Abstract
Myocardial injury is associated with excess mortality in severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infections, but the mechanisms of injury are diverse. We describe a case of stress-induced cardiomyopathy in the setting of SARS-CoV-2 and influenza A coinfection. (Level of Difficulty: Intermediate.)
Key Words: coronavirus disease 2019, dilated cardiomyopathy, influenza, myocarditis, severe acute respiratory syndrome coronavirus 2, stress-induced cardiomyopathy
Abbreviations and Acronyms: COVID-19, coronavirus disease-2019; ECG, electrocardiogram; hs-troponin I, high-sensitivity troponin I; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2
Graphical abstract
Myocardial injury is associated with excess mortality in severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infections, but the mechanisms of…
Case Presentation
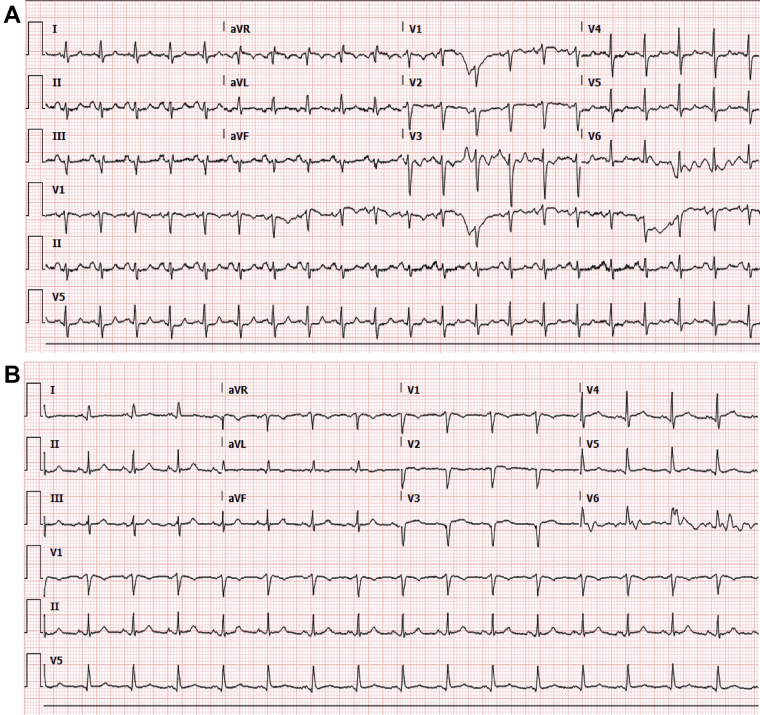
A 58-year-old woman with rheumatoid arthritis, interstitial lung disease, chronic obstructive pulmonary disease, and hypertension presented with fever and respiratory distress. A viral respiratory panel confirmed influenza virus type A H1–2009. An electrocardiogram (ECG) showed loss of anterior septal forces (Figure 1A). Her high-sensitivity troponin I (hs-troponin I) level was 9 ng/l (0 to 14 ng/l), and her brain natriuretic peptide level was 13 pg/ml (0 to 100 pg/ml). A chest radiograph revealed bronchiectasis with interstitial thickening. The result of a severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) reverse-transcriptase–polymerase chain reaction assay was positive. Broad-spectrum antibiotics and oseltamivir were initiated. She had progressive hypoxemia and hypotension requiring intubation. Supraventricular tachycardia developed, with a heart rate higher than 200 beats/min. She became pulseless and was successfully resuscitated. Two hours later she had a second episode; sinus rhythm was restored with adenosine, and an amiodarone infusion was started. A subsequent ECG suggested progression to a septal infarction pattern (Figure 1B). Repeat hs-troponin I was 2,273 ng/l, and brain natriuretic peptide was 299 pg/ml. A transthoracic echocardiogram demonstrated severely reduced left ventricular systolic function with global hypokinesis of the left ventricle. The apical segments had disproportionately poor function compared with the basal segments, a finding consistent with stress-induced (takotsubo) cardiomyopathy (Videos 1A and 1B). She was extubated but experienced upper airway compromise. She did not want to be reintubated and died 13 days after admission.
Figure 1.
Electrocardiograms
(A) Loss of septal forces. No prior electrocardiogram was available for comparison. (B) Progression to a septal infarction pattern.
Online Video 1A.
Transthoracic Echocardiogram
Apical views with and without Definity contrast material (Lantheus Medical Imaging). Transthoracic echocardiogram revealed a severely reduced left ventricular ejection fraction with disproportionate apical hypokinesis consistent with stress-induced cardiomyopathy.
Online Video 1B.
Transthoracic Echocardiogram
Apical views with and without Definity contrast material (Lantheus Medical Imaging). Transthoracic echocardiogram revealed a severely reduced left ventricular ejection fraction with disproportionate apical hypokinesis consistent with stress-induced cardiomyopathy.
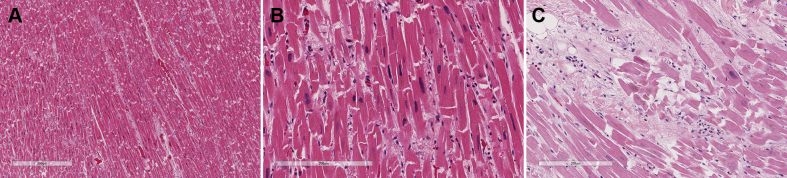
On autopsy, histopathologic examination of the heart showed mild to moderate myocyte hypertrophy with mild to moderate diffuse interstitial and perivascular fibrosis (Figures 2A and 2B). Focal areas of interstitial fibrosis exhibited mononuclear cells including lymphocytes and plasma cells in the anterior left ventricle and interventricular septum (Figure 2C). Sections of the coronary arteries did not show any pathologic abnormality. The right ventricle showed myocyte hypertrophy and normal for age intramyocardial adipose tissue.
Figure 2.
Histopathologic Examination of the Heart
(A and B) Myocyte hypertrophy with interstitial fibrosis. (C) Interstitial fibrosis, focal myocyte hypertrophy, and the presence of lymphocytes and plasma cells.
Discussion
Cardiovascular comorbidities are associated with higher rates of myocardial injury and more severe disease in coronavirus disease-2019 (COVID-19) (1). Our patient had a diagnosis of stress-induced cardiomyopathy on the basis of echocardiographic findings of diffuse apical hypokinesis. Although this patient had risk factors for atherosclerotic coronary disease, it is unknown whether she had heart failure on admission, and other common causes were not explored. Histopathologic examination of the heart showed changes associated with chronic hypertensive and atherosclerotic disease; however, in the absence of coronary disease at autopsy, we can consider a nonischemic origin. On the basis of the focal areas of interstitial fibrosis with mononuclear cells, there may have been intrinsic myocyte damage by the virus or global inflammatory deterioration. She may have also experienced true catecholamine excess with the stress of respiratory failure.
The impact of influenza co-infection in this patient with COVID-19 must also be considered because this virus is known to contribute to cardiovascular morbidity and mortality secondary to up-regulation of the inflammatory response and endothelial dysfunction (2). As such, influenza A likely had significant effects on her cardiac functioning. Coinfection with SARS-CoV-2 is of great concern, with limited data delineating the prevalence of this phenomenon (3), and positive viral panel results should not provide reassurance against coinfection.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For a supplemental video, please see the online version of this paper.
References
- 1.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020:e201017. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1286. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323:2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]