the adjudication committee (respiratory failure and acute respiratory failure). In accordance with the guidelines from the Japanese Respiratory Society,17 patients speculated to have ILD were subject to early diagnosis through imaging, laboratory tests, and pulmonary consultation. For patients with moderate to severe disease, withdrawal of study medication and management with steroids was recommended.
Statistical Analysis
The study analysis population included all patients with HER2-low breast cancer who provided informed consent, were enrolled in the dose escalation or expansion, and who received T-DXd at the 5.4- or 6.4-mg/kg doses. The safety analysis population included patients who received ≥ 1 dose.
Clinical activity endpoints were calculated separately for investigator-assessed and centrally reviewed data. Exploratory analyses of outcomes by IHC status, HR status, dose, and prior HER2-targeted therapy were performed post hoc.
Statistical computation was performed with SAS version 9.3 (SAS Institute, Cary, NC). Best percent change in the sum of the diameters of measurable tumors, demographic characteristics, and safety data were summarized with descriptive statistics. Clinical activity endpoints are defined in the Data Supplement. Objective response rate (ORR) and disease control rate were reported as point estimates and 95% exact binomial CIs. Time-to-event statistics were calculated with the Kaplan-Meier method and the associated CIs using the Brookmeyer-Crowley method.
RESULTS
Patient Disposition and Baseline Characteristics
A total of 54 patients with advanced HER2-low breast cancer were enrolled and treated with ≥ 1 dose of T-DXd at 5.4 (n = 21) or 6.4 mg/kg (n = 33). As of February 1, 2019, 11/21 (52.4%) patients in the 5.4-mg/kg group and 3/33 (9.1%) in the 6.4-mg/kg group were still receiving T-DXd. The most common reason for discontinuation in both groups was PD per RECIST (5.4 mg/kg: 9/21 [42.9%]; 6.4 mg/kg: 16/33 [48.5%]; Data Supplement). Median treatment duration for the overall population was 6.1 (range, 0.7-29.2) months and was not significantly different between dose groups (data not shown).
Baseline demographic and clinical characteristics of patients with HER2-low breast cancer are presented in Table 1. These patients were heavily pretreated; 83.3% had previously received ≥ 5 prior cancer regimens. Ten patients had prior HER2-targeted therapy, including 1 patient who received it as part of a clinical trial. Nine of these 10 patients were reported to have had past biopsies showing HER2-positive disease, with the most recent biopsies indicating HER2-low disease.
TABLE 1.
Patient Demographics and Baseline Characteristics
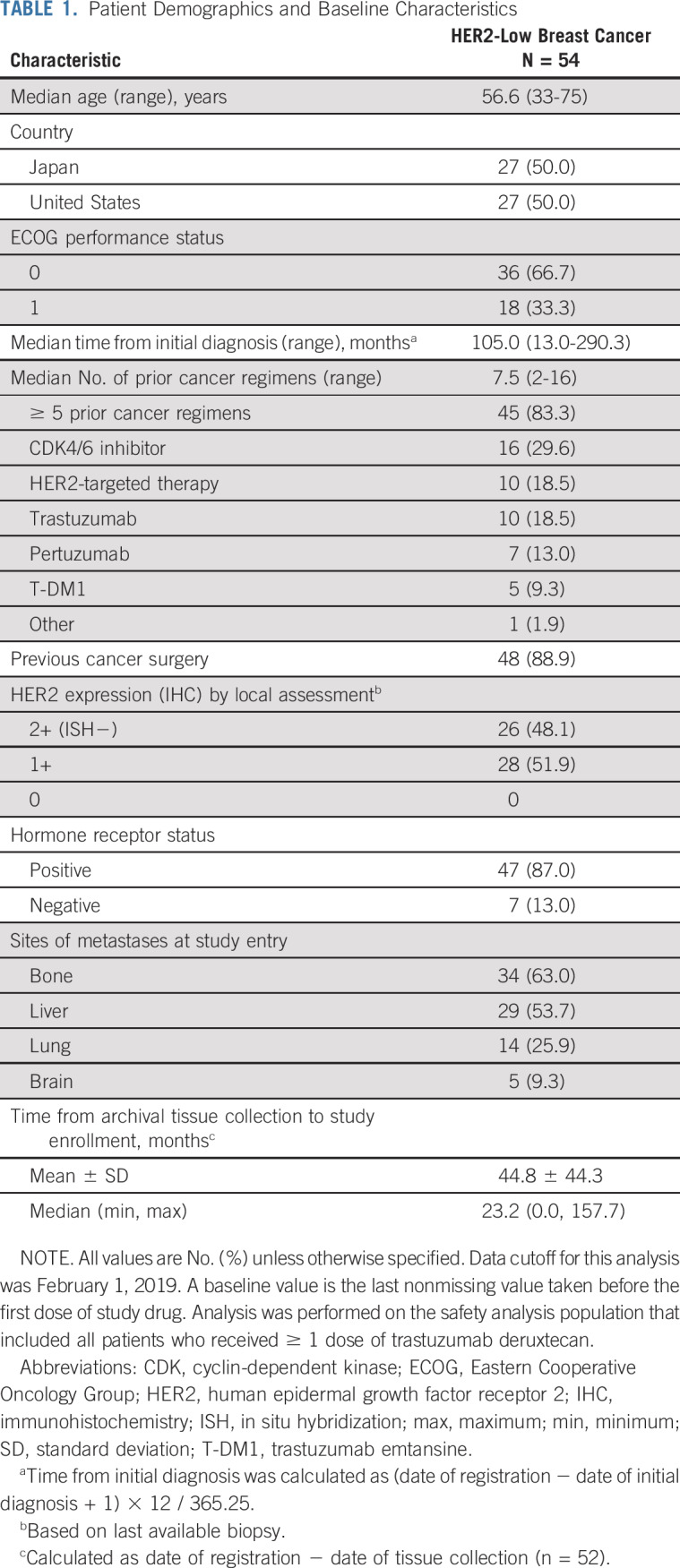
All patients had visceral disease at baseline. Common sites of metastases included bone (63.0%), liver (53.7%), and lung (25.9%). The majority of patients (87.0%) had HR-positive disease (estrogen receptor–positive, 47/54 [87.0%]; progesterone receptor–positive, 33/54 [61.1%]). Based on local assessment of target tumors, HER2 expression was IHC 1+ for 51.9% and IHC 2+ for 48.1% of patients. When target tumors were assessed retrospectively by a central laboratory, HER2 expression was IHC 0 for 5/54 (9.3%), IHC 1+ for 30/54 (55.6%), and IHC 2+ for 14/54 (25.9%) of patients. For 5 patients, tissue samples were either not submitted or unevaluable by the central laboratory. The concordance rate was 70.8% for IHC 1+ and 40.0% for IHC 2+.
Clinical Activity
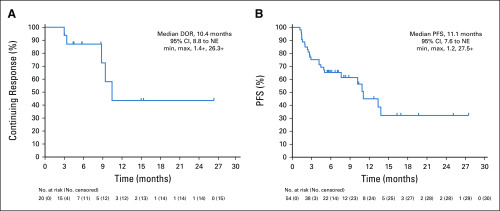
Independent central review (ICR) and investigator report of clinical activity endpoints for the overall HER2-low population are presented in Table 2. The ICR-confirmed ORR was 37.0%, and the investigator-reported confirmed ORR was 44.4%. Percentage change in tumor size from baseline per ICR is presented in Fig 1. The median duration of response was 10.4 months (Fig 2), median progression-free survival (PFS) was 11.1 months (Fig 2), and median overall survival was 29.4 (95% CI, 12.9 to 29.4) months.
TABLE 2.
Clinical Activity Outcomes for HER2-Low Patients
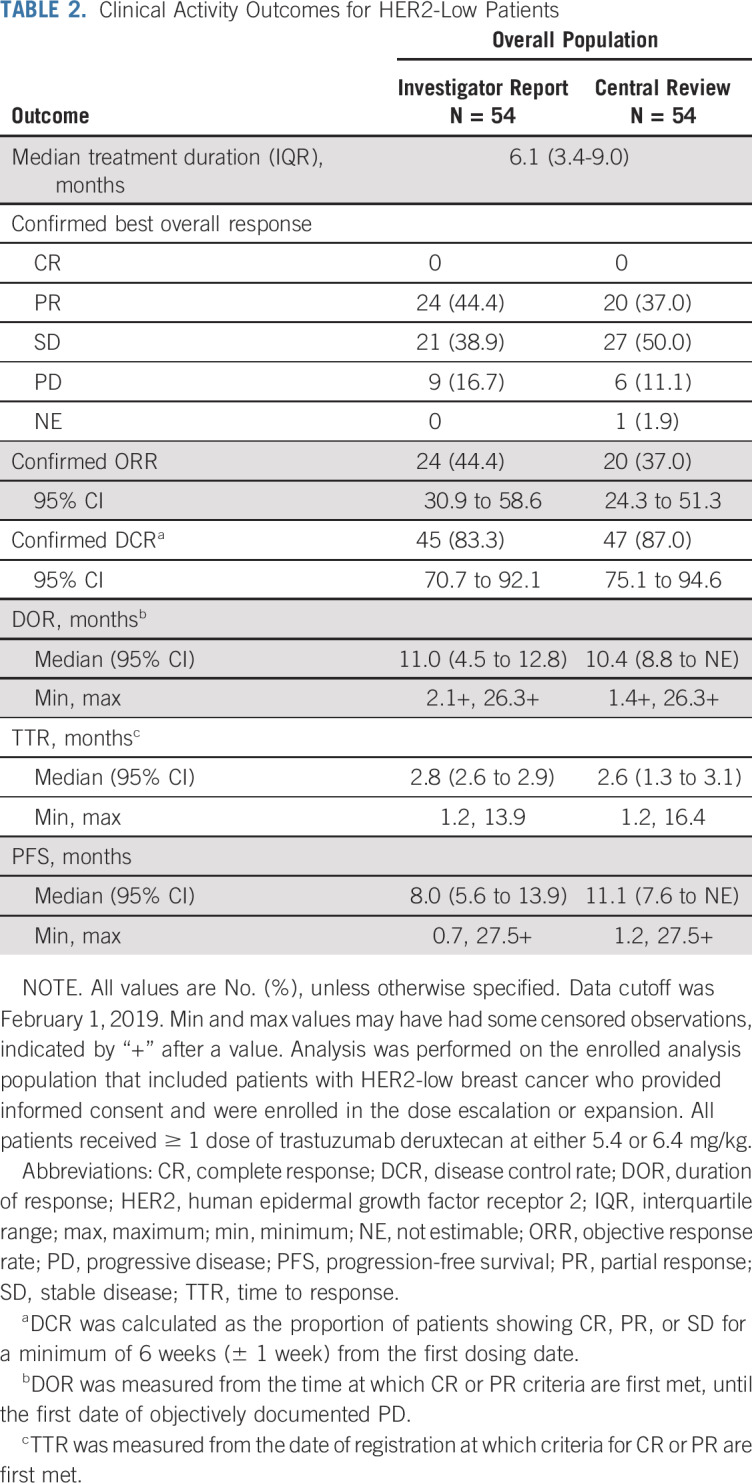
FIG 2.
The Kaplan-Meir estimates for (A) duration of response (DOR) and (B) progression-free survival (PFS) based on independent central assessment. Data cutoff was February 1, 2019. Tick marks within the graphs and a “+” in the text indicate censoring. NE, not estimable.
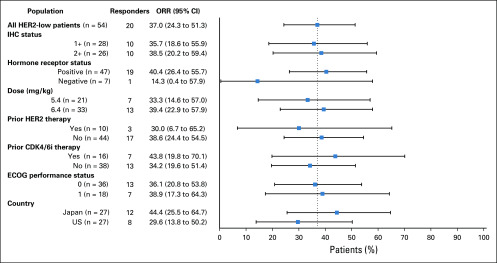
Activity of T-DXd was observed across multiple patient subgroups. The ICR-confirmed ORRs for key subgroups based on IHC status, HR status, dose, prior HER2-targeted therapy, prior cyclin-dependent kinase (CDK) 4/6 inhibitor therapy, ECOG status, and country are reported in Fig 3.
FIG 3.
Objective response rates and 95% CIs for subgroups of patients with human epidermal growth factor receptor 2 (HER2)-low breast cancer. Data cutoff was February 1, 2019. Dashed line separates the objective response rate (ORR) for the overall HER2-low population from the that for the subgroups. ORR was calculated with tumor responses per independent central review. The immunohistochemistry (IHC) status subgroups represent the IHC status as determined by local assessment. CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; ECOG, Eastern Cooperative Oncology Group.
Safety
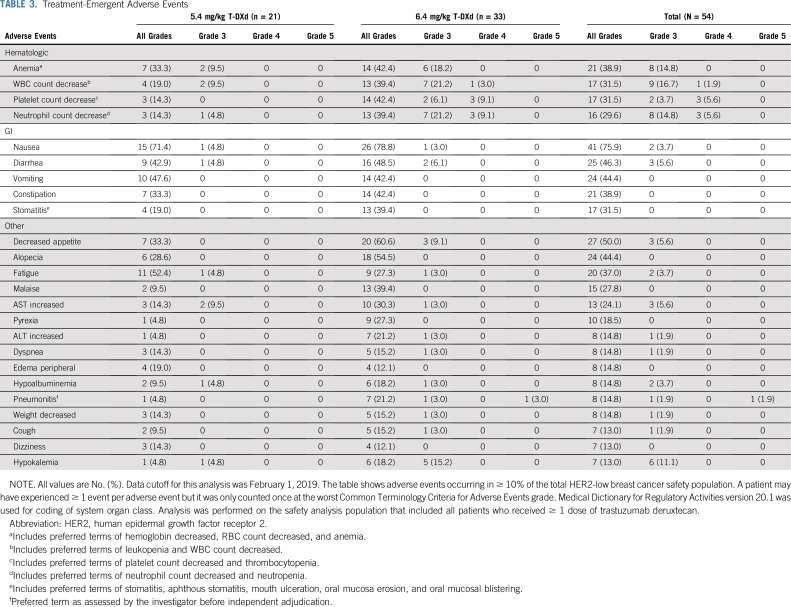
Fifty-three of 54 patients (98.1%) experienced ≥ 1 TEAE. The most common any-grade TEAEs (> 30% of patients) were nausea, decreased appetite, diarrhea, alopecia, vomiting, anemia, constipation, fatigue, WBC count decrease, platelet count decrease, and stomatitis. Grade ≥ 3 TEAEs occurred in 34/54 (63.0%) patients (Table 3), with neutrophil count decrease, WBC count decrease, anemia, hypokalemia, platelet count decrease, AST increase, decreased appetite, febrile neutropenia, cellulitis, and diarrhea occurring in ≥ 5% of patients. Any TEAE occurring in ≥ 10% of the HER2-low population is presented by dose and total in Table 3.
TABLE 3.
Treatment-Emergent Adverse Events
TEAEs leading to treatment discontinuation occurred in 11/54 (20.4%) patients; the most common events were pneumonitis (n = 7) and ILD (n = 3). T-DXd dose was reduced because of a TEAE for 12/54 (22.2%) patients. Treatment interruption because of a TEAE occurred for 21/54 (38.9%) patients. A summary of TEAEs by dose group is reported in the Data Supplement. Overall, patients who had 6.4 mg/kg T-DXd had a higher proportion of grade ≥ 3 TEAEs, serious TEAEs, TEAEs leading to discontinuation, TEAEs resulting in dose reduction, and deaths.
Adverse events were associated with the death of 3 patients with HER2-low breast cancer: 2 TEAEs of pneumonitis and 1 TEAE of ILD. All occurred with the 6.4-mg/kg dose and were drug related.
One patient (1.9%) experienced 2 grade 1 infusion-related reactions, and both resolved the same day the event occurred. There were no patients with a decrease in ejection fraction.
Eleven (20.4%; 1 with 5.4 mg/kg and 10 with 6.4 mg/kg) potential ILD events per the predefined list of 44 preferred terms were reviewed by the independent adjudication committee, and 8 (14.8%; all 6.4 mg/kg) were adjudicated as T-DXd–induced ILD, including 3 (5.6%) grade 1, 2 (3.7%) grade 2, and 3 (5.6%) grade 5 events. Seven of these 8 were Japanese patients from Japan. At the time of data cutoff, T-DXd–induced ILD status was recovering for 1 of the 5 surviving patients (grade 2; received steroids), 1 was recovered (grade 2; received steroids), and 3 had not been reported as recovered (all grade 1; none received steroids). Of the 3 patients who died as a result of ILD, 2 were Japanese patients from Japan and 1 was a white patient from the United States; all of these patients received steroids.
DISCUSSION
This first-in-human clinical study investigating the novel HER2-targeting ADC, T-DXd, demonstrated substantial antitumor activity and a generally manageable safety profile for the treatment of patients with advanced/unresectable or metastatic HER2-low–expressing breast cancer. Based on current standards, the majority of patients in this trial would be classified as having HR-positive, HER2-negative breast cancer in the clinic, with extensive prior treatment. For this patient population, the current standard of care after exhaustion of hormonal therapies consists of single-agent chemotherapies.10 These agents provide limited benefit in this setting, with a median PFS of about 3 to 5 months in many studies.18-20 Although limited in sample size, the PFS of T-DXd in this study of heavily pretreated patients compares favorably with these previous reports and is encouraging for patients with breast cancer with few or no remaining treatment options.
Results of the dose escalation (part 1) have been previously reported, with doses of 5.4 and 6.4 mg/kg given intravenously once every 3 weeks, identified as the RDE.14 In the dose expansion (part 2), the confirmed ORR by investigator assessment was 59.5% and 43.2% in patients with refractory HER2-positive breast or gastric cancer, respectively, and T-DXd exhibited a manageable safety profile.15,16 We report a confirmed ORR of 37.0% in patients with HER2-low–expressing breast cancer.
CDK4/6i therapies are clinically effective in combination with an aromatase inhibitor or fulvestrant, or as monotherapy, and are recommended as part of first-line and second-line treatments for patients with HR-positive metastatic breast cancer.9,10 Although only 16 patients in our study had any prior CDK4/6i therapy, approximately 44% responded to treatment with T-DXd, suggesting that prior CDK4/6i may not interfere with clinical activity of T-DXd. Additional studies with more patients are needed to confirm these results.
Other HER2-targeted therapies, including trastuzumab and ado-trastuzumab emtansine (T-DM1), which are clinically effective in HER2-positive breast cancer, have, thus far, failed to show activity in the context of HER2-low disease.8,21 It may be hypothesized that the unique aspects of T-DXd, including a high drug-to-antibody ratio and the observed bystander effect, may be of particular importance in driving the antitumor activity reported here. Preclinical in vitro and in vivo studies demonstrated that the released payload of T-DXd, unlike T-DM1, is cell membrane permeable and that T-DXd induces a bystander cytotoxic effect on cells in close proximity to targeted HER2-expressing tumor cells, regardless of their HER2 status.13 Given the lack of benefit of trastuzumab in HER2-low breast cancers, it is likely that in these cancers, T-DXd uses the HER2 protein primarily as a means for delivery of the topoisomerase I inhibitor payload, as opposed to direct inhibition of HER2 signaling.
In our study, confirmed tumor responses were similar in both the HER2 IHC 1+ and IHC 2+ subgroups. Although preliminary, this suggests that HER2 IHC may not be the optimal test to define the lower boundary of expression level needed to predict clinical activity of T-DXd. Furthermore, even though there are standardized guidelines for HER2 IHC testing, results may be influenced by other factors, including changes in tumor HER2 expression over time, tumor heterogeneity, or discordance between individual laboratories and readers. Local and central assessment of IHC status in our study was mostly in agreement, with discordance mainly seen as lower IHC scores by central assessment. Future studies with more patients will provide additional insight into the relationship between IHC status and clinical activity of T-DXd.
It is of interest that antitumor activity was also seen in patients in this trial who had received anti-HER2 therapy for past HER2-positive disease, but subsequently had HER2-low disease on biopsy prior to study entry. Even when these patients were excluded, antitumor activity was similar to the overall population.
The safety profile of T-DXd in patients with HER2-low breast cancer was consistent with that previously reported in HER2-positive breast and gastric cancers.15,16 The 2 most common classes of TEAEs were GI and hematologic in nature. Overall, there were numerically fewer TEAEs reported in patients who received the 5.4-mg/kg dose compared with the 6.4-mg/kg dose.
Drug-induced ILD is a potentially life-threatening adverse event associated with other HER2-targeted therapies.22,23 The incidence of ILD with T-DM1, trastuzumab, and irinotecan are 1.2%, 0.2%-0.5%, and 0.9%, respectively.23-25 However, it is difficult to compare rates of ILD, because there is variation in factors, including study populations, ILD definition (broad v narrow terms), and measurement methods (chest imaging frequency). Reported incidence of grade 1 events could be linked to frequency of chest imaging, because these events are mainly diagnosed through imaging and are otherwise asymptomatic.26 Patient demographics may also affect observed ILD rates, because ILD incidence for many drugs is higher in Japan than in other countries.27-29 Here, we report 3 fatal cases of ILD, 2 in Japanese patients from Japan, and 1 in a white patient from the United States. Similar to patients with HER2-positive breast cancer, ILD was less frequently observed with 5.4 mg/kg versus 6.4 mg/kg.16 All patients with potential ILD/pneumonitis are closely monitored in the T-DXd clinical program on an ongoing basis and adjudicated by an independent committee. Analyses across multiple studies of T-DXd are further characterizing the risk and potential predictors of drug-related ILD.
Comparison of the 5.4- and 6.4-mg/kg T-DXd doses showed a numerically higher rate of tumor response with 6.4 mg/kg dose; however, this was accompanied by a numerically greater proportion of safety events, including grade ≥ 3 TEAEs, serious TEAEs, TEAEs leading to discontinuation, TEAEs resulting in dose reduction, and deaths. This trend was consistent with a previously reported analysis of patients with HER2-positive breast cancer from this study.16 Based on analysis of antitumor activity and safety from the overall T-DXd program, which includes the current study, the 5.4-mg/kg dose was chosen for future clinical trials of HER2-low breast cancer.
Limitations of the analysis include that it was nonrandomized with a relatively small number of patients with HER2-low breast cancer. There was a lack of prospective HER2 assessment by a central laboratory, and a post hoc central laboratory assessment of HER2 status could not be performed in 5 patients. Additionally, HER2 status was not confirmed by biopsy immediately before initiation of treatment.
T-DXd is the first HER2-targeted agent to demonstrate promising clinical antitumor activity with a manageable safety profile in patients considered to be HER2 negative. A phase III, randomized, multicenter study (DESTINY-Breast04; ClinicalTrials.gov identifier: NCT03734029) has been initiated to compare the efficacy and safety of T-DXd 5.4 mg/kg versus physician’s choice (capecitabine, eribulin, gemcitabine, paclitaxel, or nab-paclitaxel) in patients with HER2-low, unresectable, and/or metastatic breast cancer. If the phase III study confirms the results reported here, T-DXd may provide a new and novel targeted treatment option for advanced HER2-low breast cancer that has progressed on prior lines of therapy.
ACKNOWLEDGMENT
We thank the patients who participated in this study and their families and caregivers. We also thank the staff and investigators at all study sites. Medical writing and editorial support was provided by Nicole Seneca, PhD, and Stefan Kolata, PhD, of AlphaBioCom, and funded by Daiichi Sankyo.
PRIOR PRESENTATION
Preliminary results presented in part at the 2018 ASCO Annual Meeting, Chicago, IL, June 1-5, 2018; 2018 San Antonio Breast Cancer Symposium, San Antonio, TX, December 4-8, 2018; and 2017 San Antonio Breast Cancer Symposium, San Antonio, TX, December 5-9, 2017.
SUPPORT
Daiichi Sankyo funded the study and participated in the study design, data collection, data analysis, and data interpretation, and provided the study drug; assisted in writing the report; and approved the final version of the manuscript for publication in conjunction with the authors. The authors had full access to all data in the study and provided final approval to submit the manuscript for publication.
AUTHOR CONTRIBUTIONS
Conception and design: Shanu Modi, Hiroji Iwata, Toshihiko Doi, Caleb Lee, Yoshihiko Fujisaki, Masahiro Sugihara, Javad Shahidi
Provision of study materials or patients: Haeseong Park, Hiroji Iwata, Kenji Tamura, Junji Tsurutani, Alvaro Moreno-Aspitia, Yasuaki Sagara
Collection and assembly of data: Shanu Modi, Haeseong Park, Hiroji Iwata, Kenji Tamura, Junji Tsurutani, Toshihiko Doi, Yasuaki Sagara, Caleb Lee, Lin Zhang, Shunji Takahashi
Data analysis and interpretation: Shanu Modi, Haeseong Park, Rashmi K. Murthy, Junji Tsurutani, Alvaro Moreno-Aspitia, Toshihiko Doi, Yasuaki Sagara, Ian E. Krop, Caleb Lee, Yoshihiko Fujisaki, Masahiro Sugihara, Lin Zhang, Javad Shahidi, Shunji Takahashi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low–Expressing Advanced Breast Cancer: Results From a Phase Ib Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Shanu Modi
Consulting or Advisory Role: Daiichi Sankyo
Speakers' Bureau: Genentech
Research Funding: Genentech, Novartis, Seattle Genetics, Synta, Daiichi Sankyo
Haeseong Park
Research Funding: Amgen (Inst), AstraZeneca (Inst), Bayer (Inst), BeiGene (Inst), Bristol-Myers Squibb (Inst), Daiichi Sankyo (Inst), Lilly (Inst), EMD Serono (Inst), Gilead Sciences (Inst), Incyte (Inst), Macrogenics (Inst), MedImmune (Inst), Medivation (Inst), Merck (Inst), Millennium (Inst), Novartis (Inst), Pfizer (Inst), Puma Biotechnology (Inst), Regeneron (Inst), Taiho Pharmaceutical (Inst), Vertex (Inst), Ambrx (Inst), GlaxoSmithKline (Inst), Array BioPharma (Inst), Genentech (Inst), Oncologie (Inst), Turning Point Therapeutics (Inst), Xencor (Inst)
Travel, Accommodations, Expenses: Bayer
Rashmi K. Murthy
Honoraria: Puma Biotechnology, Genentech, Daiichi Sankyo, Seattle Genetics
Consulting or Advisory Role: Puma Biotechnology, Genentech, Daiichi Sankyo, Seattle Genetics
Research Funding: Genentech (Inst), Daiichi Sankyo (Inst), Pfizer (Inst), EMD Serono (Inst), Seattle Genetics (Inst)
Travel, Accommodations, Expenses: Seattle Genetics, Puma Biotechnology, Genentech, Daiichi Sankyo
Hiroji Iwata
Honoraria: Chugai Pharma, AstraZeneca, Eisai, Pfizer, Daiichi Sankyo
Consulting or Advisory Role: Chugai Pharma, Daiichi Sankyo, Pfizer, AstraZeneca, Lilly Japan, Kyowa Hakko Kirin
Research Funding: MSD (Inst), AstraZeneca (Inst), Eisai (Inst), Kyowa Hakko Kirin (Inst), GlaxoSmithKline (Inst), Daiichi Sankyo (Inst), Chugai Pharma (Inst), Nihonkayaku (Inst), Lilly Japan (Inst), Novartis (Inst), Bayer (Inst), Pfizer (Inst)
Kenji Tamura
Research Funding: Daiichi Sankyo, Pfizer, Lilly
Junji Tsurutani
Honoraria: Kyowa Kirin, Eisai, Novartis, Chugai Pharma, Taiho Pharmaceutical, AstraZeneca, Nihon Kayaku, Lilly Japan, Pfizer, Daiichi Sankyo
Consulting or Advisory Role: Asahikasei, Daiichi Sankyo
Research Funding: Eisai (Inst), Boehringer Ingelheim (Inst), Eli Lilly (Inst), MSD Oncology (Inst), Kyowa Kirin (Inst), Daiichi Sankyo (Inst), Chugai Pharma (Inst), Nihon Kayaku (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo, Chugai Pharma, Eisai, Novartis
Alvaro Moreno-Aspitia
Stock and Other Ownership Interests: Merrimack
Patents, Royalties, Other Intellectual Property: Royalties from the development of a xenograft model. Royalties were assigned to go to my institution and not personally
Toshihiko Doi
Honoraria: Bristol-Myers Squibb Japan, Ono Pharmaceutical, AbbVie, Astellas Pharma, Oncolys BioPharma, Taiho Pharmaceutical
Consulting or Advisory Role: MSD, Daiichi Sankyo, Amgen, Sumitomo Dainippon, Taiho Pharmaceutical, Takeda, AbbVie, Novartis, Bayer, Boeringer Ingelheim, Rakuten Medical
Research Funding: Taiho Pharmaceutical (Inst), Novartis (Inst), Merck Serono (Inst), MSD (Inst), Boehringer Ingelheim (Inst), Pfizer (Inst), Lilly Japan (Inst), Sumitomo Group (Inst), Kyowa Hakko Kirin (Inst), Daiichi Sankyo (Inst), Bristol-Myers Squibb (Inst), AbbVie (Inst), Quintiles (Inst), Eisai (Inst)
Yasuaki Sagara
Speakers' Bureau: AstraZeneca, Chugai Pharma, Eisai, Novartis, Takeda, Taiho Pharmaceutical, Celltrion
Research Funding: Gencruix (Inst)
Charles Redfern
Stock and Other Ownership Interests: Pfizer
Ian E. Krop
Employment: Amag Pharmaceuticals (I)
Leadership: Amag Pharmaceuticals (I)
Stock and Other Ownership Interests: Amag Pharmaceuticals (I)
Honoraria: Genentech
Consulting or Advisory Role: Genentech, Daiichi Sankyo, Context Therapeutics, Macrogenics, Taiho Pharmaceutical
Research Funding: Genentech (Inst), Seattle Genetics (Inst), Pfizer (Inst), Daiichi Sankyo (Inst)
Caleb Lee
Employment: Daiichi Sankyo
Stock and Other Ownership Interests: Daiichi Sankyo
Yoshihiko Fujisaki
Employment: Daiichi Sankyo, Daiichi Sankyo (I)
Stock and Other Ownership Interests: Daiichi Sankyo, Daiichi Sankyo (I)
Travel, Accommodations, Expenses: Daiichi Sankyo, Daiichi Sankyo (I)
Masahiro Sugihara
Employment: Daiichi Sankyo
Lin Zhang
Employment: Daiichi Sankyo
Stock and Other Ownership Interests: Daiichi Sankyo
Javad Shahidi
Employment: Daiichi Sankyo
Stock and Other Ownership Interests: Daiichi Sankyo
Shunji Takahashi
Honoraria: Daiichi Sankyo, Sanofi, Eisai, Bayer, Taiho Pharmaceutical, MSD, Novartis, Chugai Pharma, AstraZeneca, Astellas Pharma, Bristol-Myers Squibb Japan, Ono Pharmaceutical, Kyowa Hakko Kirin, Nihonkayaku, Daiichi Sankyo (Inst), Sanofi (Inst), Eisai (Inst), Bayer (Inst), Taiho Pharmaceutical (Inst), MSD (Inst), Novartis (Inst), Chugai Pharma (Inst), AstraZeneca (Inst), Bristol-Myers Squibb (Inst), Lilly (Inst), Ono Pharmaceutical (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo, Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Koleva-Kolarova RG, Oktora MP, Robijn AL, et al. Increased life expectancy as a result of non-hormonal targeted therapies for HER2 or hormone receptor positive metastatic breast cancer: A systematic review and meta-analysis. Cancer Treat Rev. 2017;55:16–25. doi: 10.1016/j.ctrv.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Mendes D, Alves C, Afonso N, et al. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer--a systematic review. Breast Cancer Res. 2015;17:140. doi: 10.1186/s13058-015-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rakha EA, Pinder SE, Bartlett JM, et al. Updated UK recommendations for HER2 assessment in breast cancer. J Clin Pathol. 2015;68:93–99. doi: 10.1136/jclinpath-2014-202571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 5.Giuliani S, Ciniselli CM, Leonardi E, et al. In a cohort of breast cancer screened patients the proportion of HER2 positive cases is lower than that earlier reported and pathological characteristics differ between HER2 3+ and HER2 2+/HER2 amplified cases. Virchows Arch. 2016;469:45–50. doi: 10.1007/s00428-016-1940-y. [DOI] [PubMed] [Google Scholar]
- 6.Lal P, Salazar PA, Hudis CA, et al. HER-2 testing in breast cancer using immunohistochemical analysis and fluorescence in situ hybridization: A single-institution experience of 2,279 cases and comparison of dual-color and single-color scoring. Am J Clin Pathol. 2004;121:631–636. doi: 10.1309/VE78-62V2-646B-R6EX. [DOI] [PubMed] [Google Scholar]
- 7.Schalper KA, Kumar S, Hui P, et al. A retrospective population-based comparison of HER2 immunohistochemistry and fluorescence in situ hybridization in breast carcinomas: Impact of 2007 American Society of Clinical Oncology/College of American Pathologists criteria. Arch Pathol Lab Med. 2014;138:213–219. doi: 10.5858/arpa.2012-0617-OA. [DOI] [PubMed] [Google Scholar]
- 8. Fehrenbacher L, Cecchini RS, Geyer CE, et al: Abstract GS1-02: NSABP B-47 (NRG oncology): Phase III randomized trial comparing adjuvant chemotherapy with adriamycin (A) and cyclophosphamide (C) → weekly paclitaxel (WP), or docetaxel (T) and C with or without a year of trastuzumab (H) in women with node-positive or high-risk node-negative invasive breast cancer (IBC) expressing HER2 staining intensity of IHC 1+ or 2+ with negative FISH (HER2-Low IBC). Cancer Res 78:GS1-02-GS1-02, 2018 (suppl)
- 9.Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4) Ann Oncol. 2018;29:1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. NCCN Clinical Practical Guidelines in Oncology: Breast Cancer, Version 2.2019. https://www.nccn.org/professionals/physician_gls/default.aspx.
- 11.Nakada T, Masuda T, Naito H, et al. Novel antibody drug conjugates containing exatecan derivative-based cytotoxic payloads. Bioorg Med Chem Lett. 2016;26:1542–1545. doi: 10.1016/j.bmcl.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 13.Ogitani Y, Hagihara K, Oitate M, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039–1046. doi: 10.1111/cas.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet Oncol. 2017;18:1512–1522. doi: 10.1016/S1470-2045(17)30604-6. [DOI] [PubMed] [Google Scholar]
- 15.Shitara K, Iwata H, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: A dose-expansion, phase 1 study. Lancet Oncol. 2019;20:827–836. doi: 10.1016/S1470-2045(19)30088-9. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K, Tsurutani J, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: A dose-expansion, phase 1 study. Lancet Oncol. 2019;20:816–826. doi: 10.1016/S1470-2045(19)30097-X. [DOI] [PubMed] [Google Scholar]
- 17.Kubo K, Azuma A, Kanazawa M, et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Investig. 2013;51:260–277. doi: 10.1016/j.resinv.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman PA, Awada A, Twelves C, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015;33:594–601. doi: 10.1200/JCO.2013.52.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25:3407–3414. doi: 10.1200/JCO.2006.09.3849. [DOI] [PubMed] [Google Scholar]
- 21.Yazaki S, Hashimoto J, Ogita S, et al. 308P Lower response to T-DM1 in metastatic breast cancer patients with HER2 IHC score of 2 and FISH positive compared with IHC score of 3. Ann Oncol. 2017;28(suppl 5):v74–v108. [Google Scholar]
- 22.Pepels MJ, Boomars KA, van Kimmenade R, et al. Life-threatening interstitial lung disease associated with trastuzumab: Case report. Breast Cancer Res Treat. 2009;113:609–612. doi: 10.1007/s10549-008-9966-8. [DOI] [PubMed] [Google Scholar]
- 23.KADCYLA (ado-trastuzumab emtansine) for injection, for intravenous use [package insert] Full prescribing information. South San Francisco, CA, Genentech; 2016. [Google Scholar]
- 24.HERCEPTIN . trastuzumab) [package insert] Intravenous infusion. Full prescribing information. South San Francisco, CA, Genentech; 2017. [Google Scholar]
- 25.Tadokoro J, Kakihata K, Shimazaki M, et al. Post-marketing surveillance (PMS) of all patients treated with irinotecan in Japan: Clinical experience and ADR profile of 13,935 patients. Jpn J Clin Oncol. 2011;41:1101–1111. doi: 10.1093/jjco/hyr105. [DOI] [PubMed] [Google Scholar]
- 26.Skeoch S, Weatherley N, Swift AJ, et al. Drug-induced interstitial lung disease: A systematic review. J Clin Med. 2018;7:E356. doi: 10.3390/jcm7100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azuma A, Kudo S. Drug-induced pneumonia and Japanese [in Japanese] Nippon Naika Gakkai Zasshi. 2007;96:1077–1082. doi: 10.2169/naika.96.1077. [DOI] [PubMed] [Google Scholar]
- 28.Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer. 2004;91(S2) suppl 2:S3–S10. doi: 10.1038/sj.bjc.6602061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: A cohort and nested case-control study. Am J Respir Crit Care Med. 2008;177:1348–1357. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]