Abstract
Overexpression of adenosine triphosphate (ATP)-binding cassette subfamily G member 2 (ABCG2) in cancer cells is known to cause multidrug resistance (MDR), which severely limits the clinical efficacy of chemotherapy. Currently, there is no FDA-approved MDR modulator for clinical use. In this study, rociletinib (CO-1686), a mutant-selective epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), was found to significantly improve the efficacy of ABCG2 substrate chemotherapeutic agents in the transporter-overexpressing cancer cells in vitro and in MDR tumor xenografts in nude mice, without incurring additional toxicity. Mechanistic studies revealed that in ABCG2-overexpressing cancer cells, rociletinib inhibited ABCG2-mediated drug efflux and increased intracellular accumulation of ABCG2 probe substrates. Moreover, rociletinib, inhibited the ATPase activity, and competed with [125I] iodoarylazidoprazosin (IAAP) photolabeling of ABCG2. However, ABCG2 expression at mRNA and protein levels was not altered in the ABCG2-overexpressing cells after treatment with rociletinib. In addition, rociletinib did not inhibit EGFR downstream signaling and phosphorylation of protein kinase B (AKT) and extracellular signal-regulated kinase (ERK). Our results collectively showed that rociletinib reversed ABCG2-mediated MDR by inhibiting ABCG2 efflux function, thus increasing the cellular accumulation of the transporter substrate anticancer drugs. The findings advocated the combination use of rociletinib and other chemotherapeutic drugs in cancer patients with ABCG2-overexpressing MDR tumors.
KEY WORDS: Rociletinib, Tyrosine kinase inhibitor, Multidrug resistance, ABCG2, ATPase
Abbreviations: ABC, adenosine triphosphate-binding cassette; ABCB1, ABC transporter subfamily B member 1; ABCG2, ABC transporter subfamily G member 2; AKT, protein kinase B; ATP, adenosine triphosphate; DDP, cisplatin; DOX, doxorubicin; DMEM, Dulbecco's modified Eagle's medium; DMSO, dimethyl sulfoxide; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; FBS, fetal bovine serum; FTC, fumitremorgin C; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IAAP, iodoarylazidoprazosin; IC50, half maximal (50%) inhibitory concentration; MDR, multidrug resistance; MTT, 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazoliumbromide; MX, mitoxantrone; PTK, protein tyrosine kinases; PBS, phosphate buffer saline; Rho 123, rhodamine 123; TKIs, tyrosine kinase inhibitors; VCR, vincristine; VRP, verapamil
Graphical abstract
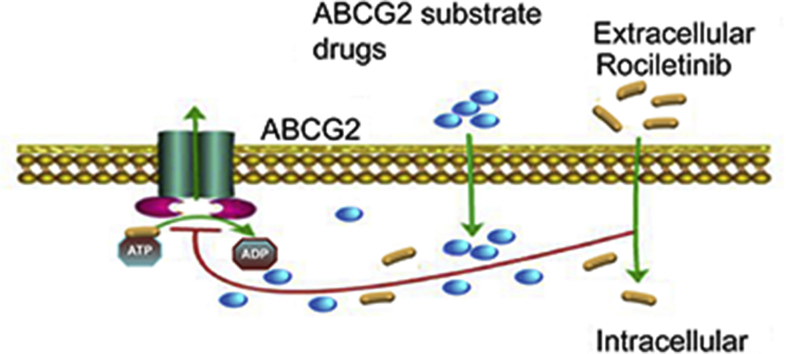
Rociletinib may bind to the ATP binding site of ABCG2, thereby blocking the efflux of the transporter substrates and increasing the intracellular accumulation of the substrate drugs. Our findings advocated the combination use of rociletinib and other chemotherapeutic drugs in cancer patients with ABCG2-overexpressing multidrug-resistant tumors.
1. Introduction
Multidrug resistance (MDR) is the major medical problem hindering successful chemotherapy in cancer treatment. The development of MDR is a multifactorial process, which involves changes in lipid bilayer membrane permeability, apoptosis, target gene amplification, detoxification of chemotherapeutic drugs by enzymes, and/or changes in the number of membrane receptors or transporters involved in drug accumulation or pharmacological response1,2. Among the various mechanisms, overexpression of ATP-binding cassette (ABC) transmembrane transporter superfamily is usually the most commonly reported and earliest cellular event contributing MDR in cancer cells, which significantly reduce intracellular drug accumulation, and decrease anticancer activity3, 4, 5.
To date, 49 ABC transporter proteins have been identified in human, 16 of which are known to mediate MDR6. According to the similarity in gene structure and sequence homolog, the ABC transporter family can be divided into seven subfamilies, ABCA to ABCG. ABC transporter subfamily G member 2 (ABCG2), also known as breast cancer resistance protein (BCRP), is considered one of the most important transporters leading to MDR, which is highly expressed in many cancer types7.
Doyle et al.8 first discovered ABCG2 in doxorubicin (DOX)-resistant breast cancer cell line MCF-7/Adr-Vp3000. Subsequently, other research teams also reported ABCG2 in mitoxantrone (MX)-resistant colon cancer cell line S1-MI-80 and non-small cell lung cancer cell line H460/MX209,10. ABCG2 is a 655-amino acid polypeptide with a molecular weight of 72 kDa. It contains only one nucleotide binding domain (NBD) and one transmembrane binding domain (TMD), so ABCG2 is called a half-transporter. ABCG2 has to form homologous dimers with itself or heterodimers with other members of ABCG subfamily before it can function as an efflux pump11. ABCG2 recognizes and effectively extrudes a wide variety of chemotherapeutic drugs with different chemical structures, including anthracyclines (daunorubicin and DOX), Topo II inhibitors (topotecan and irinotecan), antimetabolic drugs (methotrexate), and tyrosine kinase inhibitors (TKIs), to mediate their drug resistance.
Protein tyrosine kinases (PTK) play a key role in cellular signal transduction in cancer cells12. They catalyze the transfer of γ-phosphoryl group of ATP to tyrosine residues of many important oncogenic proteins, thereby activating them to promote cancer progression12. TKIs represent a new class of targeted anticancer drugs that inhibit specific PTK to elicit their anticancer activities. They work by competing with ATP for binding with ATP binding sites on oncogenic PTKs13. The fact that ABC transporters depend on the energy derived from ATP hydrolysis at their ATP binding sites to provide the drug efflux effect has prompted the initial investigation about the inhibition of ABC transporters by TKIs. To date, a number of TKIs, including lapatinib14, crizotinib15, alectinib16, and dacomitinib17 have been shown to inhibit ABC transporters to overcome the efflux transporters-mediated MDR. TKIs inhibit drug efflux and therefore increase the cellular accumulation of the concomitantly administered chemotherapeutic drugs from MDR cancer cells to enhance the overall anticancer effect.
Rociletinib (CO-1686) is an oral, irreversible epidermal growth factor receptor (EGFR) inhibitor that covalently binds to Cys797 in the ATP-binding pocket of the EGFR kinase domain18. It has been shown that rociletinib selectively targets the mutant forms of EGFR (T790M and exon 19 deletion) but spares the wild-type EGFR, therefore causing less adverse effects than the earlier generations of EGFR TKIs19,20. There has not been any report about the interaction between rociletinib and ABC transporters. In this study, the effects of rociletinib on enhancing the efficacy of chemotherapeutic drugs in ABCG2-overexpressing MDR cancer cells were investigated in vitro and in vivo.
2. Materials and methods
2.1. Reagents
Rociletinib (CO-1686) was purchased from MCE Inc. (Monmouth Junction, NJ, USA). The chemical structure of rociletinib is showed in Fig. 1A. Dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-yl)-2,5-diphenyltetrazolium bromide (MTT), MX, topotecan, DOX, rhodamine 123 (Rho 123), verapamil (VRP), fumitremorgin C (FTC), and cisplatin (DDP) were obtained from Chemical Co. (St. Louis, MO, USA). Antibodies used for flow cytometric assays, including the mouse anti-human BCRP1/ABCG2 antibody and the control anti-human immunoglobin G2b (IgG2b) antibody, were from Santa Cruz Biotechnology Inc. (Paso Robles, CA, USA). The antibodies used for Western blot analysis to detect AKT, p-AKT, ERK, p-ERK, and ABCG2 were purchased from Santa Cruz Biotechnology Inc. The antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was purchased from Kangcheng Inc. (Shanghai, China). SYBR Green qPCR Master Mix was bought from ExCell Bio Inc. (Shanghai, China). Dulbecco's modified Eagle's medium (DMEM), RPMI-1640, and fetal bovine serum (FBS) were obtained from Gibco BRL Inc. (Gaithersburg, MD, USA).
Figure 1.
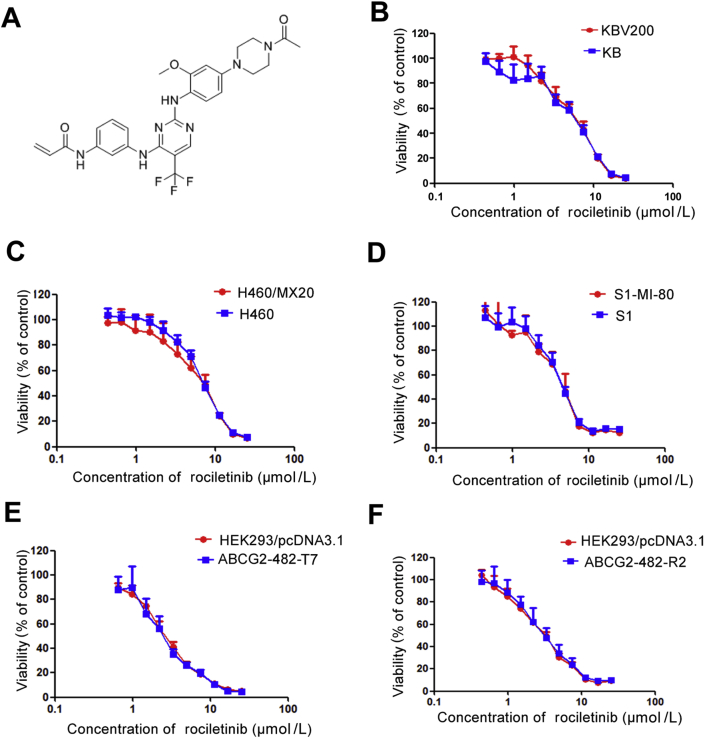
The structure of rociletinib and cytotoxicity of rociletinib. (A) The structure of rociletinib. MTT cytotoxicity assay was conducted in ABCG2 and ABCB1-overexpressing cells and their parental sensitive cells; (B) ABCB1-overexpressing KBv200 cells and their parental drug sensitive KB cells; (C) ABCG2-overexpressing H460/MX20 cells and their parental drug sensitive H460 cells; (D) ABCG2-negative S1 and ABCG2-overexpressing S1-MI-80 cells; (E) ABCB1-negative HEK293/pcDNA3.1 and ABCG2-overexpressing wild type ABCG2-482-R2; (F) ABCG2-negative HEK293/pcDNA3.1 and ABCG2-overexpressing mutant ABCG2-482-T7 cells. Cells were treated with a range of concentrations of rociletinib for 72 h. Results from three independent experiments are expressed as the mean ± SD.
2.2. Cell lines and cell culture
The following cell lines are generous gifts provided by Dr. Susan Bates (Columbia University/New York Presbyterian Hospital, Manhattan, NY, USA). KBv200 cells are vincristine (VCR)-induced ABC transporter subfamily B member 1 (ABCB1)-overexpressing resistant subline derived from human oral epidermoid carcinoma cell line KB21. H460/MX20 cells are MX-induced ABCG2-overexpressing resistant subline derived from human non-small cell lung carcinoma cell line H46022. S1-MI-80 cells are MX-induced ABCG2-overexpressing resistant subline derived from human colon carcinoma cell line S123. Human embryonic kidney stably transfected cell line HEK293/pcDNA3.1, wild-type ABCG2-482-R2, and mutant ABCG2-482-T7 cells24 were also used. H460 and H460/MX20, S1 and S1-MI-80, HEK293/pcDNA3.1, HEK293/ABCG2-482-R2, and HEK293/ABCG2-482-T7 were cultured in DMEM medium. KB and KBv200 were maintained in RPMI-1640 medium. All cell lines were maintained in a humidified incubator containing 5% CO2 at 37 °C.
2.3. Drug sensitivity assays
The MTT colorimetric cell proliferation assay was used to determine drug sensitivity in the cell models in vitro25. Single cell suspension was prepared and inoculated on 96-well plates at density of 3000–8000 per well. The cells were then placed in an incubator at 37 °C with 5% CO2 for 24 h. A range of rociletinib concentrations was tested in the cytotoxicity experiment. In the MDR reversal experiment, after 1 h incubation with 0, 0.25, 0.5, and 1 μmol/L rociletinib, 2.5 μmol/L FTC or 2.5 μmol/L VRP, a range of different concentrations of anticancer drugs was added and followed by incubation for another 68 h. At the end of the assay, 20 μL 0.5% MTT was added to each well and allowed to incubate for an additional 4 h. The supernatant was then removed and 150 μL DMSO was added into each well to dissolve the MTT crystals. The absorbance at 540 nm/655 nm dual wavelength was detected by a Model 550 Microplate Reader (Bio-Rad, Hercules, CA, USA), and the half maximal (50%) inhibitory concentration (IC50) was calculated by Bliss method26. VRP and FTC were used as positive control inhibitors for ABCB1 and ABCG2, respectively.
2.4. In vivo tumour xenograft experiment
Our team has carried out animal experimental study on rociletinib and olmutinib at the same time. The results of olmutinib research have been published by Zhang et al27. The ABCG2-overexpressing S1-MI-80 cell xenograft model was established according to our published protocol28. Athymic nude mice (BALB/c-nu/nu, female, 4–6 weeks old, 16–20 g) were purchased from the Vital River Inc. (Beijing, China). About 1 × 107 S1-MI-80 cells were implanted subcutaneously into the right flank of athymic nude mice. When the average tumor diameter reached 5 mm, the mice were randomly divided into six groups and given different drug regimens: (1) control (saline, 10 mL/kg, i.p., q5d); (2) rociletinib (5 mg/kg, p.o., q5d); (3) olmutinib (30 mg/kg, p.o., q5d); (4) topotecan (2 mg/kg, i.p., q5d); (5) rociletinib (5 mg/kg, p.o., q5d) + topotecan (2 mg/kg, i.p., q5d, given after rociletinib administration 1 h); (6) olmutinib (30 mg/kg, p.o., q5d) + topotecan (2 mg/kg, i.p., q5d, given after olmutinib administration 1 h). In this experiment, relevant experimental results of (1), (2), (4) and (5) groups were used for data analysis. Mice were measured every five days, including their body weight, the maximum diameter (a) of the tumor, and its maximum transverse diameter (b) in the vertical direction. The tumor volume (V) was calculated according to Eq. (1):
| (1) |
When the average tumor weight of the mice in the control group reached 1 g, the mice were euthanized, the xenografts were excised and weighed. The ratio of growth inhibition (IR) was estimated according to Eq. (2):
| (2) |
All experiments were performed in accordance with the guidelines for use of laboratory animals of the Sun Yat-Sen University Institutional Animal Care and Use Committee (Guangzhou, China).
2.5. Accumulation of DOX and Rho 123
Flow cytometry assays were used to detect the effect of rociletinib on the intracellular accumulation of DOX and Rho 123 in cancer cells as previously described29. Logarithmic phase cells were inoculated in 6-well plates and kept at 37 °C in incubator for 24 h before the drug accumulation experiment. Different concentrations of rociletinib or 2.5 μmol/L FTC were added to the cells and allowed to incubate for 3 h. After this pre-incubation with rociletinib or FTC, the cells were treated with Rho 123 (5 μmol/L) or DOX (10 μmol/L) and allowed to incubate at 37 °C for additional 0.5 or 3 h, respectively. The cells were then collected, washed 3 times with ice-cold phosphate buffer saline (PBS), and resuspended in 200 L PBS to make single cell suspension for flow cytometry analysis (Cytomics FC500; Beckman Coulter Inc., Brea, CA, USA). FTC was used as positive control inhibitor for ABCG2.
2.6. Rho 123 efflux assay
The effect of rociletinib on Rho 123 efflux was evaluated as described previously30. S1 and S1-MI-80 cells were treated with 5 μmol/L Rho 123 at 37 °C for 0.5 h, the cells were washed 3 times with ice-cold PBS, then cultured at 37 °C with fresh medium containing 1 μmol/L rociletinib but without Rho 123. The cells were then collected at 0, 15, 30, 60, or 90 min and washed three times with ice-cold PBS. Finally, cells were resuspended in ice-cold PBS and analyzed flow cytometry immediately.
2.7. Examination of cell surface expression of ABCG2 by flow cytometry
A flow cytometry assay was used to detect whether rociletinib affects the expression of ABCG2 on cell surface as described previously14. S1-MI-80 and H460/MX20 cells were treated with 1 μmol/L rociletinib, then incubated at 4 °C for 48 h. The cells were harvested, washed with cold PBS containing 0.5% BSA for 3 times, and then resuspended into single cell suspension (4 × 106 cells/mL). The single cell suspension (25 μL) was mixed with 10 μL of FITC-conjugated anti-human BCRP1/ABCG2 antibody and incubated in the dark for 45 min at 4 °C. Afterwards, the cells were washed twice with ice-cold PBS and resuspended in 400 μL of ice-cold PBS for flow cytometric analysis. The cell samples incubated with FITC-labeled mouse IgG2b antibody were used as negative control for comparison.
2.8. Immunofluorescence analyses
Immunofluorescence confocal microscopy was used to examine whether treatment of rociletinib leads to internalization of ABCG2 as the previously described31 with slight modifications. In brief, S1-MI-80 cells (3 × 104) were seeded on 13 mm square glass cover slides in 24-well plates, treated with 1 μmol/L rociletinib, then incubated at 4 °C for 48 h. The cells were washed with PBS, and then fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.2% Triton X-100 for 10 min. After washing three times with PBS, cells were blocked with 5% BSA for 45 min, and incubated with anti-ABCG2 primary antibody (1:100; Cat#ABP0148; Abbkine, CA, USA) overnight at 4 °C. Then stained with an Alexa Fluor 488-conjugated anti-rabbit IgG F (ab')2 fragment (1:100; Cat#4412; CST, Beverly, MA, USA) at room temperature in dark for 1 h. Nuclei was stained with 4′,6-diamidino-2-phenylindole (DAPI, 2.5 μg/mL) for 5 min and cells were observed and imaged using confocal microscope (LSM 880 with fast AirysCan; Carl Zeiss, Jena, Germany).
2.9. ABCG2 ATPase assay
ABCG2 ATPase activity was determined by a colorimetric method as previously described32 with slight modifications. Crude membranes isolated from ABCG2-overexpressing MCF7/FLV1000 cells (100 μg protein/mL) were incubated with rociletinib (0–10 μmol/L) for 5 min at 37 °C in the assay buffer (50 mmol/L KCl, 5 mmol/L sodium azide, 2 mmol/L EDTA, 10 mmol/L MgCl2, 1 mmol/L DTT, pH 6.8) with or without of 1.2 mmol/L sodium orthovanadate. ATP hydrolysis reaction started by adding 5 mmol/L Mg-ATP (concentration in a final volume of 60 μL) and then incubated for 10 min. The reaction was terminated by adding 30 μL of 10% SDS solution. Afterwards, a detection reagent (35 mmol/L ammonium molybdate, 15 mmol/L zinc acetate, 10% ascorbic acid) was added and incubated at 37 °C for 20 min. UV absorbance of the reaction mixture was then measured at 750 nm. The release of inorganic phosphate was quantitatively determined by reading from calibration curve. The activity of ABCG2 ATPase stimulated by rociletinib was determined as the difference between the amounts of inorganic phosphate released from ATP with or without sodium orthovanadate.
2.10. Photoaffinity labeling of ABCG2 with [125I]-IAAP
The photoaffinity labeling of ABCG2 with [125I]-IAAP was examined as our previously described33. In brief, crude membrane from ABCG2-overexpressing MCF7/FLV1000 cells (50 μg protein) was incubated with 0–10 μmol/L rociletinib for 5 min at room temperature in 50 mmol/L Tris-HCl (pH 7.5). [125I]-IAAP (2200 Ci/nmole, 3 nmol/L) was added and the mixture was incubated for an additional 5 min under subdued light. The samples were then cross-linked by UV illumination (365 nm) on ice. BXP21 antibody (Abcam, Cambridge, MA, USA) was used to immunoprecipitate the radio-labeled ABCG2. The samples were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 7% Tris-acetate NuPAGE gel, dried and exposed to Bio-Max MR film (Eastman Kodak Co., Rochester, NY, USA) at −80 °C for 4 h. Radioactivity incorporated into the transporter protein was visualized using the Storm 860 PhosphorImager system (Molecular Dynamics, Sunnyvale, CA, USA).
2.11. Western blot analysis
Western blot was used to examine whether the protein level of ABCG2 in ABCG2-overexpressing cells was affected by rociletinib. Moreover, the effect of different concentrations of rociletinib on the protein expression of AKT, ERK, and their phosphorylations were evaluated in S1-MI-80 and its parental S1 cells. The cells were treated with rociletinib (0, 0.25, 0.5, 1, or 5 μmol/L) and incubated for 48 h. Protein samples were then extracted from the cell lysate. Equal amounts of proteins were resolved on 10% SDS-PAGE gel and transferred to polyvinylidene fluoride (PVDF) membranes. PVDF membranes were blocked at room temperature with 5% non-fat milk for 2 h and then incubated with the primary antibody overnight at 4 °C. After washing three times with TBST, these membranes were incubated with the secondary antibody at room temperature for 2 h. The protein–antibody complexes were visualized by the enhanced chemiluminescence solutions and exposed to a Kodak medical X-ray processor (Kodak, Rochester, NY, USA). GAPDH was used as a loading control.
2.12. Real-time quantitative RT-PCR
ABCG2 mRNA expression level was analyzed as described previously34. Total RNA was extracted from cells after treatment with 0, 0.25, 0.5, 1, or 5 μmol/L rociletinib for 48 h by Trizol reagent RNA extraction kit (Molecular Research Center, Cincinnati, OH, USA). The PCR primers used are listed as follows: 5′-TGGCTGTCATGGCTTCAGTA-3′ (forward) and 5′-GCCACGTGATTCTTCCACAA-3′ (reverse) for ABCG2, 5′-CTTTGGTATCGTGGAAGGA-3′ (forward) and 5′-CACCCTGTTGCTGTAGCC-3′ (reverse) for GAPDH. The relative expression of ABCG2 was quantified after normalization with GAPDH expression in each sample.
2.13. Statistical analysis
All results were presented as mean values ± standard deviation (SD). All experiments were repeated at least three times. The SPSS statistical software (SPSS 16.0) was used in statistical analyses. The statistical differences were determined by using the Student's t test. P < 0.05 was considered statistically significant (*P < 0.05; **P < 0.01). The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number as RDDB2020000782.
3. Results
3.1. Rociletinib enhanced the efficacy of transporter substrate anticancer drugs in ABCG2-overexpressing cells in vitro
The cytotoxicity of rociletinib in different cell lines was first evaluated by MTT assay (Fig. 1). Rociletinib was found to exhibit similar cytotoxicity in the different pairs of sensitive parental (KB, H460, and S1) and their respective resistant cell lines (KBv200, H460/MX20, and S1-MI-80) and also within the panel of transfected HEK293 cells (Fig. 1). In all cell lines, cell survival was more than 80% upon treatment with 1 μmol/L rociletinib alone. Therefore, three concentrations of rociletinib (0.25, 0.5, and 1 μmol/L) were selected for the MDR reversal experiments when combination rociletinib and chemotherapeutic drugs were evaluated.
Table 1 summarizes the IC50 values of anticancer drugs in sensitive and drug-resistant cells in the absence or presence of rociletinib. Rociletinib remarkably reduced the IC50 values of the two ABCG2 substrate anticancer drugs (topotecan or MX) in H460/MX20 and S1-MI-80 cells in a concentration-dependent manner, and giving rise to synergistic cytotoxic effects. However, rociletinib did not alter the cytotoxic effect of topotecan or MX notably in the parental H460 and S1 cells. It is also noteworthy that the combination of rociletinib and the ABCB1-substrate anticancer drug DOX did not give rise to appreciable enhancement effect in the ABCB1-overexpressing KBv200 cells. Moreover, rociletinib was also found to potentiate the cytotoxicity of MX in ABCG2-482-R2 or ABCG2-482-T7 stably transfected HEK293 cells, but not in the backbone vector transfected in HEK293 cells (Table 2). The results suggested that rociletinib specifically potentiated the cytotoxic effect of conventional ABCG2-substrate chemotherapeutic drugs in ABCG2-mediated MDR cells.
Table 1.
Effect of rociletinib on enhancement of conventional chemotherapeutic agents.
| Compound | IC50 ± SD (μmol/L) (fold-reversal) |
||
|---|---|---|---|
| KB | KBv200 | ||
| DOX | 0.1221 ± 0.0088 (1.00) | 0.7872 ± 0.1878 (1.00) | |
| +0.25 μmol/L Rociletinib | 0.1036 ± 0.0043 (1.18) | 0.7662 ± 0.0086 (1.03) | |
| +0.5 μmol/L Rociletinib | 0.1246 ± 0.0102 (0.98) | 0.6655 ± 0.0408 (1.18) | |
| +1 μmol/L Rociletinib | 0.1345 ± 0.0030 (0.91) | 0.7602 ± 0.0258 (1.04) | |
| +2.5 μmol/L VRP | 0.1130 ± 0.0035 (1.08) | 0.1396 ± 0.0065 (5.64)** | |
| DDP | 2.4329 ± 0.4249 (1.00) | 14.9862 ± 1.6204 (1.00) | |
| +1 μmol/L Rociletinib |
2.2357 ± 0.0569 (1.09) |
13.1278 ± 0.0062 (1.14) |
|
| H460 |
H460/MX20 (ABCG2) |
||
| MX | 0.0190 ± 0.0051 (1.00) | 0.8214 ± 0.0668 (1.00) | |
| +0.25 μmol/L Rociletinib | 0.0232 ± 0.0061 (0.82) | 0.7341 ± 0.1103 (1.12) | |
| +0.5 μmol/L Rociletinib | 0.0122 ± 0.0078 (1.56) | 0.3971 ± 0.0244 (2.07)** | |
| +1 μmol/L Rociletinib | 0.0135 ± 0.0050 (1.41) | 0.2911 ± 0.0206 (2.82)** | |
| +2.5 μmol/L FTC | 0.0144 ± 0.0008 (1.32) | 0.0618 ± 0.0023 (13.29)** | |
| DDP | 11.4225 ± 2.0030 (1.00) | 18.8356 ± 0.3269 (1.00) | |
| +1 μmol/L Rociletinib |
14.4671 ± 2.9494 (0.79) |
21.8462 ± 0.7533 (0.86) |
|
| S1 |
S1-MI-80 (ABCG2) |
||
| MX | 0.3429 ± 0.0525 (1.00) | 9.3423 ± 0.1651 (1.00) | |
| +0.25 μmol/L Rociletinib | 0.3096 ± 0.0288 (1.11) | 4.3993 ± 0.1371 (2.12)** | |
| +0.5 μmol/L Rociletinib | 0.2737 ± 0.0084 (1.25) | 2.1458 ± 0.3145 (4.35)** | |
| +1 μmol/L Rociletinib | 0.2380 ± 0.0036 (1.44) | 1.1523 ± 0.2084 (8.11)** | |
| +2.5 μmol/L FTC | 0.3231 ± 0.0988 (1.06) | 0.0468 ± 0.0012 (199)** | |
| Topotecan | 0.0823 ± 0.0095 (1.00) | 49.9142 ± 1.4799 (1.00) | |
| +0.25 μmol/L Rociletinib | 0.0953 ± 0.0014 (0.86) | 24.0980 ± 2.6864 (2.07) | |
| +0.5 μmol/L Rociletinib | 0.0364 ± 0.0144 (2.26) | 9.5927 ± 1.1706 (5.20)** | |
| +1 μmol/L Rociletinib | 0.0507 ± 0.0034 (1.26) | 6.8783 ± 0.6357 (7.26)** | |
| +2.5 μmol/L FTC | 0.0128 ± 0.0988 (6.34) | 4.7313 ± 0.3521 (10.55)** | |
| DDP | 5.5692 ± 0.8301 (1.00) | 4.0395 ± 0.4164 (1.00) | |
| +1 μmol/L Rociletinib | 5.4199 ± 0.5084 (1.03) | 3.9445 ± 0.2253 (1.02) | |
Cell survival was performed by MTT assay as described in ‘‘Materials and methods’’. VRP (specific inhibitor of ABCB1) and FTC (specific inhibitor of ABCG2) were used as the positive control. The fold reversal of MDR (values given in parentheses) was calculated by dividing the IC50 value for cells with the anticancer agent in the absence of rociletinib by that obtained in the presence of rociletinib. Data were shown as the mean ± SD of at least three independent experiments performed in triplicate. *P < 0.05, **P < 0.01.
Table 2.
Effect of rociletinib on reversing ABCG2-mediated MDR in stable-transfected cells.
| Compound | IC50 ± SD (μmol/L) (fold-reversal) |
||
|---|---|---|---|
| HEK293/pcDNA3.1 | ABCG2-482-R2 | ABCG2-482-T7 | |
| MX | 0.0074 ± 0.0029 (1.00) | 0.0591 ± 0.0133 (1.00) | 0.0279 ± 0.0085 (1.00) |
| +0.25 μmol/L Rociletinib | 0.0052 ± 0.0014 (1.42) | 0.0269 ± 0.0089 (2.20)** | 0.0133 ± 0.0033 (2.10)** |
| +0.5 μmol/L Rociletinib | 0.0062 ± 0.0064 (1.19) | 0.0179 ± 0.0034 (3.30)** | 0.0074 ± 0.0010 (3.77)** |
| +1 μmol/L Rociletinib | 0.0055 ± 0.0014 (1.35) | 0.0119 ± 0.0048 (4.97)** | 0.0072 ± 0.0025 (3.88)** |
| +2.5 μmol/L FTC | 0.0093 ± 0.0030 (0.80) | 0.0047 ± 0.3193 (12.57)** | 0.0118 ± 0.0053 (2.36)** |
| DDP | 2.2131 ± 0.4176 (1.00) | 0.9374 ± 0.2273 (1.00) | 0.7426 ± 0.1449 (1.00) |
| +1 μmol/L Rociletinib | 1.9302 ± 0.2961 (1.15) | 0.7982 ± 0.1226 (1.17) | 0.8032 ± 0.2545 (0.92) |
Cell survival was performed by MTT assay as described in “Materials and methods”. FTC (specific inhibitor of ABCG2) was used as the positive control. The fold reversal of MDR (values given in parentheses) was calculated by dividing the IC50 value for cells with the anticancer agent in the absence of rociletinib by that obtained in the presence of rociletinib. Data were shown as the mean ± SD of at least three independent experiments performed in triplicate. *P < 0.05, **P < 0.01.
3.2. Rociletinib significantly enhanced therapeutic effect of topotecan in ABCG2-overexpressing S1-MI-80 tumor xenografts in vivo
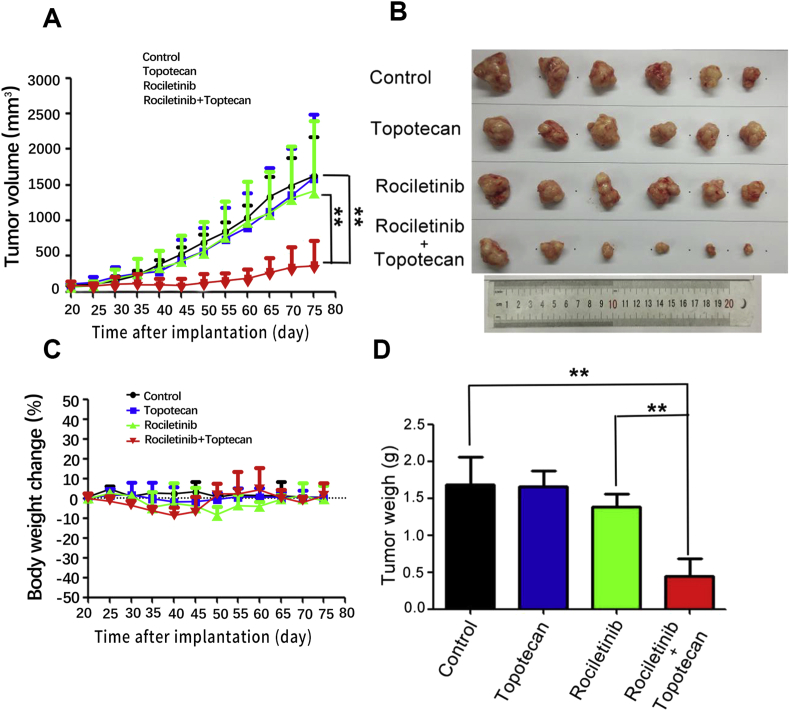
The combination of rociletinib and an ABCG2 substrate anticancer drug (topotecan) was further evaluated in vivo in our established ABCG2-overexpressing S1-MI-80 tumor xenograft model in nude mice. Mice bearing S1-MI-80 tumors were administered with 30 mg/kg rociletinib, 2 mg/kg topotecan, or their combination. As shown in Fig. 2D, no significant difference in tumor size was observed among animal groups treated with saline, topotecan and rociletinib. However, the group treated with rociletinib (30 mg/kg, p.o., given before topotecan administration 1 h) in combination with topotecan (2 mg/kg, i.p.) produced a significantly greater inhibitory effect on tumor growth, when compared with groups treated with saline, topotecan, or rociletinib alone. Importantly, no appreciable weight loss or drug-related deaths were observed in the rociletinib and topotecan combination group.
Figure 2.
Rociletinib enhanced the anticancer effect of topotecan in the S1-MI-80 tumor xenograft model in nude mice. (A) The changes in tumor volume over time after the S1-MI-80 cell implantation (n = 6). Data shown are mean ± SD of tumor volumes for each group. (B) The image of tumors size in four groups excised from the mice on the 75th day after implantation. (C) Average percentage change in body weight after treatments. (D) Mean tumor weight after excising from the mice on the 75th day after implantation (n = 6). The four treatment groups were: (1) control (saline, 10 mL/kg, i.p., q5d); (2) topotecan (2 mg/kg, i.p., q5d); (3) rociletinib (5 mg/kg, p.o., q5d) and (4) topotecan (2 mg/kg, i.p., q5d) + rociletinib (5 mg/kg, p.o., q5d given before topotecan administration 1 h). Results are presented as the mean ± SD. *P < 0.05, **P < 0.01.
3.3. Rociletinib enhanced the cellular accumulation of DOX and Rho 123 in ABCG2-overexpressing cells
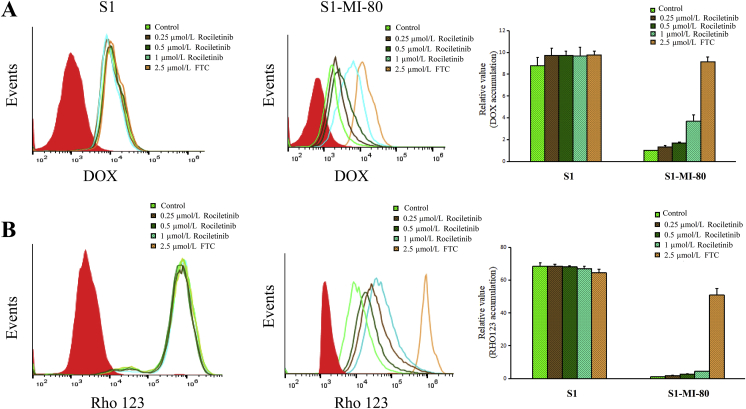
The results above showed that rociletinib can significantly improve the sensitivity of MDR cancer cells to ABCG2 substrate chemotherapeutic drugs in ABCG2-overexpressing cells in vitro and in vivo. In order to further investigate the MDR reversal mechanism of rociletinib, the intracellular accumulation of model fluorescent ABCG2 substrates (DOX and Rho 123) was measured in the presence or absence of rociletinib. As shown in Fig. 3, the cellular accumulation of DOX and Rho 123 in ABCG2-overexpressing S1-MI-80 cells were significantly lower than those in parental S1 cells, indicating that ABCG2-mediatd drug efflux results in MDR. Importantly, in drug-resistant S1-MI-80 cells, rociletinib was found to increase DOX and Rho 123 accumulation in a concentration-dependent manner. However, there was no significant effect in sensitive S1 cells. The results showed that rociletinib could reverse MDR by increasing the accumulation of substrate chemotherapeutic drugs in ABCG2-overexpressing cancer cells.
Figure 3.
Effect of rociletinib on the intracellular accumulation of DOX, Rho 123 in MDR cells and their parental cells. The accumulation of DOX (A), Rho 123 (B) in S1 and S1-MI-80 cells were measured by flow cytometric analysis as described in ‘‘Materials and methods’’ section. The results were presented as fold change in fluorescence intensity relative to control MDR cells. Data are expressed as mean ± SD from three independent experiments.
3.4. Rociletinib inhibited ABCG2-mediated Rho 123 efflux
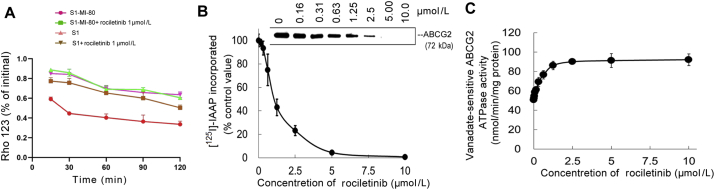
The Rho 123 efflux assay was conducted to further confirm that rociletinib increased the intracellular accumulation of DOX and Rho 123 by interfering with the transporter-mediated efflux. As shown in Fig. 4A, the efflux of Rho 123 (a fluorescent and ABCG2 substrate dye) was measured at different time points in the presence or absence of rociletinib. Due to the active efflux function of ABCG2, the retention of Rho 123 in S1-MI-80 cells was significantly lower than that in the parental S1 cells after the efflux period. Importantly, in the presence of 1 μmol/L rociletinib, intracellular retention of Rho 123 was greatly increased in S1-MI-80 cells, but no significant change was observed in S1 cells. These results suggested that rociletinib increased intracellular accumulation of Rho 123 by inhibiting ABCG2-mediated efflux in S1-MI-80 cells.
Figure 4.
Effect of rociletinib on the efflux of Rho 123, ATPase activity and [125I]-IAAPphotoaffinity labeling of ABCG2. (A) Time course of Rho 123 efflux was measured in S1 and S1-MI-80 cells, with or without 1 μmol/L rociletinib. (B) Effect of rociletinib on ABCG2 ATPase activity. The vanadate-sensitive ABCG2 ATPase activity in the presence of the indicated concentrations of rociletinib was evaluated. The mean ± SD values from three independent experiments are shown. (C) Rociletinib competed for photolabeling of ABCG2 by [125I]-IAAP. Crude membranes from ABCG2-overexpressing MCF7/FLV1000 cells were incubated with [125I]-IAAP and a range of different concentration (0–10 μmol/L) of rociletinib. The samples were then cross-linked by UV illumination, subjected to SDS-PAGE, and analyzed as described in the method section. A representative autoradiogram from three independent experiments is shown. The relative amount of [125I]-IAAP incorporated was plotted against the concentration of rociletinib used in the competition. 100% incorporation refers to the absence of rociletinib. Data are expressed as mean ± SD from three independent experiments.
3.5. Rociletinib modulated ATPase activity of ABCG2
Drug transport activity of ABCG2 is related to ATP hydrolysis, which is stimulated in the presence of transport substrate. In order to further investigate the mechanism of ABCG2 inhibition by rociletinib, vanadate-sensitive ATPase activity of ABCG2 was measured at different concentrations of rociletinib. Rociletinib was found to stimulate ABCG2 ATPase activity in a concentration-dependent manner up to about 2.5 μmol/L (Fig. 4B). No further change of ABCG2 ATPase activity was observed at rociletinib above 2.5 μmol/L (Fig. 4B). These results suggested that rociletinib stimulated the ABCG2 ATPase activity by interacting with the drug substrate binding sites on the transporter.
3.6. Rociletinib inhibited the [125I]-IAAP photoaffinity labeling of ABCG2
In order to further investigate the interaction between rociletinib and the drug-binding pocket of ABCG2, the photo-labeling of ABCG2 by [125I]-IAAP in the presence of different concentrations of rociletinib was examined. Rociletinib was shown to strongly inhibited the [125I]-IAAP photolabeling of ABCG2 in a concentration-dependent manner (Fig. 4B). The concentration of rociletinib required for 100% inhibition of photolabeling of ABCG2 by [125I]-IAAP was approximately 5 μmol/L. These suggested that rociletinib interacted with the drug binding pocket of ABCG2 and competed with other transporter substrates for drug efflux, thereby leading to higher accumulation of ABCG2 substrate anticancer drugs in MDR cancer cells.
3.7. Rociletinib did not alter the expression of ABCG2
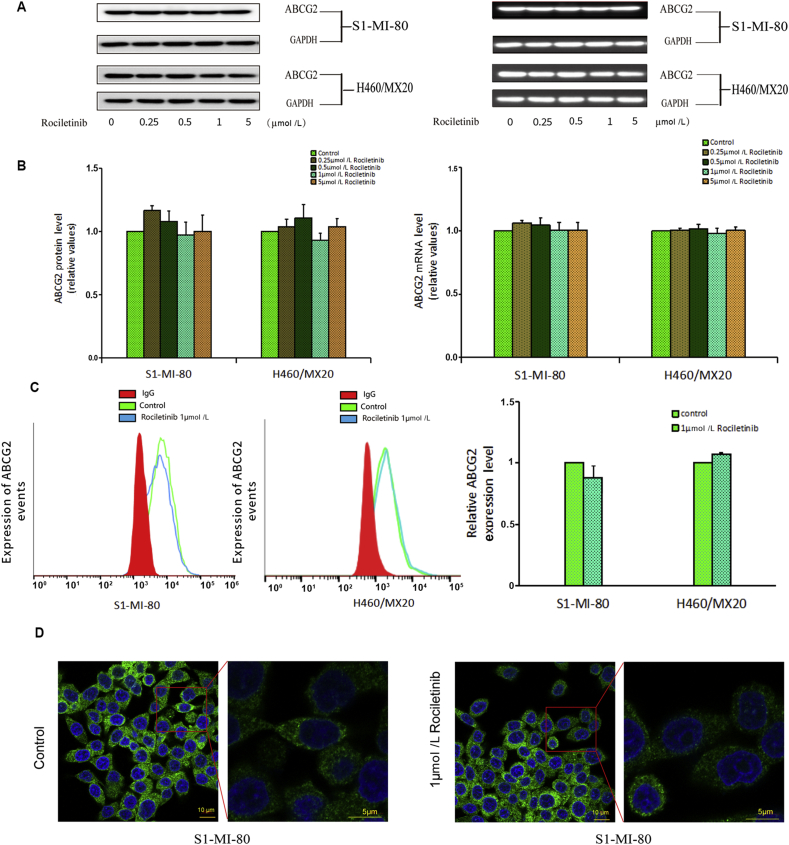
The reversal of ABCG2-mediated MDR could be achieved by inhibiting the transporter function or down-regulating the transporter expression level35. RT-PCR and Western blot were conducted to detect whether rociletinib altered the mRNA or protein levels of ABCG2. As shown in Fig. 5, no significant difference in ABCG2 expression at mRNA and protein level was observed in S1-MI-80 and H460/MX20 cells after treatment with rociletinib up to the concentration of 5 μmol/L. Of note, MDR reversal by rociletinib could be achieved in these cells at much lower concentration (as low as 0.25 μmol/L). Therefore, the circumvention of ABCG2-mediated MDR by rociletinib was likely contributed by the inhibition of ABCG2 transport function rather than down-regulation of ABCG2 expression by rociletinib.
Figure 5.
Effect of rociletinib on the expression of ABCG2 in MDR cells. (A) The protein level of ABCG2 on MDR cells after 0, 0.25, 0.5 and 1 μmol/L rociletinib stimulation for 48 h were measured by Western blot analysis. (B) The level of mRNA was measured by PCR (GAPDH as loading control), real time-PCR was further applied to confirm the unaltered mRNA levels in MDR cells. Rociletinib did not alter the mRNA and protein levels in MDR cells in concentration dependent manner. (C) The cell surface expression of ABCG2 was measured by flow cytometry before and after rociletinib stimulation on MDR cells and their parental cells. (D) The internalization of ABCG2 on MDR cells was measured by immunofluorescence confocal microscopy in the presence or absence of 1 μmol/L rociletinib. All experiments were repeated at least three times, and representative images and densitometry results were shown in each panel.
3.8. Rociletinib did not significantly alter the cell surface localization of ABCG2
To further understand whether rociletinib could influence the subcellular localization of ABCG2, the cell surface expression of ABCG2 was detected by flow cytometry. As shown in Fig. 5C, there was no significant change in the cell surface localization of ABCG2 in ABCG2-overexpressing cancer cells in the presence or absence of 1 μmol/L rociletinib.
3.9. Rociletinib did not lead to internalization of ABCG2
The above results showed that treatment of rociletinib will not alter express of ABCG2 in cancer cells. In order to further investigate the effect of rociletinib on subcellular distribution of ABCG2, immunofluorescence confocal microscopy was used to check whether treatment of rociletinib leads to internalization of ABCG2. As shown in Fig. 5D, there was no significant change in the intracellular distribution of ABCG2 in the presence or absence of rociletinib in S1-MI-80 cells. The result suggested that rociletinib did not lead to internalization of ABCG2.
3.10. Rociletinib did not block the phosphorylation of AKT and ERK at the concentration of reversal MDR
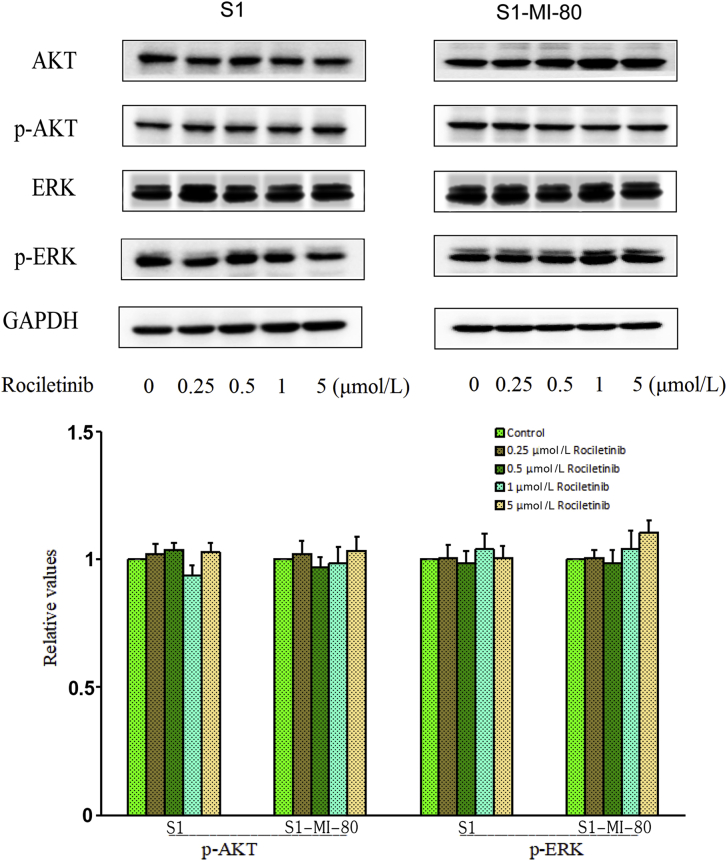
Previous studies have shown that inhibition of AKT and ERK pathway could help overcome resistance to chemotherapeutic agents in cancer cells36. Western blot analysis was performed to investigate whether rociletinib altered the expression level of AKT, ERK, and their phosphorylation at MDR reversal concentration in S1 and S1-MI-80 cells. As shown in Fig. 6, rociletinib did not alter the phosphorylation nor total levels of AKT and ERK in S1 and S1-MI-80 cells. These results indicated that the reversal of ABCG2-mediated MDR by rociletinib was not caused by the blockade of AKT and ERK signaling pathways.
Figure 6.
Effect of rociletinib on AKT, ERK, and their phosphorylations in MDR and the parental cells. S1 and S1-MI-80 cells were treated with different concentrations of rociletinib for 48 h. The protein expression level of AKT and ERK and phosphorylations were detected by Western blot (GAPDH as loading control). All these experiments were repeated at least three times.
4. Discussion
MDR is a major obstacle limiting the clinical efficacy of cancer chemotherapy, which is usually mediated by the overexpression of ABC transporters. Despite various attempts to reverse MDR, so far, no an ABC transporter inhibitor is applied in clinical practice. Novel ABC-transporter inhibitors, especially third- and fourth-generation ABCB1 inhibitors (such as zosuiqudar, elacridar, tariquidar, and resveratrol) show good efficacy in vitro and in vivo models. Phase I trial showed that tariquidar is well tolerated when combined with doxorubicin, docetaxel, or vinorelbine37. However, two phase III clinical trials of tariquidar in combination with paclitaxel plus carboplatin or vinorelbine alone for non-small cell lung cancer were discontinued. These decisions have been made due to high levels of toxicity observed in the tariquidar arms (QLT Inc.). Another ABCB1 inhibitor biricodar showed acceptable levels toxicity and good tolerability38, but was not very efficient39. Unfortunately, these efforts have failed to produce clinical trial data with the desired outcomes, due to issues with pharmacokinetic or pharmacodynamic interactions and toxicities. As ABCB1 inhibitors, several research groups continuously contribute to generating novel ABCG2 inhibitors. Among them, febuxostat will be one of the most promising candidates for clinical use40. In spite of the issues MDR modulator development poses, the problem of clinical anticancer drug resistance remains a significant issue and thus we should continue our efforts to overcome this.
In recent years, our research team has been studying the inhibition of multiple ABC transporters by various TKIs. A number of clinically approved TKIs, such as erlotinib41, osimertinib42, afatinib43, apatinib44, vandetanib45, and lapatinib14, have been reported to inhibit the efflux activity of ABC transporters at low concentrations and enhance the cytotoxicity of transporter substrate chemotherapeutic drugs to MDR cancer cells. We proposed that more specific TKIs may be identified and that their combination regimens with chemotherapeutic drugs may be further optimized to achieve MDR reversal in cancer chemotherapy in the clinical setting.
Rociletinib (CO-1686) is a small-molecule, mutant-selective and covalent EGFR inhibitor. Rociletinib exhibits potent anticancer activity in non-small cell lung cancer (NSCLC) cell lines bearing both sensitizing and resistance-causing EGFR mutations (T790M and exon 19 deletion) in vitro. Rociletinib is currently being evaluated in phase III clinical trials in EGFR-mutant NSCLC46. In this study, rociletinib was evaluated for MDR reversal in ABCG2-overexpressing cancer cell models. We are the first to show that rociletinib reversed ABCG2-mediated MDR, both in vitro and in vivo.
Results from in vitro experiments showed that rociletinib enhanced the cytotoxicity of ABCG2 substrate chemotherapeutic drugs in ABCG2-overexpressing MDR cancer cells (H460/MX20 and S1-MI-80) and ABCG2-stably transfected HEK293 cells at non-toxic concentrations. The ABCG2-inhibiting MDR reversal effect by rociletinib is likely specific because no significant effect was observed in the parental sensitive cells (S1 and H460) or HEK293/backbone vector cells. Moreover, rociletinib was found to have no effect on ABCB1-mediated MDR.
In order to further investigate the MDR reversal effect of rociletinib in vivo, the combination of rociletinib and topotecan (ABCG2 substrate anticancer drug) was also evaluated in our established ABCG2-overexpressing S1-MI-80 tumor xenograft model. While the S1-MI-80 tumor xenografts did not respond to topotecan treatment, the addition of rociletinib to topotecan treatment remarkably enhanced the resulting anticancer effect. Moreover and importantly, the enhancement of anticancer effect by rociletinib in the MDR xenograft model was achieved without additional toxicity.
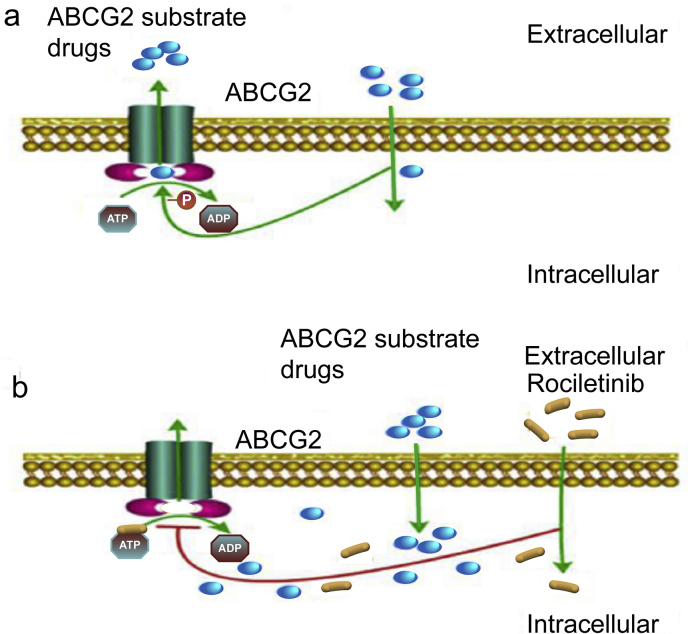
ABCG2-mediated drug transport activity, and expression and cellular localization of the transporter protein were also investigated to shed light on the mechanisms for MDR reversal by rociletinib in the model ABCG2-overexpressing cancer cells. Rociletinib was shown to increase the intracellular accumulation of DOX and Rho 123, presumably by inhibiting the drug efflux activity of ABCG2. ABC transporters are known to generate energy from their ATPase activity to drive the drug efflux function. Rociletinib was found to stimulate the vanadate-sensitive ABCG2 ATPase hydrolysis, which suggests that rociletinib may function as a competitive inhibitor of ABCG2. Further investigation revealed that rociletinib competed with [125I]-IAAP for the photoaffinity labeling of ABCG2, thus confirming the binding of rociletinib to the drug binding sites of the transporter. Furthermore, neither expression nor localization of ABCG2 was altered in MDR cancer cells even treated at high concentrations of rociletinib up to 1 μmol/L. Therefore, the increase in drug accumulation and the subsequent circumvention of MDR by rociletinib in ABCG2-overexpressing cancer cells are likely due to the inhibition of ABCG2 efflux function, but not alteration in protein expression and cellular localization of the transporter protein. Previous studies reported that blockade of PI3K/AKT and/or ERK pathways could improve the efficacy of chemotherapeutic drugs47, 48, 49. The schematic diagram summarizing the reversal of MDR by rociletinib was showed in Fig. 7.
Figure 7.
Schematic diagram showing the mechanism of rociletinib. (A) ABCG2 transporters utilize energy derived from the hydrolysis of ATP to efflux its substrates agents across the cell membrane in the absence of rociletinib. (B) Rociletinib may bind to the ATP binding site of ABCG2, thereby blocking the efflux of the transporter substrates and increasing the intracellular accumulation of the substrate drugs. Therefore, rociletinib could increase the efficacy of conventional chemotherapeutic drugs in ABCG2-overexpressing MDR cancer cells.
5. Conclusions
Our study showed that rociletinib inhibited the drug efflux function of ABCG2 and enhanced the efficacy of substrate anticancer drugs in MDR cell models without additional unwanted toxic effect both in vitro and in vivo. Therefore, our results advocate the combination use of rociletinib with conventional ABCG2 substrate chemotherapeutics to overcome ABCG2-mediated MDR.
Acknowledgments
We would like to thank Dr. Susan Bates (Columbia University/New York Presbyterian Hospital, Manhattan, NY, USA) for the ABCG2-overexpressing cell lines as a gift. This work was supported by the National Natural Science Foundation of China (grant number 81673463); and the National Science and Technology Major Project (grant number 2018ZX09711002-003-011, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2020.01.008.
Contributor Information
Zongheng Zheng, Email: 13318837281@163.com.
Liwu Fu, Email: fulw@mail.sysu.edu.cn.
Author contributions
Conceptualization: Liwu Fu and Zongheng Zheng; Methodology: Liwu Fu, Fang Wang, Fanpu Zeng, Zhen Chen and Kenneth Kin Wah To; Data curation: Fanpu Zeng, Zhen Chen, Kenneth Kin Wah To, Hong Zhang and Qian Han; Supervision: Liwu Fu and Zongheng Zheng; Writing original draft: Fanpu Zeng and Kenneth Kin Wah To; Review and editing: Liwu Fu, Fanpu Zeng and Kenneth Kin Wah To.
Conflicts of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gottesman M.M. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 2.Sodani K., Patel A., Kathawala R.J., Chen Z.S. Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer. 2012;31:58–72. doi: 10.5732/cjc.011.10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland I.B., Blight M.A. ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. J Mol Biol. 1999;293:381–399. doi: 10.1006/jmbi.1999.2993. [DOI] [PubMed] [Google Scholar]
- 4.Reese M.J., Savina P.M., Generaux G.T., Tracey H., Humphreys J.E., Kanaoka E. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos. 2013;41:353–361. doi: 10.1124/dmd.112.048918. [DOI] [PubMed] [Google Scholar]
- 5.Rochat B. Importance of influx and efflux systems and xenobiotic metabolizing enzymes in intratumoral disposition of anticancer agents. Curr Cancer Drug Targets. 2009;9:652–674. doi: 10.2174/156800909789056999. [DOI] [PubMed] [Google Scholar]
- 6.Vasiliou V., Vasiliou K., Nebert D.W. Human ATP-binding cassette (ABC) transporter family. Hum Genom. 2009;3:281–290. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhuri S., Klaassen C.D. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int J Toxicol. 2006;25:231–259. doi: 10.1080/10915810600746023. [DOI] [PubMed] [Google Scholar]
- 8.Doyle L.A., Yang W., Abruzzo L.V., Krogmann T., Gao Y., Rishi A.K. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyake K., Mickley L., Litman T., Zhan Z., Robey R., Cristensen B. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 10.Robey R.W., Honjo Y., van de Laar A., Miyake K., Regis J.T., Litman T. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2) Biochim Biophys Acta. 2001;1512:171–182. doi: 10.1016/s0005-2736(01)00308-x. [DOI] [PubMed] [Google Scholar]
- 11.Natarajan K., Xie Y., Baer M.R., Ross D.D. Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochem Pharmacol. 2012;83:1084–1103. doi: 10.1016/j.bcp.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter T. The role of tyrosine phosphorylation in cell growth and disease. Harvey Lect. 1998;94:81–119. [PubMed] [Google Scholar]
- 13.Yaish P., Gazit A., Gilon C., Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988;242:933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]
- 14.Dai C.L., Tiwari A.K., Wu C.P., Su X.D., Wang S.R., Liu D.G. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W.J., Zhang X., Cheng C., Wang F., Wang X.K., Liang Y.J. Crizotinib (PF-02341066) reverses multidrug resistance in cancer cells by inhibiting the function of P-glycoprotein. Br J Pharmacol. 2012;166:1669–1683. doi: 10.1111/j.1476-5381.2012.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang K., Chen Y., To K.K., Wang F., Li D., Chen L. Alectinib (CH5424802) antagonizes ABCB1- and ABCG2-mediated multidrug resistance in vitro, in vivo and ex vivo. Exp Mol Med. 2017;49:e303. doi: 10.1038/emm.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X., To K.K.W., Chen Z., Wang X., Zhang J., Luo M. Dacomitinib potentiates the efficacy of conventional chemotherapeutic agents via inhibiting the drug efflux function of ABCG2 in vitro and in vivo. J Exp Clin Cancer Res. 2018;37:31. doi: 10.1186/s13046-018-0690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passaro A., Guerini-Rocco E., Pochesci A., Vacirca D., Spitaleri G., Catania C.M. Targeting EGFR T790M mutation in NSCLC: from biology to evaluation and treatment. Pharmacol Res. 2017;117:406–415. doi: 10.1016/j.phrs.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Liao B.C., Lin C.C., Lee J.H., Yang J.C. Update on recent preclinical and clinical studies of T790M mutant-specific irreversible epidermal growth factor receptor tyrosine kinase inhibitors. J Biomed Sci. 2016;23:86. doi: 10.1186/s12929-016-0305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proto C., Imbimbo M., Gallucci R., Brissa A., Signorelli D., Vitali M. Epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of central nervous system metastases from non-small cell lung cancer: the present and the future. Transl Lung Cancer Res. 2016;5:563–578. doi: 10.21037/tlcr.2016.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J.Y., Wu H.Y., Xia X.K., Liang Y.J., Yan Y.Y., She Z.G. Anthracenedione derivative 1403P-3 induces apoptosis in KB and KBv200 cells via reactive oxygen species-independent mitochondrial pathway and death receptor pathway. Cancer Biol Ther. 2007;6:1413–1421. doi: 10.4161/cbt.6.9.4543. [DOI] [PubMed] [Google Scholar]
- 22.Henrich C.J., Bokesch H.R., Dean M., Bates S.E., Robey R.W., Goncharova E.I. A high-throughput cell-based assay for inhibitors of ABCG2 activity. J Biomol Screen. 2006;11:176–183. doi: 10.1177/1087057105284576. [DOI] [PubMed] [Google Scholar]
- 23.Litman T., Brangi M., Hudson E., Fetsch P., Abati A., Ross D.D. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) J Cell Sci. 2000;113:2011–2021. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- 24.Robey R.W., Honjo Y., Morisaki K., Nadjem T.A., Runge S., Risbood M. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–1978. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmichael J., DeGraff W.G., Gazdar A.F., Minna J.D., Mitchell J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:943–946. [PubMed] [Google Scholar]
- 26.Shi Z., Liang Y.J., Chen Z.S., Wang X.W., Wang X.H., Ding Y. Reversal of MDR1/P-glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivo. Cancer Biol Ther. 2006;5:39–47. doi: 10.4161/cbt.5.1.2236. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z., Guo X., To K.K.W., Chen Z., Fang X., Luo M. Olmutinib (HM61713) reversed multidrug resistance by inhibiting the activity of ATP-binding cassette subfamily G member 2 in vitro and in vivo. Acta Pharm Sin B. 2018;8:563–574. doi: 10.1016/j.apsb.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F., Mi Y.J., Chen X.G., Wu X.P., Liu Z., Chen S.P. Axitinib targeted cancer stemlike cells to enhance efficacy of chemotherapeutic drugs via inhibiting the drug transport function of ABCG2. Mol Med. 2012;18:887–898. doi: 10.2119/molmed.2011.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu L., Liang Y., Deng L., Ding Y., Chen L., Ye Y. Characterization of tetrandrine, a potent inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Chemother Pharmacol. 2004;53:349–356. doi: 10.1007/s00280-003-0742-5. [DOI] [PubMed] [Google Scholar]
- 30.Dai C.L., Liang Y.J., Wang Y.S., Tiwari A.K., Yan Y.Y., Wang F. Sensitization of ABCG2-overexpressing cells to conventional chemotherapeutic agent by sunitinib was associated with inhibiting the function of ABCG2. Cancer Lett. 2009;279:74–83. doi: 10.1016/j.canlet.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Litman T., Jensen U., Hansen A., Covitz K.M., Zhan Z., Fetsch P. Use of peptide antibodies to probe for the mitoxantrone resistance-associated protein MXR/BCRP/ABCP/ABCG2. Biochim Biophys Acta. 2002;1565:6–16. doi: 10.1016/s0005-2736(02)00492-3. [DOI] [PubMed] [Google Scholar]
- 32.Hrycyna C.A., Ramachandra M., Ambudkar S.V., Ko Y.H., Pedersen P.L., Pastan I. Mechanism of action of human P-glycoprotein ATPase activity photochemical cleavage during a catalytic transition state using orthovanadate reveals cross-talk between the two ATP sites. J Biol Chem. 1998;273:16631–16634. doi: 10.1074/jbc.273.27.16631. [DOI] [PubMed] [Google Scholar]
- 33.Shukla S., Robey R.W., Bates S.E., Ambudkar S.V. The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry. 2006;45:8940–8951. doi: 10.1021/bi060552f. [DOI] [PubMed] [Google Scholar]
- 34.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2012;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Sarkadi B., Homolya L., Szakacs G., Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 36.Dey A., Wong E., Kua N., Teo H.L., Tergaonkar V., Lane D. Hexamethylene bisacetamide (HMBA) simultaneously targets AKT and MAPK pathway and represses NF-κB activity: implications for cancer therapy. Cell Cycle. 2008;7:3759–3767. doi: 10.4161/cc.7.23.7213. [DOI] [PubMed] [Google Scholar]
- 37.Fox E., Widemann B.C., Pastakia D., Chen C.C., Yang S.X., Cole D. Pharmacokinetic and pharmacodynamic study of tariquidar (XR9576), a P-glycoprotein inhibitor, in combination with doxorubicin, vinorelbine, or docetaxel in children and adolescents with refractory solid tumors. Cancer Chemother Pharmacol. 2015;76:1273–1283. doi: 10.1007/s00280-015-2845-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peck R.A., Hewett J., Harding M.W., Wang Y.M., Chaturvedi P.R., Bhatnagar A. Phase I and pharmacokinetic study of the novel MDR1 and MRP1 inhibitor biricodar administered alone and in combination with doxorubicin. J Clin Oncol. 2001;19:3130–3141. doi: 10.1200/JCO.2001.19.12.3130. [DOI] [PubMed] [Google Scholar]
- 39.Gandhi L., Harding M.W., Neubauer M., Langer C.J., Moore M., Ross H.J. A phase II study of the safety and efficacy of the multidrug resistance inhibitor VX-710 combined with doxorubicin and vincristine in patients with recurrent small cell lung cancer. Cancer. 2007;109:924–932. doi: 10.1002/cncr.22492. [DOI] [PubMed] [Google Scholar]
- 40.Miyata H., Takada T., Toyoda Y., Matsuo H., Ichida K., Suzuki H. Identification of febuxostat as a new strong ABCG2 inhibitor: potential applications and risks in clinical situations. Front Pharmacol. 2016;7:518. doi: 10.3389/fphar.2016.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Z., Peng X.X., Kim I.W., Shukla S., Si Q.S., Robey R.W. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007;67:11012–11020. doi: 10.1158/0008-5472.CAN-07-2686. [DOI] [PubMed] [Google Scholar]
- 42.Chen Z., Chen Y., Xu M., Chen L., Zhang X., To K.K. Osimertinib (AZD9291) enhanced the efficacy of chemotherapeutic agents in ABCB1-and ABCG2-overexpressing cells in vitro, in vivo, and ex vivo. Mol Cancer Ther. 2016;15:1845–1858. doi: 10.1158/1535-7163.MCT-15-0939. [DOI] [PubMed] [Google Scholar]
- 43.Wang X.K., To K.K., Huang L.Y., Xu J.H., Yang K., Wang F. Afatinib circumvents multidrug resistance via dually inhibiting ATP binding cassette subfamily G member 2 in vitro and in vivo. Oncotarget. 2014;5:11971–11985. doi: 10.18632/oncotarget.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mi Y.J., Liang Y.J., Huang H.B., Zhao H.Y., Wu C.P., Wang F. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70:7981–7991. doi: 10.1158/0008-5472.CAN-10-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng L.S., Wang F., Li Y.H., Zhang X., Chen L.M., Liang Y.J. Vandetanib (Zactima, ZD6474) antagonizes ABCC1- and ABCG2-mediated multidrug resistance by inhibition of their transport function. PLoS One. 2009;4:e5172. doi: 10.1371/journal.pone.0005172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nukaga S., Yasuda H., Tsuchihara K., Hamamoto J., Masuzawa K., Kawada I. Amplification of EGFR wild-type alleles in non-small cell lung cancer cells confers acquired resistance to mutation-selective EGFR tyrosine kinase inhibitor. Cancer Res. 2017;77:2078–2089. doi: 10.1158/0008-5472.CAN-16-2359. [DOI] [PubMed] [Google Scholar]
- 47.Knuefermann C., Lu Y., Liu B., Jin W., Liang K., Wu L. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22:3205–3212. doi: 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]
- 48.Normanno N., De Luca A., Bianco C., Strizzi L., Mancino M., Maiello M.R. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 49.Li Q.Q., Wang W.J., Xu J.D., Cao X.X., Chen Q., Yang J.M. Involvement of CD147 in regulation of multidrug resistance to P-gp substrate drugs and in vitro invasion in breast cancer cells. Cancer Sci. 2007;98:1064–1069. doi: 10.1111/j.1349-7006.2007.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.