Abstract
Background
When Belgium's coronavirus disease 2019 (COVID-19) outbreak began in March 2020, our neurosurgical department followed the protocol of most surgical departments in the world and postponed elective surgery. However, patients with tumor-like brain lesions requiring urgent surgery still received treatment as usual, in order to ensure ongoing neurooncologic care. From a series of 31 patients admitted for brain surgery, 3 were confirmed as infected by the novel severe acute respiratory syndrome coronavirus 2.
Case Description
We present the clinical outcomes of these 3 COVID-19 patients, who underwent an intracerebral biopsy in our department during April 2020. All suffered from a diffuse intraparenchymal hemorrhage postoperatively. Unfortunately, we were not able to identify a clear etiology of these postoperative complications. It could be hypothesized that an active COVID-19 infection status may be related to a higher bleeding risk. The remaining 28 neurooncologic non-COVID-19 patients underwent uneventful surgery during the same period.
Conclusions
This case series reports the previously unreported and unexpected outcomes of COVID-19 patients suffering from acute hemorrhage after intracerebral biopsy procedures. Although no direct relation can yet be established, we recommend the neurosurgical community be cautious in such cases.
Key words: Case report, COVID-19, Intracerebral biopsy, Intraparenchymal hemorrhage
Abbreviations and Acronyms: ACE, Angiotensin-converting enzyme; CO-RADS, COVID-19 Reporting and Data System; CT, Computed tomography; COVID-19, Coronavirus disease 2019; IPH, Intraparenchymal hemorrhage; LM, Leptomeningeal metastasis; MRI, Magnetic resonance imaging; PCR, Polymerase chain reaction; SARS-CoV, Severe acute respiratory syndrome coronavirus 2
Background and Importance
When Belgium's coronavirus 2019 (COVID-19) outbreak began in March 2020, our department followed the protocol of most surgical departments in the world and postponed elective surgery. However, patients presenting with tumor-like brain lesions requiring urgent surgery received treatment as usual to ensure ongoing neurooncologic care, a practice that has been advocated in recent literature.1, 2, 3, 4 Nonetheless, we faced a high rate of unexpected complications in 3 COVID-19 patients. All 3 patients who underwent an intracerebral biopsy experienced postoperative hemorrhage. We feel the obligation to report the rare and unexpected outcome of these cases.
Clinical Presentation
From a series of 31 patients admitted for brain surgery, 3 were confirmed as infected by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV). We describe 3 consecutive cases of COVID-19 patients who underwent an intracerebral biopsy in April 2020 for histopathologic diagnosis. The patients were operated on by 3 separate, experienced neurosurgeons from our department.
Case 1
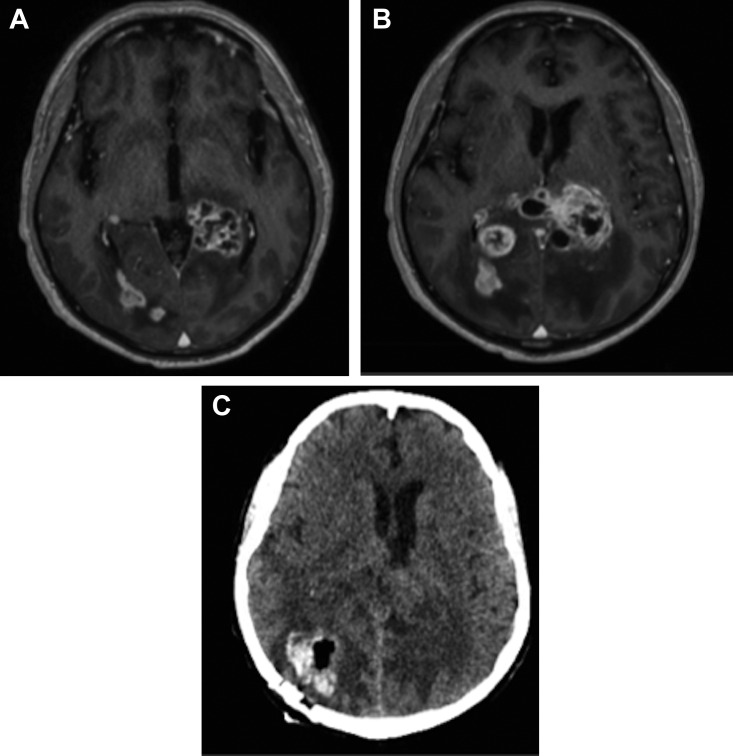
A 72-year-old male patient with history of repetitive falls was admitted to our department. He presented with gait disturbance, disequilibrium along with multiple weeks of headaches, and cognitive changes. Neurologic examination showed bradyphrenia, and 1-step commands were followed with great difficulty. Brain magnetic resonance imaging revealed a voluminous tumorous cystic-necrotic lesion centered on the corpus callosum (Figure 1A and B ), which was suspected to be a butterfly glioblastoma. 18F-Fluorodeoxyglucose−positron emission tomography/computed tomography imaging showed hypermetabolism in the left thalamus, corpus callosum and right parieto-occipital: suspicious hypermetabolism of the prostate and a T12 compression fracture were identified as well. One day after admission polymerase chain reaction (PCR) SARS CoV 2 appeared positive, but chest computed tomography (CT) was normal (CO-RADS 6).5 The patient presented neither fever nor respiratory symptoms. Coagulation tests were normal (Table 1 ), and he was not taking any anticoagulant nor undergoing antiplatelet therapy. Following protocol, he was admitted to our hospital's COVID department. Ten days after admission, biopsy of the lesion was performed. A neuro-navigation-guided biopsy was performed first, but quality of the obtained tissue was insufficient. Subsequently, a 15-mm diameter occipital craniotomy was conducted for an open biopsy given the adequate access for tissue sampling. Several grayish tumoral samples were taken, and proper hemostasis was obtained at the end of the procedure. Histopathologic analysis confirmed the diagnosis glioblastoma multiforme, World Health Organization grade 4. On the third postoperative day, the patient presented with left hemiparesis and an altered level of consciousness. Brain CT found an intraparenchymal hemorrhage (IPH) of 2.5 cm × 3 cm × 3 cm in the operative site with midline shift (see Figure 1C). Given the gloomy prognosis and limited hematoma size, no surgical evacuation was performed and no subsequent CT scans were carried out. The patient passed away on postoperative day 6 with the aid of comfort care.
Figure 1.
Case 1. (A and B) Axial brain magnetic resonance imaging T1-weighted contrast-enhanced images showing a voluminous tumorous cystic-necrotic lesion centered on the left side of the splenium of the corpus callosum with bilateral temporoparietaloccipital extension. There is mass effect causing effacement of the left occipital and temporal horn. Imaging is suspected to show a butterfly glioblastoma. (C) Axial brain computed tomography image demonstrates an intraparenchymal hemorrhage located in the operative site, responsible for mass effect and 6-mm midline shift.
Table 1.
Coagulation Laboratory Findings
| Number | Platelet Count (150–440 × 103/mm3) | D-Dimer Concentration (<500 ng/mL) | PT (9.8–12.5 seconds) | INR (0.95–1.31) |
|---|---|---|---|---|
| Case 1 | 307 | Unknown | 12.4 | 1.17 |
| Case 2 | 200 | 243 | 11.9 | 1.12 |
| Case 3 | 91 | 2412 | 13.5 | 1.28 |
Normal values are indicated within parentheses.
INR, international normalized ratio; PT, prothrombin time.
Case 2
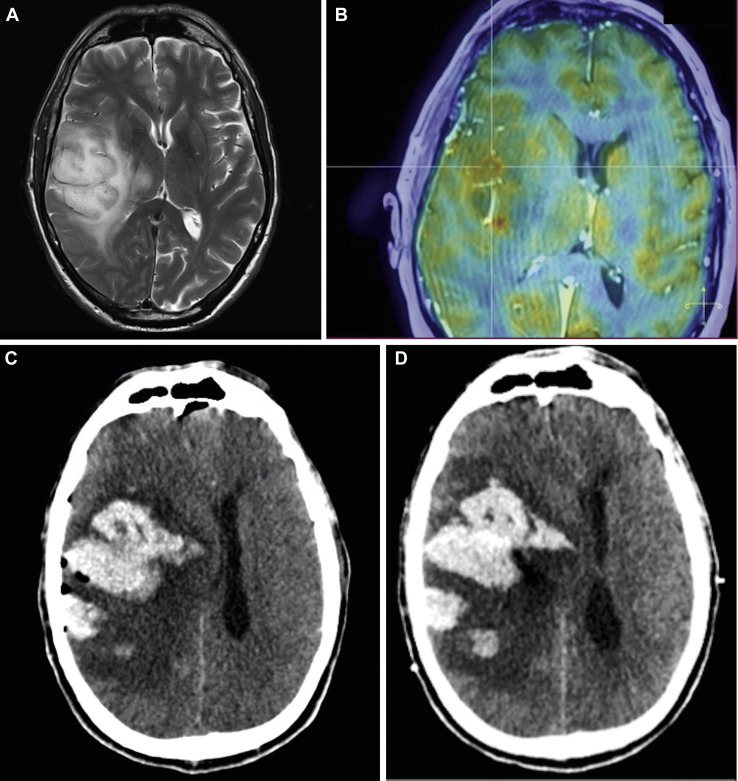
A 63-year-old male patient with history of an inaugural tonic-clonic seizure was transferred to our center. The patient was on antiplatelet therapy (acetylsalicylic acid), which was stopped on admission. Neurologic examination revealed dysarthria, left facial paralysis, and left hemiparesis. Coagulation tests were normal (see Table 1). Brain magnetic resonance imaging (MRI) revealed an infiltrative tumorous cystic-necrotic lesion, right frontoparietotemporoinsular, suspected to be a high-grade glioma (Figure 2A ). 18F-Fluorodeoxyglucose positron emission tomography−CT showed hypermetabolism in the cerebral lesion, confirming the suspicion of high-grade glioma; bilateral hypermetabolic pulmonary consolidations were identified as well. Although PCR SARS-CoV was negative 3 times, thorax CT was highly suspicious for COVID-19 including the typical COVID-19 CT findings according to the CO-RADS classification.5 The patient presented only once an episode of fever and no respiratory symptoms. He was then transferred to the COVID ward. Twelve days after admission, a stereotactic needle biopsy of the lesion was performed through a right frontal burr hole (see Figure 2B). Histopathologic analysis confirmed diagnosis of an anaplastic astrocytoma, World Health Organization grade 3. Postoperatively, alteration of consciousness was observed in the form of Glasgow Coma Scale 8/15. CT imaging showed a large multifocal IPH of 4.5 × 6 × 6.8 cm in the right frontal lobe with major perilesional edema and midline shift (see Figure 2C). Subsequently, intracranial pressure, brain tissue oxygen, and intracerebral electroencephalography monitoring were inserted right frontally. In the following hours, the IPH continued to enlarge discretely (see Figure 2D) and the patient developed an intraventricular hemorrhage associated with a ventricular dilatation; a left extraventricular drain was then inserted. After discussion with the patient's family about the gloomy prognosis, it was decided that a surgical evacuation of the hematoma would not be performed. The patient passed away on the third day post biopsy.
Figure 2.
Case 2. (A) Axial brain magnetic resonance imaging (MRI) T2-weighted image showing an infiltrative tumorous cystic-necrotic lesion right frontoparietotemporoinsular with thalamopeduncular and isthmus involvement, suspected to be a high-grade glioma. (B) MRI and positron emission tomography−computed tomography (CT) fused image demonstrating the target for ROSA robot-guided stereotactic biopsy. (C) Axial CT image demonstrating a large intraparenchymal hemorrhage in the right frontal lobe with major perilesional edema, responsible for mass effect, 18-mm midline-shift, right subfalcine, and infratentorial herniation. (D) Axial CT image demonstrating discrete enlargement of the intra-parenchymal hemorrhage at day 1 post biopsy.
Case 3
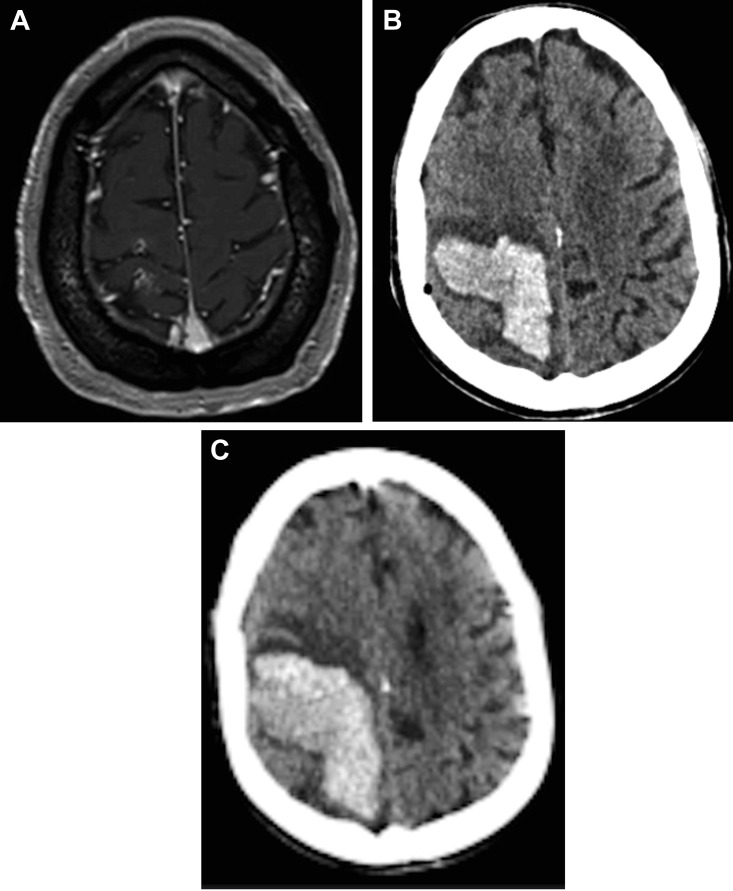
A 78-year-old male with a month-long history of headaches was admitted with confusion, reduced verbal fluency, and left hemihypoesthesia. Two months earlier, the patient was diagnosed with a prostatic adenocarcinoma with right iliac lymph node invasion. Brain MRI revealed a cortical and meningeal contrast enhancement in the right parietal region (Figure 3A ). This could be compatible with leptomeningeal metastasis (LM). 18F-Fluorodeoxyglucose positron emission tomography−CT showed hypometabolic activity in the left parietotemporal region, a hypermetabolic thyroid lesion, and a hypermetabolic lymph node in the right iliac. These findings were not in favor of LM. Two lumbar punctures did not reveal malignant cells. Two PCR SARS-CoV were positive, as was thorax-CT (CO-RADS 6).5 The patient presented neither fever nor respiratory symptoms. Fourteen days after admission, meningeal biopsy was performed through a right parietal burr hole. His antiplatelet therapy (acetylsalicylic acid) was terminated 7 days before biopsy. We observed mild thrombocytopenia, increased D-dimer concentrations, and slightly prolonged prothrombin time (see Table 1). Histopathologic analysis was not compatible with LM or vasculitis. Despite uneventful awakening at 24 hours postoperatively, the patient developed left hemiplegia. Brain CT showed a large right parietal IPH of 7.8 cm × 3.0 cm × 5.4 cm with perilesional edema and midline shift (see Figure 3B). The family requested no surgical evacuation of the hematoma due to the precarious preoperative state of the patient and the risk of the procedure posed by eloquent localization of the hematoma. The hematoma continued to enlarge in the following days (see Figure 3C), and the patient passed away on the 11th postoperative day with the aid of comfort care.
Figure 3.
Case 3. (A) Axial brain magnetic resonance imaging T1-weighted contrast-enhanced images showing a cortical and meningeal contrast enhancement in the right parietal region, which could be compatible with leptomeningeal metastasis. (B) Axial brain computed tomography (CT) image demonstrating an intraparenchymal hemorrhage located in the operative site, responsible for mass effect and 6-mm midline shift. (C) Axial brain CT image performed at day 6 postoperatively, demonstrating enlargement of the intraparenchymal hemorrhage.
Immunohistochemistry analyses for anti-SARS-CoV were all negative on these 3 cerebral tissue samples.
Discussion
We have reported 3 cases of acute hemorrhage in COVID-19 patients after intracerebral biopsy by different surgeons using different techniques.
To date, literature is scarce concerning COVID-19 patients who have undergone surgery for brain tumors.3 No cases of perioperative hemorrhage after neurooncologic procedures in COVID-19 patients have been described. However, several cases of spontaneous intraparenchymal bleeding in COVID-19 patients have been reported. Poyiadji et al6 presented a female COVID-19 patient with acute hemorrhagic necrotizing encephalopathy; brain MRI demonstrated hemorrhagic rim-enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions. Sharifi-Razavi et al7 reported a massive right hemispheric intracerebral hemorrhage, accompanied by intraventricular hemorrhage and subarachnoid hemorrhage, in a COVID-19 male without history of hypertension or anticoagulation therapy. Vu et al8 reported a male with a coincidental presentation of COVID-19 and a left basal ganglia hemorrhage. No vascular malformation or other etiology was discovered.8
COVID-19 has been associated with coagulopathies such as disseminated intravascular coagulation leading to thrombosis.9, 10, 11 Subsequently, consumption of platelets in disseminated intravascular coagulation can result in hemorrhage.12 Panciani et al13 described 4 COVID patients undergoing surgery or embolization for chronic subdural hematoma with a mortality rate of 100%. In 2 cases, a thrombocytopenia was observed and led to rebleeding postoperatively. Thrombocytopenia, elevated D-dimer, and prolonged prothrombin time have been associated with COVID-19.11 , 14 In our series, we observed alteration of these coagulation parameters in 1 case. Acetylsalicylic acid was discontinued 7 days before surgery, as is recommended.
The overall hemorrhage complication rate in nonimmunocompromised patients after stereotactic biopsy ranges from 1%–3%. All 3 COVID-19 patients who underwent surgery during a 1-month-period suffered from a hemorrhagic complication. Also throughout this month, 28 non-COVID-patients underwent uneventful operations for brain tumors.
We emphasize that in these described cases only a small amount of brain tissue was sampled, a fact that resulted in an unusually large, diffuse hematoma. Two cases became symptomatic after 24 hours, which is unusual. We were not able to identify a clear etiology for these presentations.
Sharifi-Razavi et al7 hypothesized that angiotensin-converting-enzyme (ACE) II receptors used by COVID-19 for cell entry are responsible for dysfunction of the cerebrovascular endothelial cells. This may be leading to the disruption of autoregulation, as well as blood pressure spikes due to arterial wall rupture.7 ACE-II is widely expressed throughout the human brain, mostly in glial cells.15 , 16 Two of our patients underwent biopsy of a high-grade glioma; it could be suggested that bleeding risk is higher due to dysregulation in these ACE-II-receptors within the glial cells, although our immunohistochemistry analyses for anti-SARS-CoV were all negative on the obtained cerebral tissues.
In addition, the surgical procedures themselves present a complication: They are more difficult to perform while wearing mandatory personal protective equipment.3 The face shield, especially, may impair vision and does not facilitate the use of a microscope. However, the authors do not consider technical difficulties to be responsible for these hemorrhagic complications.
No causal relationship can be confirmed for tumor-biopsy surgery in COVID-19-patients on the basis of 3 cases alone. Nonetheless, we urge the neurosurgical community to take our findings into consideration before pursuing such procedures in this particular population.
Conclusion
With this case series we want to alert the neurosurgical community to the unexpected high risk of postoperative hematoma after tumor biopsy in COVID-19 patients.
Acknowledgments
The authors would like to express our gratitude to Dr. Claire Royer-Chardon, who provided additional information regarding the interpretation of the immunohistochemistry analyses for anti-SARS-CoV.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Perin A., Servadei F., Dimeco F. May we deliver neuro-oncology in difficult times (e.g., COVID-19)? J Neurooncol. 2020;148:203–205. doi: 10.1007/s11060-020-03496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramakrishna R., Zadeh G., Sheehan J.P. Inpatient and outpatient case prioritization for patients with neuro-oncologic disease amid the COVID-19 pandemic: general guidance for neuro-oncology practitioners from the AANS/CNS Tumor Section and Society for Neuro-Oncology. J Neurooncol. 2020;147:525–529. doi: 10.1007/s11060-020-03488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu Y., Zhang J., Chen Z. Experiences of practicing surgical neuro-oncology during the COVID-19 pandemic. J Neurooncol. 2020;148:199–200. doi: 10.1007/s11060-020-03489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zacharia B.E., Eichberg D.G., Ivan M.E. Letter: surgical management of brain tumor patients in the COVID-19 era [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa162 Neurosurgery. [DOI] [PMC free article] [PubMed]
- 5.Prokop M., van Everdingen W., van Rees Vellinga T. CO-RADS—a categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation [e-pub ahead of print] https://doi.org/10.1148/radiol.2020201473 Radiology. accessed May 19, 2020. [DOI] [PMC free article] [PubMed]
- 6.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [e-pub ahead of print] https://doi.org/10.1148/radiol.2020201187 Radiology. accessed May 19, 2020. [DOI] [PMC free article] [PubMed]
- 7.Sharifi-Razavi A., Karimi N., Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? https://doi.org/10.1016/j.nmni.2020.100669 N Microbes N Infect. accessed May 19, 2020. [DOI] [PMC free article] [PubMed]
- 8.Vu D., Ruggiero M., Choi W.S. Three unsuspected CT diagnoses of COVID-19. https://doi.org/10.1007/s10140-020-01775-4 Emerg Radiol. accessed May 19, 2020. [DOI] [PMC free article] [PubMed]
- 9.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia [e-pub ahead of print] https://doi.org/10.1111/jth.14768 J Thromb Haemost. accessed May 19, 2020. [DOI] [PMC free article] [PubMed]
- 11.Levi M., Jecko T., Toshiaki I. Comment: coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papageorgiou C., Jourdi G., Adjambri E. Disseminated intravascular coagulation: an update on pathogenesis, diagnosis, and therapeutic strategies. Clin Appl Thromb. 2018;24:8S–28S. doi: 10.1177/1076029618806424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panciani P.P., Saraceno G., Zanin L., Renisi G., Signorini L., Fontanella M.M. Letter: COVID-19 infection affects surgical outcome of chronic subdural hematoma [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa140 Neurosurgery. [DOI] [PMC free article] [PubMed]
- 14.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mankad K., Perry M.D., Mirsky D.M. COVID-19: a primer for neuroradiologists. Neuroradiology. 2020;62:647–648. doi: 10.1007/s00234-020-02437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia H., Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107:1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]