Abstract
Objectives
To determine the association between frailty and short-term mortality in older adults hospitalized for coronavirus disease 2019 (COVID-19).
Design
Retrospective single-center observational study.
Setting and participants
Eighty-one patients with COVID-19 confirmed by reverse-transcriptase polymerase chain reaction (RT-PCR), at the Geriatrics department of a general hospital in Belgium.
Measurements
Frailty was graded according to the Rockwood Clinical Frailty Scale (CFS). Demographic, biochemical, and radiologic variables, comorbidities, symptoms, and treatment were extracted from electronic medical records.
Results
Participants (N = 48 women, 59%) had a median age of 85 years (range 65-97 years) and a median CFS score of 7 (range 2-9); 42 (52%) were long-term care residents. Within 6 weeks, 18 patients died. Mortality was significantly but weakly associated with age (Spearman r = 0.241, P = .03) and CFS score (r = 0.282, P = .011), baseline lactate dehydrogenase (LDH; r = 0.301, P = .009), lymphocyte count (r = −0.262, P = .02), and RT-PCR cycle threshold (Ct, r = −0.285, P = .015). Mortality was not associated with long-term care residence, dementia, delirium, or polypharmacy. In multivariable logistic regression analyses, CFS, LDH, and RT-PCR Ct (but not age) remained independently associated with mortality. Both age and frailty had poor specificity to predict survival. A multivariable model combining age, CFS, LDH, and viral load significantly predicted survival.
Conclusions and Implications
Although their prognosis is worse, even the oldest and most severely frail patients may benefit from hospitalization for COVID-19, if sufficient resources are available.
Keywords: COVID-19, frailty, hospitalization, older adults, severe acute respiratory syndrome coronavirus 2
Coronavirus disease 2019 (COVID-19) is a global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 Older adults are at increased risk of hospitalization and mortality due to COVID-19.2, 3, 4, 5
Different ethical guidelines deal with triage in case a surge in hospital admissions due to COVID-19 overwhelms scarce hospital resources.6, 7, 8 Likelihood of benefit, age, and frailty are among the most commonly used triage criteria.9 , 10 In the United Kingdom and in Belgium (among other countries), intensive care unit (ICU) admission is not recommended for frail older adults aged 65 years and older.11 , 12 These guidelines rely on frailty assessment according to the Rockwood Clinical Frailty Scale (CFS). Patients can be classified on the CFS as not frail (scores 1-4), mildly frail (score 5), moderately frail (score 6), or severely frail (score 7-9).13 ICU admission is discouraged for frail older adults, that is, those with a CFS score of 5 or higher, in the United Kingdom and Belgium.11 , 12 Hospital admission is discouraged for nursing home residents with suspected or confirmed COVID-19 and a CFS score of 7 or higher.12
Previous studies have shown that frailty is associated with worse outcomes in hospitalized older adults.13 , 14 However, little is known about the outcomes in frail older adults or long-term care residents hospitalized for COVID-19. Therefore, the aim of this retrospective observational study was to describe outcomes in hospitalized geriatric COVID-19 patients according to their age, degree of frailty, and place of residence.
Methods
Study Design
A retrospective, single-center observational study was performed among COVID-19 patients at the geriatrics department of our General Hospital in Belgium, admitted between March 12 and April 30, 2020. Demographic, clinical, laboratory, and radiographic parameters were extracted from electronic health records. Laboratory values included C-reactive protein (CRP; reference values <5 mg/L); ferritin; d-dimers; lactate dehydrogenase (LDH); 25-hydroxyvitamin D levels; and white blood cell, platelet, and lymphocyte counts. Polypharmacy was defined as the use of 5 or more medications.
Ethics
The Ethical Committee approved the research protocol and waived the need for informed consent, because it did not constitute a clinical study according to national and European regulations.
Clinical Procedures
COVID-19 was confirmed by reverse transcriptase–polymerase chain reaction (RT-PCR) testing on nasopharyngeal swabs, using protocols validated within our national SARS-CoV-2 reference network.15 All patients admitted through the emergency department were screened for COVID-19 by low-dose chest computed tomography (CT). Findings on COVID-19 likelihood and extent of pulmonary involvement (CT score ranging 0-25) were reported using a standardized radiologic protocol as described previously.15
All COVID-19 patients in our hospital were hospitalized on dedicated wards under the care of a staff pulmonologist, nephrologist, infectious disease specialist or geriatrician, depending on their usual care team (eg, nephrology in dialysis patients). Additional local criteria to admit patients under geriatric care were age 85 years or older, long-term care residence or equivalent home care (ie, complete dependency on assistance for activities of daily living), patients with dementia or delirium, or patients aged 75 years and older with multiple comorbidities and polypharmacy.
On admission, an experienced geriatrician scored premorbid frailty according to the CFS based on information from patients, their families, and caregivers, primary care referral letters, or long-term care records.
Statistics
Results for continuous and categorical variables are reported as median and interquartile range and number (percentage), respectively. Differences between survivors and nonsurvivors were examined using Mann-Whitney U test and chi-square test for continuous and categorical variables, respectively. Association of age, frailty, and other baseline characteristics with mortality were evaluated by Spearman r and multiple logistic regression. Survival according to frailty status was examined using odds ratios, survival analyses (log-rank Mantel-Cox test) and receiver operating characteristic (ROC) curve analysis. Two-tailed P values <.05 were considered significant. All analyses were performed using GraphPad Prism v8.4.2.
Results
Baseline characteristics of our cohort are shown in Table 1 . Median age was 85 years (minimum 65, maximum 97 years), and 48 were women (59%). Median CFS score was 7 (range 2-9). Dementia had been diagnosed in 36 patients (44%), and 42 (52%) were long-term care residents. Polypharmacy was present in 52 (64%) subjects.
Table 1.
Characteristics of the Population According to Survival Status
| All (N = 81) | Survivors (n = 62) | Nonsurvivors (n = 19) | P | Spearman r | |
|---|---|---|---|---|---|
| Sex | .89 | ||||
| Men | 33 (41) | 25 (40) | 8 | ||
| Women | 48 (59) | 37 (60) | 11 | ||
| Age | 85 (81-90) | 84.5 (79-89) | 88 (84-90) | .03 | 0.241 |
| LTC resident | .27 | ||||
| Yes | 42 (52) | 30 (48) | 12 | ||
| No | 39 (48) | 32 (52) | 7 | ||
| CFS score | 7 (5-7) | 6 (4-7) | 7 (6-8) | .011 | 0.282 |
| Dementia | .77 | ||||
| Yes | 36 (44) | 26 (43) | 10 | ||
| No | 45 (56) | 36 (57) | 9 | ||
| Polypharmacy | .52 | ||||
| Yes | 52 (64) | 41 (65) | 11 | ||
| No | 29 (36) | 21 (35) | 8 | ||
| CT score | 9 (5-12) | 9 (5-12) | 9.5 (5-16.5) | .39 | |
| RT-PCR Ct value | 24.65 (20.25-28.78) | 25.95 (21.50-29.38) | 21.65 (19.28-22.95) | .015 | −0.285 |
| CRP, mg/L | 58 (32-89) | 56 (32-88) | 75 (39-130) | .20 | |
| CRPmax, mg/L | 110 (59-155) | 88 (47-140) | 190 (110-270) | <.001 | 0.453 |
| LDH, U/L | 305 (235-396) | 286 (229-367) | 390 (315-493) | .009 | 0.301 |
| Lymphocytes, 103/μL | 0.9 (0.6-1.2) | 0.9 (0.7-1.25) | 0.55 (0.4-1.05) | .020 | −0.262 |
| Lymphocyte nadir, 103/μL | 0.7 (0.5-0.95) | 0.8 (0.6-1.1) | 0.4 (0.275-0.65) | <.001 | −0.419 |
| Delirium | .88 | ||||
| Yes | 34 (42) | 26 (42) | 8 | ||
| No | 47 (58) | 36 (58) | 11 | ||
| Length of stay, d | 13 (8-18.5) | 13 (8-21) | 7 (3.75-15) | .050 |
LTC, long-term care.
Values are median (interquartile range) or n (%).
Sex, place of residence, dementia, polypharmacy, extent of affected lung tissue on CT, or CRP values at baseline did not differ between survivors and nonsurvivors. However, compared to survivors of COVID-19, nonsurvivors were significantly older (88.5 vs 85 years, median age) and frailer (median CFS 7 vs 6). Their RT-PCR cycling threshold (Ct) values were also significantly lower (indicating higher viral load). Baseline LDH was significantly higher and baseline lymphocyte count lower in nonsurvivors. Baseline CRP, ferritin, d-dimer, white blood cell, platelet, or 25-hydroxyvitamin D levels were not different (latter data not shown). Lymphopenia was present on admission in 48 patients (60%) and occurred during admission in 60 of 80 patients (75%; 1 patient was excluded because of chronic lymphocytic leukemia). The peak CRP and lymphocyte nadir reached during admission was higher among nonsurvivors, and these differences were highly significant. Length of stay tended to be shorter in those who died (P = .05).
Among these variables, the CFS score was associated with dementia (P < .0001, r = 0.602), long-term care residence (P < .0001, r = 0.465), and weakly with sex (lower frailty in males, P = .007, r = −0.296) and incident delirium (P = .043, r = 0.230). There was no significant association between CFS and older age in our cohort.
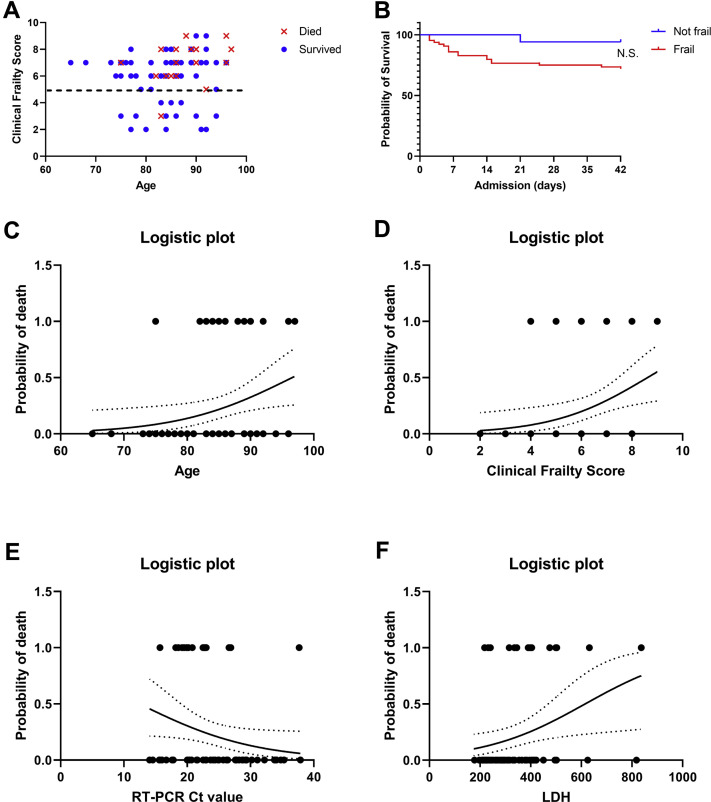
One of 17 patients died in the nonfrail group (CFS score 1-4), compared with 18 deaths among 64 frail patients; however, this difference did not reach significance (P = .054). Supplementary Figure 1A shows survivors and nonsurvivors according to their age and CFS. Most deaths occurred in older, frailer patients. However, this group overlapped considerably with many surviving frail older patients. Kaplan-Meier curves also showed only a trend towards higher mortality in frail vs nonfrail subjects (Mantel-Cox log-rank P = .06, Supplementary Figure 1B).
Supplementary Figure 1.
(A) Visual outline showing survivors and nonsurvivors according to their age and clinical frailty score. (B) Kaplan-Meier curves for frail vs nonfrail individuals. Logistic plots showing probability of death from COVID-19 according to baseline (C) age, (D) CFS, (E) reverse transcriptase polymerase chain reaction cycling threshold (RT-PCR Ct) values, or (F) lactate dehydrogenase (LDH) plasma concentration in single-factor logistic regression models.
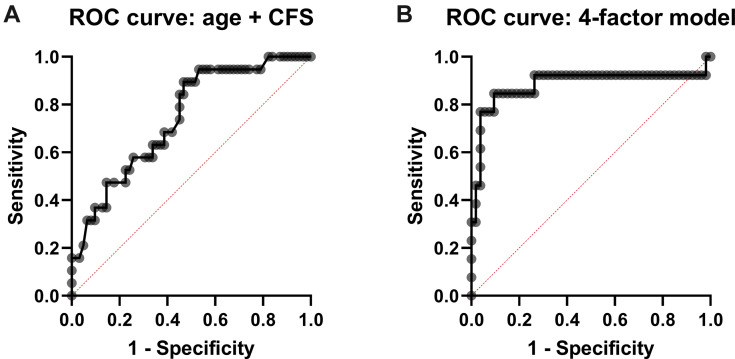
Next, we examined the clinical diagnostic utility of the individual variables that were significantly associated with mortality, in multiple logistic regression analyses. Again, age, CFS, RT-PCR Ct values, and LDH were significantly associated with higher odds of mortality (Table 2 and Supplementary Figure 1 C-F), whereas baseline lymphocyte count was no longer significant. In a bivariate model with age and CFS score combined, only the CFS remained significantly associated with mortality. The area under the ROC curve (AUROC) was 0.7443 (95% confidence interval 0.6213-0.8673) for this model (Figure 1 A), with a positive and negative predictive value of 57% and 80%, respectively. When age and CFS were combined with RT-PCR Ct values and LDH, the latter 3 variables remained significantly associated with mortality. The 4-factor model predicted the probability of mortality (range 0-1) as follows (intercept + β1∗age + β2∗CFS + β3∗RT-PCR Ct value + β4∗LDH): −13.89 + 0.126∗years + 0.561∗CFS-score + (−0.1623)∗Ct + 0.5275∗[U/L]/100 (Supplementary Table 1). The AUROC for this model was 0.8824 (0.7384-1.000, P < .0001, Figure 1B), with a negative predictive power of 89.5% and a positive predictive power of 78%, sensitivity of 54% and specificity of 78%.
Table 2.
Odds Ratios of 4 Independent Variables That Predict Mortality (Outcome Variable) in a Univariate and 2 Multivariate Models (Logistic Regression Analyses)
| Single-Factor Model | Model Age + CFS | Four-Factor Model | |
|---|---|---|---|
| Age | |||
| OR | 1.117 (1.021-1.240)∗ | 1.096 (1.002-1.218) | 1.134 (1.002-1.333) |
| AUROC | 0.664 (0.531-0.797)∗ | ||
| CFS score | |||
| OR | 1.705 (1.173-2.750)∗ | 1.588 (1.105-2.524)∗ | 1.752 (1.096-3.435)∗ |
| AUROC | 0.688 (0.555-0.821)∗ | ||
| RT-PCR Ct value | |||
| OR | 0.897 (0.798-0.994)∗ | 0.850 (0.723-0.972)∗ | |
| AUROC | 0.698 (0.559-0.836)∗ | ||
| LDH (U/L) | |||
| OR | 1.005 (1.001-1.010)∗ | 1.005 (1.000-1.011)∗ | |
| AUROC | 0.7124 (0.571-0.853)∗ |
OR, odds ratio; AUROC, area under the receiver operating characteristic curve.
Values are listed with 95% confidence intervals between brackets.
P < .05.
Figure 1.
(A) Receiver operating characteristic curves for model with age + clinical frailty score (CFS). (B) Receiver operating characteristic curve for model including age, CFS, reverse-transcriptase polymerase chain reaction cycling threshold (RT-PCR Ct values), and lactate dehydrogenase (LDH) levels.
Seven patients were treated with hydroxychloroquine, 60 (74%) with antibiotics, 46 (57%) with intravenous fluid support, and 25 with glucocorticoids (31%). Seven patients were admitted to the ICU, 5 of whom died. The odds ratio for mortality was significantly higher in patients requiring ICU admission (P = .0017).
There were 13 cases of presumably hospital-acquired COVID-19 (taking into account negative RT-PCR on admission, incubation time, and a local outbreak in 1 of our non-COVID-19 wards). Four of these patients died. There was no significantly higher or lower mortality between presumed hospital-acquired or community-acquired COVID-19 cases.
Discussion
The current COVID-19 pandemic particularly strikes frail older adults and/or long-term care residents, posing considerable medical and ethical challenges for our overwhelmed health care systems. Different guidelines have been released to assist in the triage in this population.9 , 11 , 16 Belgian and UK guidelines recommend the CFS to inform decision making regarding hospital referral of nursing home residents with suspected or confirmed COVID-19. However, empirical evidence supporting the use of frailty instruments to predict treatment outcomes and thus apply triage restrictions has remained lacking.17
The short-term mortality (∼23%) in this case series is similar to mortality rates reported for hospitalized older adults in Wuhan or California,3 , 4 but lower than that reported by Sun et al18 or than in the New York City area.2 This may be considered unexpected, given the greater frailty and older age of our patients compared with previous cohorts. Similar or higher mortality rates have been reported in long-term care residents19 or in younger ICU populations.20 These findings support the notion that it may be discriminatory and unethical to restrict hospital care based on age or frailty status alone.10 , 21 Still, mortality was higher in patients requiring ICU transfer in our cohort, suggesting that intensive care is of unclear clinical benefit in this population.22
Older age was significantly but weakly associated with increased risk of mortality, confirming recent studies.1, 2, 3, 4 Anecdotally, nonagenarians or centenarians have survived COVID-19.23 Our main finding was that frailty was also significantly but weakly associated with a higher risk of mortality in COVID-19 patients (multivariate odds ratio for mortality with each higher CFS point: 1.75.) Still, many severely frail patients survived (72%), and the CFS by itself had poor specificity and no useful cut-off for mortality prediction. A recent study from Italy showed that in a sample of 105 COVID-19 patients, frailty as assessed by the Frailty Index was associated with in-hospital mortality or ICU admission, independent of age and sex.24
Apart from age and frailty, LDH was the only circulating biomarker significantly associated with mortality in our cohort. This confirms prior studies.25, 26, 27 However, only a few patients met this criterion in our cohort, making it practically useless. Maximal CRP and nadir lymphocyte count during admission was significantly associated with mortality, but these parameters are not available at baseline. Interestingly, we observed a significant association between RT-PCR Ct values and mortality. Viral load peaks longer in patients with more severe COVID-19 and in older adults, as shown by Zheng et al.28 We speculate that a higher viral load may also be a marker for increased risk of mortality, although sampling bias needs to be excluded before we can support this conclusion. Of note, our RT-PCR method was semi-quantitative rather than quantitative, precluding extrapolation to other settings. The 4-factor model combining clinical, host, and viral parameters showed the most promising characteristics but still remained inadequate from a clinical perspective. Sun et al reported a similar logistic regression model based on older age and lymphocyte count.18 Further work is needed to establish optimal clinical, viral, and host immune system characteristics to predict mortality among COVID-19 patients.26
Our study provides the geriatric community with several novel insights into the outcomes of frail older COVID-19 patients. However, we recognize several limitations, mainly due to our retrospective study design. Because data were obtained retrospectively from electronic health records, missing data (eg, for CT scan or biochemical parameters) may have introduced bias, and follow-up was limited. However, selection bias is unlikely, because we had included consecutive cases in a country with universal health coverage. Caution should be applied to extrapolate findings from this single-center study to other health care settings. The associations we observed may not be causally related. Despite our robust findings on the association between frailty and mortality, some analyses were likely underpowered. A larger sample size would have helped reduce the size of our parameter estimate confidence intervals and increase the validity of our model; however, the first COVID-19 wave ended in our hospital and no more deaths have accumulated. We chose not to include patients with so-called radiographically confirmed COVID-19, that is, with typical clinical features and radiographic evidence on chest CT but with repeatedly negative SARS-CoV-2 RT-PCR. However, only 3 such patients were excluded, which is unlikely to have influenced the results.
Many instruments to determine frailty are available.29 We applied the CFS, which has been adopted in several national COVID-19 triage policies, most notably by UK NICE guidelines.11 Previous research has shown that CFS scores can reliably be obtained in critically ill patients based on chart review and patient and/or family interview.30 However, we recommend further research to ascertain the reproducibility and reliability before widespread implementation of the CFS during COVID-19 outbreaks. Importantly, we were unable to include younger, nonfrail patients, because frailty was not assessed in nongeriatric patients. The association between frailty and mortality would likely have been stronger if we included younger, less frail patients.
Conclusions and Implications
In summary, we showed that age and frailty were significantly but weakly associated with mortality among hospitalized older adults affected by COVID-19. However, both frailty and age alone have poor specificity to predict mortality, and many severely frail patients survived COVID-19. We recommend clinicians, ethicists, and policy makers to consider these empirical findings.
Acknowledgments
MRL has received consultancy and lecture fees from Alexion, Amgen, Kyowa Kirin, Menarini, Sandoz, Takeda, UCB, and Will-Pharma, none of which are related to this work.
The remaining authors declare no conflicts of interest.
Appendix
Supplementary Table 1.
Four-Factor Model to Predict Mortality: Parameter Estimates
| Parameter | Variable | Unit | Estimate | Standard Error | 95% Confidence Interval |
|---|---|---|---|---|---|
| Intercept | −13.89 | 6.351 | −28.27 to −2.674 | ||
| β1 | Age | Years | 0.126 | 0.071 | 0.002 to 0.287 |
| β2 | CFS | — | 0.561 | 0.281 | 0.092 to 1.234 |
| β3 | Ct value | — | −0.162 | 0.074 | −0.325 to −0.029 |
| β4 | LDH | (U/L)/100 | 0.528 | 0.268 | 0.045 to 1.130 |
CFS, Clinical Frailty Score (range 1-9); Ct value = cycling threshold of reverse transcriptase polymerase chain reaction.
References
- 1.Shahid Z., Kalayanamitra R., McClafferty B. COVID-19 and older adults: What we know. J Am Geriatr Soc. 2020;68:926–929. doi: 10.1111/jgs.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myers L.C., Parodi S.M., Escobar G.J. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323:2195–2198. doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Adamo H., Yoshikawa T., Ouslander J.G. Coronavirus disease 2019 in geriatrics and long-term care: The ABCDs of COVID-19. J Am Geriatr Soc. 2020;68:912–917. doi: 10.1111/jgs.16445. [DOI] [PubMed] [Google Scholar]
- 6.Farrell T.W., Ferrante L.E., Brown T. AGS position statement: Resource allocation strategies and age-related considerations in the COVID-19 era and beyond. J Am Geriatr Soc. 2020;68:1136–1142. doi: 10.1111/jgs.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrell T.W., Francis L., Brown T. Rationing limited health care resources in the COVID-19 era and beyond: Ethical considerations regarding older adults. J Am Geriatr Soc. 2020;68:1143–1149. doi: 10.1111/jgs.16539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cesari M., Proietti M. COVID-19 in Italy: Ageism and decision making in a pandemic. J Am Med Dir Assoc. 2020;21:576–577. doi: 10.1016/j.jamda.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matheny Antommaria A.H., Gibb T.S., McGuire A.L. Ventilator triage policies during the COVID-19 pandemic at U.S. Hospitals Associated With Members of the Association of Bioethics Program Directors. Ann Intern Med. 2020 Apr 24 doi: 10.7326/M20-1738. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bledsoe T.A., Jokela J.A., Deep N.N. Universal do-not-resuscitate orders, social worth, and life-years: Opposing discriminatory approaches to the allocation of resources during the COVID-19 pandemic and other health system catastrophes. Ann Intern Med. 2020 Apr 24 doi: 10.7326/M20-1862. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence (NICE) NICE guideline [NG159]: COVID-19 rapid guideline: Critical care in adults. https://www.nice.org.uk/guidance/ng159 2020. Available at:
- 12.Belgian Geriatric Society . 2020. Flowchart for decision making on hospital admission of possibly COVID-19 infected nursing home residents. [Google Scholar]
- 13.Ellis H.L., Wan B., Yeung M. Complementing chronic frailty assessment at hospital admission with an electronic frailty index (FI-Laboratory) comprising routine blood test results. CMAJ. 2020;192:E3–E8. doi: 10.1503/cmaj.190952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muscedere J., Waters B., Varambally A. The impact of frailty on intensive care unit outcomes: A systematic review and meta-analysis. Intensive Care Med. 2017;43:1105–1122. doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dangis A., Gieraerts C., De Breucker Y. Accuracy and reproducibility of low-dose submillisievert chest CT for the diagnosis of COVID-19. Radiol Cardiothorac Imaging. 2020:2. doi: 10.1148/ryct.2020200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong E., Chan M., Tan H.N. COVID-19: Use of the clinical frailty scale for critical care decisions. J Am Geriatr Soc. 2020 doi: 10.1111/jgs.16528. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubbard R.E., Maier A.B., Hilmer S.N. Frailty in the face of COVID-19. Age Ageing. 2020;68 doi: 10.1093/ageing/afaa095. E30-E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun H., Ning R., Tao Y. Risk factors for mortality in 244 older adults with COVID-19 in Wuhan, China: A retrospective study. J Am Geriatr Soc. 2020;68 doi: 10.1111/jgs.16533. E19-E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMichael T.M., Currie D.W., Clark S. Epidemiology of COVID-19 in a long-term care facility in king county, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White D.B., Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020 Mar 27 doi: 10.1001/jama.2020.5046. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Clarfield A.M., Dwolatzky T., Brill S. Israel ad hoc COVID 19 committee. Guidelines for care of older persons during a pandemic. J Am Geriatr Soc. 2020 May 11 doi: 10.1111/jgs.16554. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y.M., Hong X.Z., Shen J. China's oldest coronavirus survivors. J Am Geriatr Soc. 2020;68:940–942. doi: 10.1111/jgs.16462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellelli G., Rebora P., Valsecchi M.G. Frailty index predicts poor outcome in COVID-19 patients. Intensive Care Med. 2020 May 25 doi: 10.1007/s00134-020-06087-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J.J.Y., Lee K.S., Ang L.W. Risk factors of severe disease and efficacy of treatment in patients infected with COVID-19: A systematic review, meta-analysis and meta-regression analysis. Clin Infect Dis. 2020 May 14 doi: 10.1093/cid/ciaa576. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji D., Zhang D., Xu J. Prediction for progression risk in patients with COVID-19 pneumonia: The CALL Score. Clin Infect Dis. 2020 Apr 9 doi: 10.1093/cid/ciaa414. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry B.M., de Oliveira M.H.S., Benoit S. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chem Lab Med. 2020;58:1121–1128. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 28.Zheng S., Fan J., Yu F. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: Retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faller J.W., Pereira D.D.N., de Souza S. Instruments for the detection of frailty syndrome in older adults: A systematic review. PLoS One. 2019;14:e0216166. doi: 10.1371/journal.pone.0216166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shears M., Takaoka A., Rochwerg B. Assessing frailty in the intensive care unit: A reliability and validity study. J Crit Care. 2018;45:197–203. doi: 10.1016/j.jcrc.2018.02.004. [DOI] [PubMed] [Google Scholar]