Abstract
Due to the disconnection of surviving neural elements after spinal cord injury (SCI), such patients had to suffer irreversible loss of motor or sensory function, and thereafter enormous economic and emotional burdens were brought to society and family. Despite many strategies being dealing with SCI, there is still no effective regenerative therapy. To date, significant progress has been made in studies of SCI repair strategies, including gene regulation of neural regeneration, cell or cell-derived exosomes and growth factors transplantation, repair of biomaterials, and neural signal stimulation. The pathophysiology of SCI is complex and multifaceted, and its mechanisms and processes are incompletely understood. Thus, combinatorial therapies have been demonstrated to be more effective, and lead to better neural circuits reconstruction and functional recovery. Combinations of biomaterials, stem cells, growth factors, drugs, and exosomes have been widely developed. However, simply achieving axon regeneration will not spontaneously lead to meaningful functional recovery. Therefore, the formation and remodeling of functional neural circuits also depend on rehabilitation exercises, such as exercise training, electrical stimulation (ES) and Brain–Computer Interfaces (BCIs). In this review, we summarize the recent progress in biological and engineering strategies for reconstructing neural circuits and promoting functional recovery after SCI, and emphasize current challenges and future directions.
Subject terms: Spinal cord injury, Regeneration, Spinal cord diseases
Facts
A variety of therapeutic strategies, including gene regulation of neural regeneration, cell or cell-derived exosomes and growth factors transplantation, repair of biomaterials, and neural signal stimulation, lead to axonal regeneration and neural circuit reconstruction related to functional recovery.
The formation and remodeling of functional neural circuits also depend on rehabilitation exercises, such as exercise training, ES and BCIs.
Combinatorial therapies have been demonstrated to be more effective, and lead to better neural circuits reconstruction and functional recovery.
Open questions
What are the mechanisms of scar formation after SCI?
What are the mechanisms that limit the effective formation and regeneration of new neural circuits?
Is the formation and remodeling of functional neural circuits also depend on rehabilitation exercises, such as exercise training, ES and BCIs?
Introduction
Spinal cord injury (SCI) is a severely disabling disease that leads to loss of sensation, motor, and autonomic function1. Patients with SCI have a higher risk of complications, such as bladder dysfunction, sexual dysfunction, gastrointestinal and respiratory problems, and urinary tract infection2, resulting in death in severe cases. The leading cause of SCI in most regions is falls and road injuries3, and the other reasons include acts of violence, environmental heat and cold exposure, and sports/recreation activities4. About 13% of patients with SCI worldwide suffer from limb dysfunction every year5. SCI is a devastating neurodegenerative disorder, which leads to the physical and psychological problems of patients and brings an enormous economic burden on patients, their families and the social medical system6.
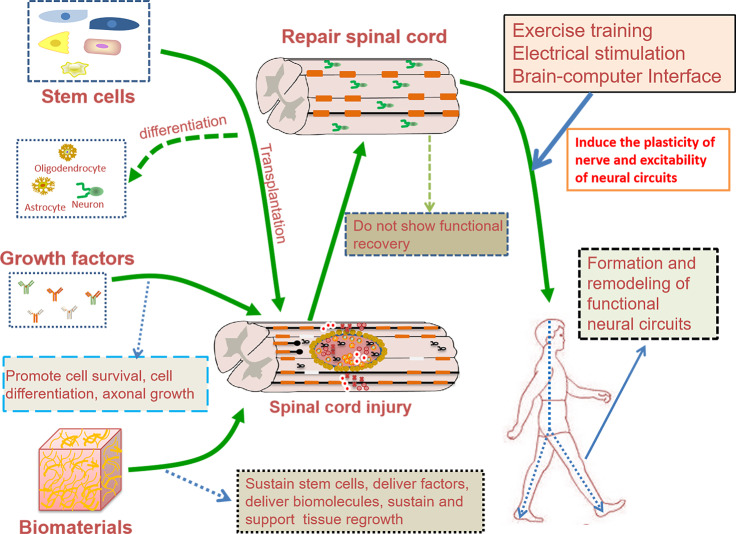
The permanent disability after SCI is related to the failure of axon regeneration and neural circuit reconstruction1. To date, there are no effective treatments that can completely regenerate axons after SCI. Over the past decade, significant progress has been made not only in traditional research fields, such as inflammation, scar formation, cell transplantation, axon regeneration, and biomaterial repair, but also in determining the mechanisms of spinal cord automation, spontaneous circuit reorganization and functional recovery after SCI7,8. Because of the complexity issues of pathological processes that occur following SCI9, combinatorial strategies that solve the problems caused by different aspects are expected to be more effective, and lead to better functional recovery10. Combinations of biomaterials, stem cells, growth factors, drugs, and exosomes have been widely developed (Fig. 1). However, functional recovery depends on strengthening neuroplasticity to promote the growth of injured and spared axons, to increase the strength of the remaining connections and to promote the formation of new spontaneous circuits10. Therefore, the formation and remodeling of functional neural circuits also depend on rehabilitation exercises, such as exercise training, ES and BCIs (Fig. 1). In this review, we summarize the recent progress in biological and engineering strategies for reconstructing neural circuits and promoting functional recovery after SCI, and emphasize current challenges and future directions.
Fig. 1. The figure of effective neural circuits reconstruction after SCI.
Combinatorial therapies have been demonstrated to be more effective, and lead to better functional recovery. However, simply achieving axon regeneration will not spontaneously lead to meaningful functional recovery. Therefore, the formation and remodeling of functional neural circuits also depend on rehabilitation exercises, such as exercise training, ES and BCIs.
Pathophysiology
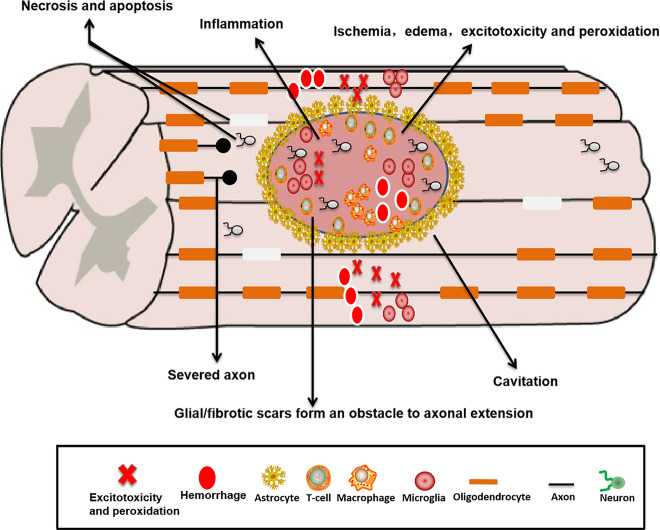
SCI is mainly caused by the mechanism of primary and secondary injury (Fig. 2). The primary injury is irreversible, which is related to the initial traumatic damage such as compression, stretch, laceration and hemorrhage, thus triggering a complex cascade of acute and chronic degenerative events that further disrupt neuronal function11. During SCI, the primary injury leads to the production of free radicals12 and a chronic state of causing ischemia and hypoxia13, resulting in glutamate excitotoxicity, lipid peroxidation, calcium influx, edema, and cellular damage14. Finally, inflammation and immune response affect the integrity of adjacent tissues14. Secondary injury leads to demyelination of the axons, glial cell proliferation, the loss of damaged cells and the disconnection of living neurons, culminating in formation of a microenvironment that is not conducive to nerve regeneration15. In addition, in the chronic stage, glial/fibrotic scars that inhibit cellular infiltration16 can form an obstacle to axonal regeneration and extension17. Astrocytic scars are widely regarded as the main cause that transected axons fail to regrow after SCI18, but when pericyte-derived fibrotic scars are reduced at the lesion site, axonal regeneration and functional recovery will be promoted19. Extracellular matrix (ECM) proteins deposited within and around glial/fibrous scars limit axon growth, such as chondroitin sulfate proteoglycans (CSPGs), fibronectin, laminin, and collagen. Among them, CSPGs play a major role in inhibiting axon regeneration20, including aggrecan, neurogen and neural/glial antigen21. Furthermore, myelin-associated inhibitors that strongly inhibit axon and myelin regeneration, including myelin-associated glycoprotein (MAG), Nogo, netrin, ephrins, and oligodendrocyte-myelin glycoprotein (OMgp), can lead to growth cone collapse after SCI22. Poor axon growth ability and inhibitory factors that hinder axon regeneration in the scar23 lead to the failure of regeneration and repair of spinal cord and reconstruction of neural circuits. Therefore, the therapeutic focus is on how to promote axon regeneration, myelinate axons and reconstruct neural circuits.
Fig. 2. Pathophysiological mechanism of SCI.
During SCI, the primary injury leads to the production of free radicals and a chronic state of causing ischemia and hypoxia, resulting in glutamate excitotoxicity, lipid peroxidation, calcium influx, edema and cellular damage. Finally, inflammation and immune response affect the integrity of adjacent tissues. Secondary injury leads to demyelination of the axons, glial cell proliferation, the loss of damaged cells and the disconnection of living neurons, culminating in formation of a microenvironment that is not conducive to nerve regeneration.
Stem cells transplantation for SCI
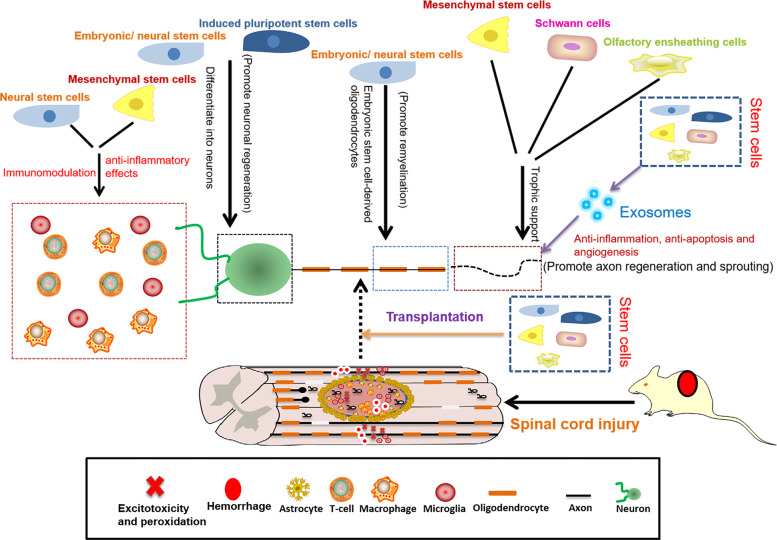
Stem cells play a neuroprotective role by differentiating into specialized cell types to replace damaged cells and secreting factors to promote the survival and activity of these cells24,25 (Fig. 3). In addition, the mechanisms of stem cells that promote repair and function improvement include immunomodulation, anti-inflammatory effect, inhibition of scar formation, axon and myelin regeneration, and prevention of vascular loss or promotion of angiogenesis24–26 (Fig. 3).
Fig. 3. Scheme summarizing the role of each type of stem cells in repair of SCI.
Stem cells play a neuroprotective role by differentiating into specialized cell types to replace damaged cells and secreting factors to promote the survival and activity of these cells. In addition, the mechanisms of stem cells that promote repair and function improvement include immunomodulation, anti-inflammatory effect, inhibition of scar formation, axon and myelin regeneration, and prevention of vascular loss or promotion of angiogenesis.
Neural stem/progenitor cells
Neural stem/progenitor cells (NSCs/NPCs) can differentiate into neurons for the repair of nerve tissue damage caused by SCI, and into oligodendrocytes to promote axon regeneration and myelination25,27. NSCs/NPCs also can regulate astrocytes to protect tissue integrity28 and improve the microenvironment of the injury site by secreting factors15,24. For instance, a study showed that transplantation of the spinal cord-derived NSCs into the injury site produced massive amounts of excitatory neurons and axons that extend a long distance, leading to the robust regeneration of corticospinal cord29. Another study showed that nine months after transplantation of human spinal cord-derived NPCs into the lesion site of the spinal cord, the grafts successfully differentiated into neurons and glial cells that reconstruct the neural tissue and local microenvironment, and the regeneration axons of the host and the grafts successfully were interconnected30. However, due to the tendency that NSCs differentiate into astrocytes31, some measures, including ES32, gene modification33, and the application of nanomaterials34, should be taken. For instance, Wnt4-modified NSCs could activate MAPK/JNK and β-catenin pathway and inhibit the activation of Notch signaling to significantly promote the differentiation of NSCs into neurons, thus effectively repairing the damaged spinal cord and promoting the recovery of motor function33.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) can be obtained from different types of tissues, including adipose tissue, placenta, umbilical cord, amniotic membrane and bone marrow25,31, which can be expanded efficiently in vitro25. Moreover, MSCs have the advantages of high biosafety, low immunogenicity, wide biological effects and immunomodulatory characteristics35. MSCs can differentiate into neurons and glial cells after transplantation into the injury site35 to reduce the area of glial scar and the size of the cavity, leading to the regeneration of nerve tissue, axon and myelin sheath36. The use of MSCs for SCI has a wide range of biological mechanisms, including regulating immune response37, promoting angiogenesis38, anti-apoptotic and anti-inflammatory effects39,40. However, the most important mechanism is the paracrine effect of MSCs, which can release a variety of nutritional factors that protect the injury spinal cord tissue and promote nerve regeneration24. In a separate study, the combination of MSCs and three-dimensional (3D) gelatin sponge could form a perineurium-like sheath that enwrapped the host myelin sheaths and axons, and form a physical and chemical barrier to protect the nerve fibers from oxidative damage by secreting nutrients, extracellular matrix and antioxidant superoxide dismutase-3 (SOD-3)41. Some strategies such as gene modification, hypoxia preconditioning and improvement of the local microenvironment of the host can promote the survival and differentiation of transplanted MSCs42,43. For instance, a recent study found that retinoic acid (RA), sonic hedgehog (SHH), and neurotrophic factors could efficiently induce human adipose-derived stem cells (hADSCs) into electrophysiologically active motoneuron-like cells (hADSC-MNs) in vitro44. After hADSC-MNs were transplanted into the injury site, hADSC-MNs could survive well and improve the microenvironment by immunosuppression, thus promoting the reconstruction of the spinal circuit.
Embryonic stem cells
Embryonic stem cells (ESCs) can differentiate into three types of germ cells due to their great developmental and unlimited self-renewal ability45. Many transcription factors, including Nanog, Sox2 and OCT3/4, have been identified in human ESCs45, which are used to induce fibroblasts and other somatic cells into pluripotent stem cells (iPSCs)46. ESCs can differentiate into neurons and glial cells to repair damaged cells, and secrete active factors to regulate the immune response and provide nutrition support, ultimately leading to the regeneration of nerve tissue and axons, remyelination of axons, and the reconstruction of injury cortical circuits35,47–49. For instance, a study conducted by Manley et al.50 showed that oligodendrocyte progenitor cells (OPCs) derived from human ESCs could reduce the cavity of the injury site, increase the sparse myelinated axons, and significantly improve the motor function. Another recent study reported that human ESCs derived-NSCs significantly promoted recovery of motor function by reducing the size of the cavity and the area of glial scar51.
Induced pluripotent stem cells
Because previous stem cell sources such as ESCs and MSCs are still plagued by the immune, ethical and clinical concern, iPSCs are an alternative with significant advantages52. iPSCs were initially obtained by viral transduction of transcription factors in differentiated somatic cells, such as tumour suppressor Krüppel-like factor 4 [KLF4], OCT3/4, Sox2, and proto-oncogene c-MYC53. IPSCs also can be obtained from somatic cells after genetic reprogramming by the combination of other transcription factors54 and reprogramming molecules55,56 that promote the expression of core transcription factors such as Oct4, Sox2 and Nanog57. iPSCs can be differentiated into neurons, astrocytes and oligodendrocytes to replace damaged cells58, regulate the microenvironment of the spinal cord by secreting growth factors59, and promote the regeneration of axons and myelin sheath, thus reconstructing the neural circuit that promotes functional recovery60. For instance, a recent study found that three months after transplantation of iPSCs-derived NSCs into the injury spinal cord, iPSCs-derived NSCs survived and successfully differentiated into neurons, which extended a large number of axons from the injury site and formed synapses with host neurons, leading to the reconstruction of the neural network61. Another study conducted by Fan and his colleagues showed that iPSCs-derived NSCs loaded in 3D gelatin methacrylate (GelMA) hydrogel reduced inflammation by reducing activated macrophages/microglia, inhibited the formation of glial scar, and reduced the area of the cavity, thus promoting axonal regeneration after SCI62.
Olfactory ensheathing cells
Olfactory ensheathing cells (OECs) promote axon regeneration and remyelination by inhibiting inflammation of the spinal cord, reducing the glial scar and cavity63, and promoting angiogenesis, contributing to the reconstruction of the injury neural network64. Moreover, OECs have shown higher levels of growth factors secretion, which can promote the survival and activity of neurons and their axons extension, thus improving tissue repair and functional recovery65,66. For example, a recent study found that intravenous OECs transplantation into the injury site significantly decreased local inflammation, thus promoting axon and myelin sheath regeneration related to function recovery67. Combinatorial therapies can be used to improve the regeneration efficiency of OECs in the SCI model. For instance, another recent study reported that combining adipose tissue-derived stromal cells (ASCs) with strong paracrine effect with OECs that can induce axonal growth could significantly improve the motor function by reducing the inflammatory response and astrocyte proliferation68.
Schwann cells
Schwann cells (SCs) are the main glial cells in the peripheral nervous system (PNS), which are widely used in the repair of SCI because of their role in neuroprotection, promoting axon regeneration and myelination25,69. SCs play a neuroprotective role by inhibiting the activity of pro-inflammatory microglia and macrophages to regulate the local microenvironment70. Besides, SCs can secrete a variety of active factors to reduce nerve cell death and the cavity formation25. However, combinatorial strategies are often used to improve the effect of SCs transplantation. For example, a study showed that ECM-derived matrices such as Matrigel were used to carry SCs, which improved the survival of SCs in the transplanted site of SCI, and significantly promoted the growth of axons to improve the motor function71.
Exosomes derived from stem cells
The mechanisms of stem cell-based therapy are mediated by exosomes released by stem cells, and microRNA in these exosomes is the main factor to play the therapeutic effect72. Exosomes can transmit biological signals between cells and participate in a variety of physiological and pathological processes73. Exosomes derived from stem cells are expected to be a new strategy for the treatment of SCI. Exosomes have the functions of neuroprotection, angiogenesis, immune regulation, anti-inflammatory, anti-apoptosis, and axon regeneration, which can promote the repair and regeneration of nerve tissue74,75. For example, a study showed that the human umbilical cord mesenchymal stem cells (hucMSC) derived exosomes could effectively induce macrophages derived from bone marrow to polarize from M1 phenotype to M2 phenotype, and reduce inflammatory factors such as IL-6, IFN-γ and TNF-α, thus promoting functional recovery after SCI76. Another study found that NSCs-derived exosomes reduced the extent of SCI, repaired the injury site, and promoted the functional recovery by reducing the neuroinflammatory response, microglia activation and neuronal apoptosis77. Combined with the advantages of exosomes, exosomes for molecular targeted drug delivery are expected to become the next generation of intelligent engineering biomaterials for precision medicine78. For instance, as shown in the study by Kim et al., iron oxide nanoparticle (IONP)-incorporated exosome-mimetic nanovesicles from IONP-treated human mesenchymal stem cells (hMSCs) could target the injury spinal cord under magnetic guidance79. Compared with the exosomes from hMSCs alone, it significantly reduced inflammation, inhibited apoptosis and promoted vascular regeneration, leading to significant improvement in functional recovery after SCI. In another study, MSCs-derived exosomes loaded with phosphatase and tensin homolog siRNA significantly reduced the proliferation of astrocytes and microglia, promoted the formation of new blood vessels and axons regeneration, thus promoting the functional recovery after SCI80.
Biomaterials
Biomaterials can improve the local microenvironment and promote the survival of nerve cells by reducing the inflammatory response, improving cell retention, inhibiting cell necrosis or apoptosis, and reducing the formation of the glial scar, thus reconnecting the injury spinal cord tissue, reconstructing the nerve conduction circuit, and ultimately promoting the motor recovery after SCI81. As a method of regenerative medicine, biomaterials containing cells, drugs and bioactive molecules can repair or replace the function and anatomical structure of the damaged tissue82. However, the main neural mechanism of functional biomaterials that promote motor function recovery depends on the formation of neuronal bridging, rather than the long-distance regeneration of the descending motor axon and ascending sensory axon83. Although various forms of biomaterials can be used, hydrogels, nanomaterials and 3D printing materials represent an attractive form for spinal cord repair.
The hydrogel has the characteristics of the porous structure, high water content and sol-gel transformation under certain conditions, which is widely used in tissue engineering, cell therapy and drug delivery84. Therefore, the hydrogel is currently an ideal carrier because it can mimic ECM to provide suitable microenvironment and support for stem cells85. Moreover, the hydrogel can provide the proper 3D networks for axonal growth, and promote axonal regeneration, synaptic reconnection and reconstruction of neural circuits by carrying various drugs and growth factors8. For instance, a recent report by Caron et al. described the use of 3D biomimetic agarose/carbomer hydrogel and hMSCs that optimize the density, vitality and paracrine transport could significantly increase M2 macrophage population to improve the microenvironment of the injury site86. Papa et al.87 further found that human chemokine (C–C motif) ligand 2 (hCCL2) secreted by hMSCs could promote macrophage recruitment and transformation to M2 phenotype to play a neuroprotective role, thus preventing motor neuron degeneration to improve the motor function after SCI. The responsive hydrogel can respond to stimuli such as ions, pH, temperature, enzyme and electromagnetic fields, resulting in changes in its physical and chemical properties to better mimic the dynamic natural 3D cell microenvironment88. For example, a study found that composite hydrogels consisting of alginate, chitosan and genipin were high sensitivity to Ca2+ concentration, which could alleviate the secondary injury caused by the overload of Ca2+ and control the astrocyte behavior, leading to the recovery of motor function89. In addition, the spinal cord can conduct and transmit bioelectric signal to play a vital role in promoting axon growth, nerve regeneration90 and nerve circuits remodeling91. For instance, a study showed that conductive hydrogels could inhibit astrocyte development and promote differentiation of NSCs into neurons, thus improving the recovery of motor function after SCI92.
3D printing technology allows the manufacture of precise anatomical and personalized scaffolds to stimulate and guide axonal regeneration and elongation. The injury host axons regenerate into 3D printing scaffolds, while implanted NPCs extend axons out of the scaffolds and form synaptic connections with each other to restore synaptic transmission, thus significantly improving the host motor function93. To improve biocompatibility or for a special purpose, 3D printing scaffolds can be modified, biochemical functionalized and mechanically tuned to promote stem cell proliferation, adhesion, and differentiation94. In a separate study, 3D printing poly-lactic-co-glycolic acid (PLGA) scaffolds provided a favorable 3D environment for the regeneration of nerve cells and neurogliocytes95. Another recent study conducted by Sun et al. showed that 3D printing collagen/chitosan scaffolds could promote neural regeneration, reduce scar formation, and partially reconstruct the permissive microenvironment of axon regeneration, ultimately promoting functional recovery96. Joung and his colleagues put NPCs and OPCs from iPSCs in the precise position of 3D printing scaffolds to construct the bionic spinal cord, which is helpful to reconstruct the functional axon connection of the injury site97.
Due to their unique structures and properties, nanomaterials can be used in the diagnosis and treatment of neurological diseases, and be designed as drug delivery carriers that cross the blood–brain barrier (BBB) and deliver specific molecules to target cells, which have better safety and efficacy than traditional simple drug treatment98. Besides, nanomaterials can promote the extravasation and system clearance of the BBB and maintain the high activity of neural cells after entering cells, and surface modification of nanomaterials can also enhance biocompatibility and selective targeting via cell surface receptor interaction99. For instance, a study conducted by Papa et al. showed that poly-ε-caprolactone (PCL) nanoparticles (NPs) loaded with minocycline could selectively target and regulate the activated pro-inflammatory microglia to reduce the secondary injury and improve the microenvironment of the injury site, thus promoting axon regeneration100. Another study conducted by the same research group showed that a nano-structured gel loaded with rolipram targeting A1 astrocytes could reduce nitric oxide synthase (iNOS) and Lipocalin 2 (Lcn2) to reduce inflammation caused by activated astrocytes, thus protecting nerve cells and promoting the recovery of motor function after SCI101. Our group constructed targeting scar tissue and esterase-responsive nano-micelles. After being activated, the micelles were endocytosed and degraded by microglia, resulting in a decrease in accumulation of reactive oxygen species (ROS) and the transformation of M1 to M2 microglia population, thus protecting nerve cells and promoting the recovery of motor function102.
Growth factors
Growth factors are a family of proteins regulating biological development and neural function, which can regulate the survival of neurons, promote the release of neurotransmitters, promote the recovery of synaptic function, and induce the growth and remodeling of axons103. However, various types of growth factors have different functions in repairing SCI. For example, neurotrophin-3 (NT-3), neurotrophin-4 (NT-4) and neurotrophin-5 (NT-5) have the function of protecting damaged neurons and promoting the growth and differentiation of neurons24. Nerve growth factor (NGF) can support the survival of neurons and promote the growth, differentiation and synapse formation of neurons, thus promoting nerve regeneration, neural network remodeling and motor function recovery104. Brain-derived neurotrophic factor (BDNF) has neuroprotective effects on 5-serotonin, dopaminergic, cholinergic and GABA neurons by promoting the growth of neurons, the regeneration and sprouting of axons, and the formation of remyelination of axons103. Glial cell-derived neurotrophic factor (GDNF) can reduce inflammation and lesion volume, improve pain, and promote axon regeneration, which also has a positive regulatory effect on astrocyte proliferation105. Fibroblast growth factors (FGFs), including FGF1, FGF2, FGF4 and FGF10, can alleviate the secondary injury such as inflammation and astrocyte activation, stimulate axon regeneration and angiogenesis, and protect damaged neurons106. Insulin-like growth factor 1 (IGF-1) plays an antioxidant and pro-survival role to promote the differentiation and survival of oligodendrocytes and the growth and survival of axons, and increase the formation of regenerated axonal myelin sheath107. Ciliary neurotrophic factor (CNTF) can promote the survival of damaged neurons and oligodendrocytes and the long-distance regeneration of axons108. Given the complexity of the pathological process following SCI, it is necessary to use multiple growth factors according to different pathological mechanisms and the effects of growth factors. For example, a study showed that osteopontin, IGF-1 and CNTF could reactivate the growth ability of spinal neurons, FGF2 and epidermal growth factor (EGF) could induce growth support, and GDNF could chemically attract axon growth. A combination of above growth factors whose cooperative effects were required to stimulate the regeneration of robust intrinsic axons, thus enhancing recovery after SCI109. Due to the short half-life of growth factors, they are often delivered in combination with biomaterials. A study found that chitosan biomaterials were used for sustained release of NT-3 into the injured spinal cords, where this combinatorial therapy could promote the activation of endogenous NSCs to differentiate into neurons, thus reconnecting the damaged axons to form functional neural networks, leading to the recovery of function110. We previously reported that loaded FGF4 heparin-laponite hydrogels could significantly reduce inflammation, inhibit astrocyte proliferation and scar formation, and promote axonal regeneration and remyelination of axons by increasing stable microtubules and activating mitochondria release111.
Rehabilitation
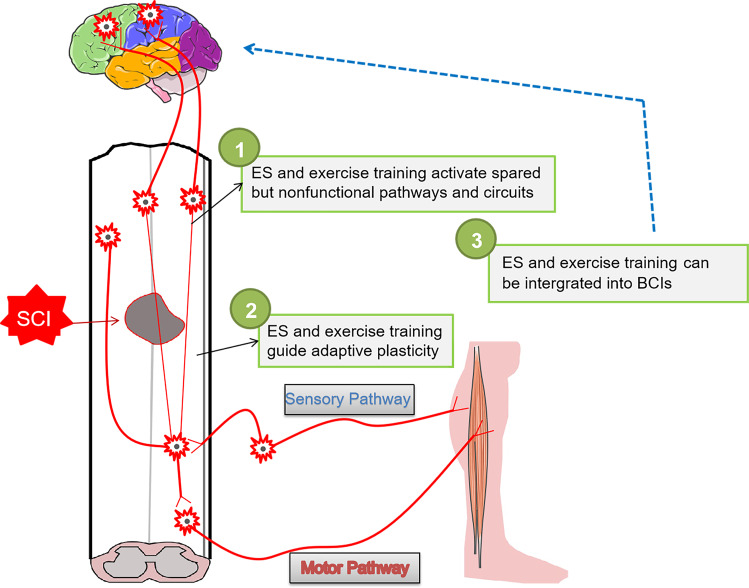
After SCI, the ascending sensation and descending input of the spinal cord is interrupted, and the axon of corticospinal cord retracts so that it cannot regenerate, resulting in permanent loss of function112. However, simply achieving axon regeneration will not spontaneously lead to meaningful functional recovery. Therefore, it is widely recognized that the formation and remodeling of functional neural circuits also depends on strengthening neuroplasticity113, such as exercise training, ES and BCIs (Fig. 4). Exercise training can improve the function of spinal motor neurons and the remodeling function of the cerebral cortex, thus increasing neuronal activity to strengthen the reorganization of neural pathways114. ES can regulate the excitability of the spinal circuit115, restore muscle strength and quality, and induce the plasticity of nerve, thus promoting the recovery of function116. BCIs are an emerging technology that translates brain activity into motor actions117, which enable direct communication between the brain and electronic devices, thus recording and modulating neural activity to restore lost function118.
Fig. 4. Rehabilitation strategies for the long-term recovery of effective neural circuits.
Exercise training, ES and BCIs can regulate the excitability of spinal circuit through the central mechanism, leading to muscle contraction, which can not only restore muscle strength and quality, but also induce the plasticity of nerve and excitability of neural circuit, thus promoting the recovery of motor function.
Exercise training
Exercise training can reduce apoptosis, increase growth factor levels such as IGF, BDNF, and NGF, decrease inflammation, and promote neurogenesis to protect neurovascular units and strengthen spared functions of the spinal cord119,120. Moreover, exercise training can reduce the size of syringomyelia and the area of glial scar to promote the regeneration of axons, the remodeling of synapses, and the remyelination of axons121. In addition, by enhancing or replacing the residual spinal cord and muscle function, exercise training can remodel the neural circuit to promote the recovery of motor function122. Through remolding the function of skeletal muscle and cerebral cortex, exercise training can regulate the physiological metabolism function of spinal motor neurons, and improve the function from end effectors such as skeletal muscle to the cerebral cortex in different degrees123.
Electrical stimulation
ES can accelerate axon growth and myelin sheath formation124, stimulate neurons to discharge bioelectric signal to strengthen muscle contraction, thus reconnecting the neural network of spinal cord115. Therefore, the baseline excitability of neural circuits is regulated by ES, such as epidural electrical stimulation (EES) and transcutaneous spinal cord stimulation (tSCS), leading to action potential within and between neural circuits by regulating excitability further or closer to the motor threshold125. ES also recruits local neurosuppressive neurotransmitters across synaptic sites by stimulating the amount of neurostimulation of afferent nerve fibers, thus enhancing the release capacity of neurotransmitters126. A clinical study has suggested that EES may promote the recovery of the sensorimotor network of the spinal cord after SCI by producing robust and coordinated spinal motor output, thus producing independent standing and gait127. In another clinical study, EES restored the voluntary control of walking in patients with severe or complete paralysis caused by SCI four years ago despite full rehabilitation128. TSCS is a new, non-invasive and promising technology, which can stimulate the spinal cord from the surface of the skin and reach the spinal cord network by using high current and unique waveform ES. For instance, Inanici and his colleagues used tSCS to significantly restore upper limb movement and sensation in an elderly patient with incomplete SCI, indicating that tSCS can promote neuroplasticity and long-term recovery129.
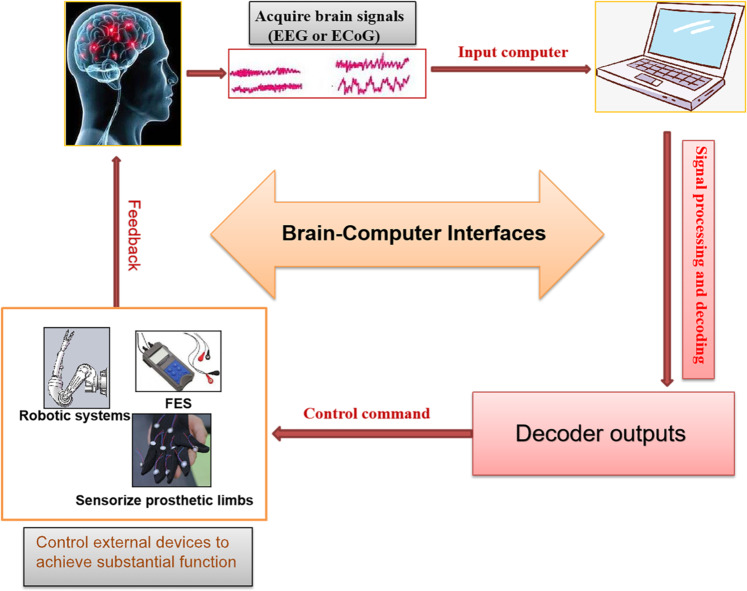
Brain–computer interfaces
In recent years BCIs technology has become a research hotspot. BCIs, as the foundation of a new generation of the neuroprosthetic device, can control external devices to achieve substantial function by recording the electrical signals from the brain such as electroencephalography (EEG) or electrocorticography (ECoG)130 (Fig. 5). The prosthesis consists of implanted electrodes that record the expected motion of the paralyzed part of the body, computer algorithm that decode the expected motion, and auxiliary devices controlled by the expected motion signal131 (Fig. 5). The computer decodes the electronic signals from the motor cortex to drive robotic systems or stimulate muscles to recover the paralyzed person’s movement132. Moreover, the combination of BCIs and sensory cortex can enhance the flexibility and fine control of limbs133.
Fig. 5. General diagram of BCIs for SCI.
BCIs can control external devices to achieve substantial function by recording the electrical signals from the brain such as EEG or ECoG. The computer decodes the electronic signals from the motor cortex to drive robotic systems or stimulate muscles to recover the paralyzed person’s movement. Moreover, the combination of BCIs and sensory cortex can enhance the flexibility and fine control of limbs.
Combining BCIs with functional ES or robotic therapies provide hope for patients without residual motor function, which can retrain the connection between central and peripheral to reconstruct nerve circuits. BCIs combined with robotic systems such as exoskeletons and orthotics have become a new therapeutic modality to help or rehabilitate patients with limb disability. For example, Hochberg et al.134 reported that two patients with long-term quadriplegia used a BCI-based robotic arm to control and perform 3D stretching and grasping movements. The results showed that they could control the arm and hand over a wide space, and even one of them could use a robotic arm to drink coffee in the bottle. Benabid and his colleagues used an exoskeleton system controlled by an epidural wireless BCI to enable patients to use virtual avatars at home, control walking simulation procedures cortically, perform different stretching and touch tasks135. BCIs combined with functional electrical stimulation (BCIs-FES) can be used to activate the paralyzed muscle through ES136, transmit the electrical pulse directly to the peripheral nerve and muscle tissue to cause contraction and assist specific body movements115. For instance, a study conducted by Capogrosso et al. showed that the BCI-FES restored non-human primates weight-bearing movement of paralyzed legs on the treadmill and the ground six days after injury without prior training137. Another clinical study by Ajiboye et al. showed that the BCI-FES could successfully control coordinated movements of single-joint and multi-joint arms, achieve point-to-point target capture, and recover both stretching and grasping movements of patients with tetraplegia due to SCI138. Compared with motor function recovery, sensory function recovery is a higher requirement for SCI patients, and also a key step of BCI technology in clinical application. Microstimulation of the somatosensory cortex provides the potential to create sensory prosthetic limbs to restore sensory function139. For instance, a study conducted by O’Doherty et al. found that BCIs based-on sensory function recovery controlled the exploratory arrival movement of the actuator, and sent out the artificial tactile feedback signal via intracortical microstimulation (ICMS)140. ICMS of the primary somatosensory cortex can produce somatosensory sensation, which may become a method of sensory prosthesis in the future141.
Combinatorial therapies
So far, due to the complexity of the pathological process of SCI, combinatorial therapies may be more effective, thus maximizing the effect of treatment of SCI. Some of these combinatorial strategies have been discussed in the previous paragraphs, and we will further develop them (Table 1). For example, MSCs improved the microenvironment of NSCs and promoted the survival of transplanted NSCs. Therefore, MSCs combined with NSCs could significantly improve the motor function after SCI142. Overexpression of IGF-1 in MSCs increased immune regulation, cell survival and myelination. Transplantation of IGF-1 overexpressed MSCs could significantly promote nerve regeneration after SCI107. IGF-1 inhibitd the inflammatory response and apoptosis via miR-219a-2-3p/YY1 mechanism and enhanced the neuroprotective effect of NSCs-derived exosomes, and their combinations significantly promoted the recovery of motor function143. The combination of gelatin sponge scaffold with NT-3 could create a favorable microenvironment, reduce inflammation and cavity formation, and induce the migration of endogenous nerve cells into the injury site to promote the regeneration of nerve and axon144. MnO2 NPs effectively enhanced the activity of MSCs by alleviating local oxidation environment, and hydrogel could promote cell adhesion growth and bridge spinal cord tissue. Therefore, combinations of MSCs and MnO2 NPs-dotted hydrogel induced neural differentiation of MSCs, resulting in the efficient regeneration of spinal cord tissue to significantly promote the recovery of motor function145. Co-culture of TrkC-gene-modified NSCs and NT-3-gene-modified SCs on the gelatin sponge scaffold to construct the neural network tissue. After transplanted into the SCI model in canine, it could significantly promote the regeneration of nerve fibers and integrate with the host neural circuit synapses, thus becoming a neuron relay to transmit the excitatory electrical signal through the lesion site146.
Table 1.
Combinatorial strategies for SCI.
| Combinatorial strategies | Animal model | SCI type | Follow up time | Comments about combination group | Reference |
|---|---|---|---|---|---|
| MSCs and NSCs | Rat | T10, contusion injury | 8 weeks | MSCs improved the microenvironment of NSCs and promoted the survival of transplanted NSCs. MSCs combined with NSCs could significantly improve the motor function. | 142 |
| IGF-1 and MSCs | Mouse | T10, contusion injury | 4 weeks | Overexpression of IGF-1 in MSCs increased immune regulation, cell survival and myelination. Transplantation of IGF-1 overexpressed MSCs could significantly promote nerve regeneration after SCI. | 107 |
| NSCs-derived exosomes and IGF-1 | Rat | T10, contusion injury | 4 weeks | IGF-1 inhibited the inflammatory response and apoptosis via miR-219a-2-3p/YY1 mechanism and enhanced the neuroprotective effect of NSCs-derived exosomes, and their combinations significantly promoted the recovery of motor function. | 143 |
| Gelatin sponge scaffold and NT-3 | Rat and canine | T10, transection and hemisection | 4 weeks | The combination of gelatin sponge scaffold with NT-3 could create a favorable microenvironment, reduce inflammation and cavity formation, and induce the migration of endogenous nerve cells into the injury site to promote the regeneration of nerve and axon. | 144 |
| MSCs and MnO2 NPs-dotted hydrogel | Rat | T10, transection | 4 weeks | MnO2 NPs effectively enhanced the activity of MSCs by alleviating local oxidation environment, and hydrogel could promote cell adhesion growth and bridge spinal cord tissue. Therefore, combinations of MSCs and MnO2 NPs-dotted hydrogel induced neural differentiation of MSCs, resulting in the efficient regeneration of spinal cord tissue to significantly promote the recovery of motor function. | 145 |
| TrkC-gene modified NSCs, NT-3-gene modified SCs and gelatin sponge scaffold | Canine | T10, transection | 24 weeks | Co-culture of TrkC-gene-modified NSCs and NT-3-gene-modified SCs on the gelatin sponge scaffold to construct the neural network tissue. it could significantly promote the regeneration of nerve fibers and integrate with the host neural circuit synapses, thus becoming a neuron relay to transmit the excitatory electrical signal through the lesion site. | 146 |
| MSCs and exercise training | Mouse | T10, aneurysm clip | 8 weeks | Combinatorial treatment of MSCs and exercise training could promote the protection of nerve tissue and enhance the recovery of motor function after SCI. | 147 |
| EES and high-intensity treadmill training | Human | Not mentioned | 85 weeks and 15 weeks | EES combined with high-intensity treadmill training enabled patients to walk on the ground, stand independently, and maintain trunk stability compared with individual exercise training. | 148 |
| BCIs, visuo-tactile feedback and assisted exercise training | Human | Not mentioned | 112 weeks | Combinations of visual-tactile feedback, noninvasive BCIs and assisted exercise training significantly restored the patients’ motor function and part of sensory function, even part of intestinal, urinary and sexual functions. | 149 |
However, the reconstruction of neural circuits after SCI also depends on rehabilitation strategies, such as exercise training, ES, and BCIs. For example, combinatorial treatment of MSCs and exercise training could promote the protection of nerve tissue and enhance the recovery of motor function after SCI147. EES combined with high-intensity treadmill training enabled patients to walk on the ground, stand independently, and maintain trunk stability compared with individual exercise training148. Combinations of visual-tactile feedback, noninvasive BCIs and assisted exercise training significantly restored the patients’ motor function and part of sensory function, even part of intestinal, urinary and sexual functions149.
Conclusions and perspectives
The pathophysiology of SCI is complex and multifaceted, and its mechanisms and processes are incompletely understood. Thus, combinatorial therapies have been demonstrated to be more effective, and lead to better neural circuits reconstruction and functional recovery. Combinations of biomaterials, stem cells, growth factors, drugs and exosomes have been widely developed. However, simply achieving axon regeneration will not spontaneously lead to meaningful functional recovery. Therefore, the formation and remodeling of functional neural circuits also depend on rehabilitation exercises, such as exercise training, ES and BCIs.
It is believed that in the future we can analyze and record the nerve signals, and regulate the nerve signals through cell transplantation and molecular regulation after SCI. By stimulating local nerve circuits related to sensorimotor control and spinal cord autonomy, the protection, maintenance and even re-bridging of nerve circuits can be achieved. The relevant nerve circuits are fine-tuned, and then the corresponding nerve circuits are trained and strengthened by various rehabilitation methods. It can help the individual with SCI to resume autonomous movement as soon as possible, and ultimately promote the research and treatment of SCI to achieve more enormous breakthroughs in many fields.
Acknowledgements
This paper was supported by grants from Medical Science and Technology Project of Zhejiang Province of China [No. 2020385155], Public welfare technology research and social development project of science and Technology Department of Zhejiang Province of China [No. LGF19H060014], Scientific Research Fund of Zhejiang Provincial Education Department [No. Y201941476], and the National Natural Science Foundation of China [NO. 81972096, NO. 81401822, NO. 81572177, and NO. 81472504].
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Edited by N. Bazan
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Biao Yang, Feng Zhang
Contributor Information
Fangcai Li, Email: lifangcai@zju.edu.cn.
Chengzhen Liang, Email: liangchengzhen@zju.edu.cn.
Qixin Chen, Email: zrcqx@zju.edu.cn.
References
- 1.Sofroniew MV. Dissecting spinal cord regeneration. Nature. 2018;557:343–350. doi: 10.1038/s41586-018-0068-4. [DOI] [PubMed] [Google Scholar]
- 2.Picoli CC, et al. Pericytes act as key players in spinal cord injury. Am. J. Pathol. 2019;189:1327–1337. doi: 10.1016/j.ajpath.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James SL, et al. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkatesh K, Ghosh SK, Mullick M, Manivasagam G, Sen D. Spinal cord injury: pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019;377:125–151. doi: 10.1007/s00441-019-03039-1. [DOI] [PubMed] [Google Scholar]
- 5.Savage N. The mind-reading devices that can free paralysed muscles. Nature. 2018;555:S12–S14. doi: 10.1038/d41586-018-02478-0. [DOI] [PubMed] [Google Scholar]
- 6.Yuan S, Shi Z, Cao F, Li J, Feng S. Epidemiological features of spinal cord injury in china: a systematic review. Front Neurol. 2018;9:683. doi: 10.3389/fneur.2018.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes D. Spinal-cord injury: spurring regrowth. Nature. 2017;552:S49. doi: 10.1038/d41586-017-07550-9. [DOI] [PubMed] [Google Scholar]
- 8.Courtine G, Sofroniew MV. Spinal cord repair: advances in biology and technology. Nat. Med. 2019;25:898–908. doi: 10.1038/s41591-019-0475-6. [DOI] [PubMed] [Google Scholar]
- 9.David G, et al. Traumatic and nontraumatic spinal cord injury: pathological insights from neuroimaging. Nat. Rev. Neurol. 2019;15:718–731. doi: 10.1038/s41582-019-0270-5. [DOI] [PubMed] [Google Scholar]
- 10.Hutson TH, Di Giovanni S. The translational landscape in spinal cord injury: focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019;15:732–745. doi: 10.1038/s41582-019-0280-3. [DOI] [PubMed] [Google Scholar]
- 11.Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15:541–553. doi: 10.1007/s13311-018-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cizkova D, et al. Spinal cord injury: animal models, imaging tools and the treatment strategies. Neurochem. Res. 2020;45:134–143. doi: 10.1007/s11064-019-02800-w. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, et al. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat. Med. 2017;23:733–741. doi: 10.1038/nm.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilton BJ, Moulson AJ, Tetzlaff W. Neuroprotection and secondary damage following spinal cord injury: concepts and methods. Neurosci. Lett. 2017;652:3–10. doi: 10.1016/j.neulet.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Dell’Anno MT, et al. Human neuroepithelial stem cell regional specificity enables spinal cord repair through a relay circuit. Nat. Commun. 2018;9:3419. doi: 10.1038/s41467-018-05844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams KL, Gallo V. The diversity and disparity of the glial scar. Nat. Neurosci. 2018;21:9–15. doi: 10.1038/s41593-017-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahar M, Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 2018;19:323–337. doi: 10.1038/s41583-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson MA, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias DO, et al. Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell. 2018;173:153–165 e122. doi: 10.1016/j.cell.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiological Rev. 2018;98:881–917. doi: 10.1152/physrev.00017.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenzweig ES, et al. Chondroitinase improves anatomical and functional outcomes after primate spinal cord injury. Nat. Neurosci. 2019;22:1269–1275. doi: 10.1038/s41593-019-0424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poplawski GHD, et al. Adult rat myelin enhances axonal outgrowth from neural stem cells. Sci. Transl. Med. 2018;10:eaal2563. doi: 10.1126/scitranslmed.aal2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruschel J, et al. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 2015;348:347–352. doi: 10.1126/science.aaa2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veneruso V, et al. Stem cell paracrine effect and delivery strategies for spinal cord injury regeneration. J. Control. Release. 2019;300:141–153. doi: 10.1016/j.jconrel.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 25.Vismara I, Papa S, Rossi F, Forloni G, Veglianese P. Current options for cell therapy in spinal cord injury. Trends Mol. Med. 2017;23:831–849. doi: 10.1016/j.molmed.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017;20:637–647. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 27.Yu C, et al. The application of neural stem/progenitor cells for regenerative therapy of spinal cord injury. Curr. Stem Cell Res Ther. 2019;14:495–503. doi: 10.2174/1574888X14666190329095638. [DOI] [PubMed] [Google Scholar]
- 28.Stenudd M, Sabelstrom H, Frisen J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol. 2015;72:235–237. doi: 10.1001/jamaneurol.2014.2927. [DOI] [PubMed] [Google Scholar]
- 29.Kumamaru H, et al. Generation and post-injury integration of human spinal cord neural stem cells. Nat. Methods. 2018;15:723–731. doi: 10.1038/s41592-018-0074-3. [DOI] [PubMed] [Google Scholar]
- 30.Rosenzweig ES, et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018;24:484–490. doi: 10.1038/nm.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong Z, et al. Stem cell transplantation: a promising therapy for spinal cord injury. Curr. Stem Cell Res. Ther. 2019;15:321–331. doi: 10.2174/1574888X14666190823144424. [DOI] [PubMed] [Google Scholar]
- 32.Zhu R, et al. Electrical stimulation affects neural stem cell fate and function in vitro. Exp. Neurol. 2019;319:112963. doi: 10.1016/j.expneurol.2019.112963. [DOI] [PubMed] [Google Scholar]
- 33.Li X, et al. Wnt4-modified NSC transplantation promotes functional recovery after spinal cord injury. FASEB J. 2020;34:82–94. doi: 10.1096/fj.201901478RR. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B, et al. Nanomaterials in neural-stem-cell-mediated regenerative medicine: imaging and treatment of neurological diseases. Adv. Mater. 2018;30:e1705694. doi: 10.1002/adma.201705694. [DOI] [PubMed] [Google Scholar]
- 35.Shao A, Tu S, Lu J, Zhang J. Crosstalk between stem cell and spinal cord injury: pathophysiology and treatment strategies. Stem Cell Res. Ther. 2019;10:238. doi: 10.1186/s13287-019-1357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hakim R, et al. Mesenchymal stem cells transplanted into spinal cord injury adopt immune cell-like characteristics. Stem Cell Res. Ther. 2019;10:115. doi: 10.1186/s13287-019-1218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao F, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maldonado-Lasuncion I, Verhaagen J, Oudega M. Mesenchymal stem cell-macrophage choreography supporting spinal cord repair. Neurotherapeutics. 2018;15:578–587. doi: 10.1007/s13311-018-0629-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 40.Park K. Functional recovery in spinal cord injury using mesenchymal stem cells. J. Control Release. 2018;278:159. doi: 10.1016/j.jconrel.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 41.Ma YH, et al. Perineurium-like sheath derived from long-term surviving mesenchymal stem cells confers nerve protection to the injured spinal cord. Biomaterials. 2018;160:37–55. doi: 10.1016/j.biomaterials.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Luo Z, et al. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival by inducing HIF-1alpha in injured neuronal cells derived exosomes culture system. Cell Death Dis. 2019;10:134. doi: 10.1038/s41419-019-1410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, et al. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics. 2019;9:2017–2035. doi: 10.7150/thno.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao S, et al. Differentiation of human adipose-derived stem cells into neuron/motoneuron-like cells for cell replacement therapy of spinal cord injury. Cell Death Dis. 2019;10:597. doi: 10.1038/s41419-019-1772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hassani SN, Moradi S, Taleahmad S, Braun T, Baharvand H. Transition of inner cell mass to embryonic stem cells: mechanisms, facts, and hypotheses. Cell Mol. Life Sci. 2019;76:873–892. doi: 10.1007/s00018-018-2965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song CG, et al. Stem cells: a promising candidate to treat neurological disorders. Neural Regen. Res. 2018;13:1294–1304. doi: 10.4103/1673-5374.235085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou P, et al. Cell therapeutic strategies for spinal cord injury. Adv. Wound care. 2019;8:585–605. doi: 10.1089/wound.2019.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng J, Zhang Y, Xie Y, Zhang L, Tang P. Cell transplantation for spinal cord injury: tumorigenicity of induced pluripotent stem cell-derived neural stem/progenitor cells. Stem Cells Int. 2018;2018:5653787. doi: 10.1155/2018/5653787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michelsen KA, et al. Area-specific reestablishment of damaged circuits in the adult cerebral cortex by cortical neurons derived from mouse embryonic stem cells. Neuron. 2015;85:982–997. doi: 10.1016/j.neuron.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Manley NC, Priest CA, Denham J, Wirth ED, 3rd, Lebkowski JS. Human embryonic stem cell-derived oligodendrocyte progenitor cells: preclinical efficacy and safety in cervical spinal cord injury. Stem Cells Transl. Med. 2017;6:1917–1929. doi: 10.1002/sctm.17-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarei-Kheirabadi M, et al. Human embryonic stem cell-derived neural stem cells encapsulated in hyaluronic acid promotes regeneration in a contusion spinal cord injured rat. Int. J. Biol. Macromol. 2020;148:1118–1129. doi: 10.1016/j.ijbiomac.2020.01.219. [DOI] [PubMed] [Google Scholar]
- 52.Trawczynski M, Liu G, David BT, Fessler RG. Restoring motor neurons in spinal cord injury with induced pluripotent stem cells. Front Cell Neurosci. 2019;13:369. doi: 10.3389/fncel.2019.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 54.Yu JY, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 55.Zhu SY, et al. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell. Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li W, et al. Identification of Oct4-activating compounds that enhance reprogramming efficiency. Proc. Natl Acad. Sci. USA. 2012;109:20853–20858. doi: 10.1073/pnas.1219181110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramotowski C, Qu X, Villa-Diaz LG. Progress in the use of induced pluripotent stem cell-derived neural cells for traumatic spinal cord injuries in animal populations: meta-analysis and review. Stem Cells Transl. Med. 2019;8:681–693. doi: 10.1002/sctm.18-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yousefifard M, et al. Neural stem/progenitor cell transplantation for spinal cord injury treatment; a systematic review and meta-analysis. Neuroscience. 2016;322:377–397. doi: 10.1016/j.neuroscience.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 60.Tsuji O, et al. Concise review: laying the groundwork for a first-in-human study of an induced pluripotent stem cell-based intervention for spinal cord injury. Stem Cells. 2019;37:6–13. doi: 10.1002/stem.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu P, et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789–796. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan L, et al. Directing induced pluripotent stem cell derived neural stem cell fate with a three-dimensional biomimetic hydrogel for spinal cord injury repair. ACS Appl Mater. Interfaces. 2018;10:17742–17755. doi: 10.1021/acsami.8b05293. [DOI] [PubMed] [Google Scholar]
- 63.Yao R, et al. Olfactory ensheathing cells for spinal cord injury: sniffing out the issues. Cell Transplant. 2018;27:879–889. doi: 10.1177/0963689718779353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomez RM, et al. Cell therapy for spinal cord injury with olfactory ensheathing glia cells (OECs) Glia. 2018;66:1267–1301. doi: 10.1002/glia.23282. [DOI] [PubMed] [Google Scholar]
- 65.Delarue Q, et al. Inhibition of ADAMTS-4 expression in olfactory ensheathing cells enhances recovery after transplantation within spinal cord injury. J. Neurotrauma. 2020;37:507–516. doi: 10.1089/neu.2019.6481. [DOI] [PubMed] [Google Scholar]
- 66.Wright AA, et al. Enhancing the therapeutic potential of olfactory ensheathing cells in spinal cord repair using neurotrophins. Cell Transplant. 2018;27:867–878. doi: 10.1177/0963689718759472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang L, Zhuang X, Chen Y, Xia H. Intravenous transplantation of olfactory bulb ensheathing cells for a spinal cord hemisection injury rat model. Cell Transplant. 2019;28:1585–1602. doi: 10.1177/0963689719883842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gomes ED, et al. Co-transplantation of adipose tissue-derived stromal cells and olfactory ensheathing cells for spinal cord injury repair. Stem Cells. 2018;36:696–708. doi: 10.1002/stem.2785. [DOI] [PubMed] [Google Scholar]
- 69.Jessen KR, Arthur-Farraj P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia. 2019;67:421–437. doi: 10.1002/glia.23532. [DOI] [PubMed] [Google Scholar]
- 70.Pearse DD, Bastidas J, Izabel SS, Ghosh M. Schwann cell Transplantation subdues the pro-inflammatory innate immune cell response after spinal cord injury. Int J. Mol. Sci. 2018;19:2550. doi: 10.3390/ijms19092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cerqueira SR, et al. Decellularized peripheral nerve supports Schwann cell transplants and axon growth following spinal cord injury. Biomaterials. 2018;177:176–185. doi: 10.1016/j.biomaterials.2018.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang ZG, Buller B, Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019;15:193–203. doi: 10.1038/s41582-018-0126-4. [DOI] [PubMed] [Google Scholar]
- 73.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 74.Huang JH, et al. Systemic administration of exosomes released from mesenchymal stromal cells attenuates apoptosis, inflammation, and promotes angiogenesis after spinal cord injury in rats. J. Neurotrauma. 2017;34:3388–3396. doi: 10.1089/neu.2017.5063. [DOI] [PubMed] [Google Scholar]
- 75.Harrell CR, et al. Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells. 2019;8:E467. doi: 10.3390/cells8050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun G, et al. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater. Sci. Eng. C. Mater. Biol. Appl. 2018;89:194–204. doi: 10.1016/j.msec.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 77.Rong Y, et al. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019;10:340. doi: 10.1038/s41419-019-1571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tran PHL, et al. Exosomes and nanoengineering: a match made for precision therapeutics. Adv. Mater. 2019;32:e1904040. doi: 10.1002/adma.201904040. [DOI] [PubMed] [Google Scholar]
- 79.Kim HY, et al. Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord injury treatment. Nano Lett. 2018;18:4965–4975. doi: 10.1021/acs.nanolett.8b01816. [DOI] [PubMed] [Google Scholar]
- 80.Guo S, et al. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano. 2019;13:10015–10028. doi: 10.1021/acsnano.9b01892. [DOI] [PubMed] [Google Scholar]
- 81.Chen X, et al. Functional multichannel poly(propylene fumarate)-collagen scaffold with collagen-binding neurotrophic factor 3 promotes neural regeneration after transected spinal cord injury. Adv. Healthc. Mater. 2018;7:e1800315. doi: 10.1002/adhm.201800315. [DOI] [PubMed] [Google Scholar]
- 82.Volpato FZ, Fuhrmann T, Migliaresi C, Hutmacher DW, Dalton PD. Using extracellular matrix for regenerative medicine in the spinal cord. Biomaterials. 2013;34:4945–4955. doi: 10.1016/j.biomaterials.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 83.Liu D, et al. Different functional bio-scaffolds share similar neurological mechanism to promote locomotor recovery of canines with complete spinal cord injury. Biomaterials. 2019;214:119230. doi: 10.1016/j.biomaterials.2019.119230. [DOI] [PubMed] [Google Scholar]
- 84.Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336:1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 85.Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356:eaaf3627. doi: 10.1126/science.aaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caron I, et al. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials. 2016;75:135–147. doi: 10.1016/j.biomaterials.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 87.Papa S, et al. Mesenchymal stem cells encapsulated into biomimetic hydrogel scaffold gradually release CCL2 chemokine in situ preserving cytoarchitecture and promoting functional recovery in spinal cord injury. J. Control. Release. 2018;278:49–56. doi: 10.1016/j.jconrel.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 88.Dong Y, et al. Engineering the cell microenvironment using novel photoresponsive hydrogels. ACS Appl Mater. Interfaces. 2018;10:12374–12389. doi: 10.1021/acsami.7b17751. [DOI] [PubMed] [Google Scholar]
- 89.McKay CA, et al. An injectable, calcium responsive composite hydrogel for the treatment of acute spinal cord injury. ACS Appl. Mater. Interfaces. 2014;6:1424–1438. doi: 10.1021/am4027423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu C, et al. Cell-laden electroconductive hydrogel simulating nerve matrix to deliver electrical cues and promote neurogenesis. ACS Appl Mater. Interfaces. 2019;11:22152–22163. doi: 10.1021/acsami.9b05520. [DOI] [PubMed] [Google Scholar]
- 91.Bonizzato M, et al. Brain-controlled modulation of spinal circuits improves recovery from spinal cord injury. Nat. Commun. 2018;9:3015. doi: 10.1038/s41467-018-05282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou L, et al. Soft conducting polymer hydrogels cross-linked and doped by tannic acid for spinal cord injury repair. ACS Nano. 2018;12:10957–10967. doi: 10.1021/acsnano.8b04609. [DOI] [PubMed] [Google Scholar]
- 93.Koffler J, et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019;25:263–269. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Z, Tang M, Zhao J, Chai R, Kang J. Looking into the future: toward advanced 3D biomaterials for stem-cell-based regenerative medicine. Adv. Mater. 2018;30:e1705388. doi: 10.1002/adma.201705388. [DOI] [PubMed] [Google Scholar]
- 95.Sun F, et al. 3D poly(lactic-co-glycolic acid) scaffolds for treating spinal cord injury. J. Biomed. Nanotechnol. 2017;13:290–302. doi: 10.1166/jbn.2017.2348. [DOI] [PubMed] [Google Scholar]
- 96.Sun Y, et al. 3D printing collagen/chitosan scaffold ameliorated axon regeneration and neurological recovery after spinal cord injury. J. Biomed. Mater. Res A. 2019;107:1898–1908. doi: 10.1002/jbm.a.36675. [DOI] [PubMed] [Google Scholar]
- 97.Joung D, et al. 3D printed stem-cell derived neural progenitors generate spinal cord scaffolds. Adv. Funct. Mater. 2018;28:10. doi: 10.1002/adfm.201801850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Papa S, Rossi F, Vismara I, Forloni G, Veglianese P. Nanovector-mediated drug delivery in spinal cord injury: a multitarget approach. ACS Chem. Neurosci. 2019;10:1173–1182. doi: 10.1021/acschemneuro.8b00700. [DOI] [PubMed] [Google Scholar]
- 99.Song YH, Agrawal NK, Griffin JM, Schmidt CE. Recent advances in nanotherapeutic strategies for spinal cord injury repair. Adv. Drug Deliv. Rev. 2018;148:38–59. doi: 10.1016/j.addr.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Papa S, et al. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials. 2016;75:13–24. doi: 10.1016/j.biomaterials.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 101.Vismara I, et al. Selective modulation of A1 astrocytes by drug-loaded nano-structured gel in spinal cord injury. ACS Nano. 2020;14:360–371. doi: 10.1021/acsnano.9b05579. [DOI] [PubMed] [Google Scholar]
- 102.Wang J, et al. Scar tissue-targeting polymer micelle for spinal cord injury treatment. Small. 2020;16:e1906415. doi: 10.1002/smll.201906415. [DOI] [PubMed] [Google Scholar]
- 103.Keefe, K. M., Sheikh, I. S. & Smith, G. M. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int. J. Mol. Sci. 18, 548 (2017). [DOI] [PMC free article] [PubMed]
- 104.Xu D, et al. Efficient delivery of nerve growth factors to the central nervous system for neural regeneration. Adv. Mater. 2019;31:e1900727. doi: 10.1002/adma.201900727. [DOI] [PubMed] [Google Scholar]
- 105.Rosich K, Hanna BF, Ibrahim RK, Hellenbrand DJ, Hanna A. The effects of glial cell line-derived neurotrophic factor after spinal cord injury. J. Neurotrauma. 2017;34:3311–3325. doi: 10.1089/neu.2017.5175. [DOI] [PubMed] [Google Scholar]
- 106.Zhou Y, Wang Z, Li J, Li X, Xiao J. Fibroblast growth factors in the management of spinal cord injury. J. Cell Mol. Med. 2018;22:25–37. doi: 10.1111/jcmm.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Allahdadi KJ, et al. IGF-1 overexpression improves mesenchymal stem cell survival and promotes neurological recovery after spinal cord injury. Stem Cell Res. Ther. 2019;10:146. doi: 10.1186/s13287-019-1223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ong W, Pinese C, Chew SY. Scaffold-mediated sequential drug/gene delivery to promote nerve regeneration and remyelination following traumatic nerve injuries. Adv. Drug Deliv. Rev. 2019;149-150:19–48. doi: 10.1016/j.addr.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 109.Anderson MA, et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature. 2018;561:396–400. doi: 10.1038/s41586-018-0467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang Z, et al. NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc. Natl Acad. Sci. USA. 2015;112:13354–13359. doi: 10.1073/pnas.1510194112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang C, et al. An injectable heparin-Laponite hydrogel bridge FGF4 for spinal cord injury by stabilizing microtubule and improving mitochondrial function. Theranostics. 2019;9:7016–7032. doi: 10.7150/thno.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Serradj N, Agger SF, Hollis ER. 2nd corticospinal circuit plasticity in motor rehabilitation from spinal cord injury. Neurosci. Lett. 2017;652:94–104. doi: 10.1016/j.neulet.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 113.Torres-Espin A, et al. Eliciting inflammation enables successful rehabilitative training in chronic spinal cord injury. Brain. 2018;141:1946–1962. doi: 10.1093/brain/awy128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell. 2014;159:1626–1639. doi: 10.1016/j.cell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 115.James ND, McMahon SB, Field-Fote EC, Bradbury EJ. Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol. 2018;17:905–917. doi: 10.1016/S1474-4422(18)30287-4. [DOI] [PubMed] [Google Scholar]
- 116.Arpin DJ, Ugiliweneza B, Forrest G, Harkema SJ, Rejc E. Optimizing neuromuscular electrical stimulation pulse width and amplitude to promote central activation in individuals with severe spinal cord injury. Front. Physiol. 2019;10:1310. doi: 10.3389/fphys.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sakellaridi S, et al. Intrinsic variable learning for brain-machine interface control by human anterior intraparietal cortex. Neuron. 2019;102:694–705 e693. doi: 10.1016/j.neuron.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shanechi MM. Brain-machine interfaces from motor to mood. Nat. Neurosci. 2019;22:1554–1564. doi: 10.1038/s41593-019-0488-y. [DOI] [PubMed] [Google Scholar]
- 119.Cote MP, Murray M, Lemay MA. Rehabilitation strategies after spinal cord injury: inquiry into the mechanisms of success and failure. J. Neurotrauma. 2017;34:1841–1857. doi: 10.1089/neu.2016.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cobianchi S, Arbat-Plana A, Lopez-Alvarez VM, Navarro X. Neuroprotective effects of exercise treatments after injury: the dual role of neurotrophic factors. Curr. Neuropharmacol. 2017;15:495–518. doi: 10.2174/1570159X14666160330105132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo LY, Lozinski B, Yong VW. Exercise in multiple sclerosis and its models: focus on the central nervous system outcomes. J. Neurosci. Res. 2020;98:509–523. doi: 10.1002/jnr.24524. [DOI] [PubMed] [Google Scholar]
- 122.Asboth L, et al. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat. Neurosci. 2018;21:576–588. doi: 10.1038/s41593-018-0093-5. [DOI] [PubMed] [Google Scholar]
- 123.Fu J, Wang H, Deng L, Li J. Exercise training promotes functional recovery after spinal cord injury. Neural Plast. 2016;2016:4039580. doi: 10.1155/2016/4039580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liang ZW, Lei T, Wang S, Luo ZJ, Hu XY. A simple electrical stimulation cell culture system on the myelination of dorsal root ganglia and Schwann cells. Biotechniques. 2019;67:11–15. doi: 10.2144/btn-2018-0175. [DOI] [PubMed] [Google Scholar]
- 125.Taccola G, et al. Acute neuromodulation restores spinally-induced motor responses after severe spinal cord injury. Exp. Neurol. 2020;327:113246. doi: 10.1016/j.expneurol.2020.113246. [DOI] [PubMed] [Google Scholar]
- 126.Hofstoetter US, et al. Transcutaneous spinal cord stimulation induces temporary attenuation of spasticity in individuals with spinal cord injury. J. Neurotrauma. 2020;37:481–493. doi: 10.1089/neu.2019.6588. [DOI] [PubMed] [Google Scholar]
- 127.Gill ML, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018;24:1677–1682. doi: 10.1038/s41591-018-0175-7. [DOI] [PubMed] [Google Scholar]
- 128.Wagner FB, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65–71. doi: 10.1038/s41586-018-0649-2. [DOI] [PubMed] [Google Scholar]
- 129.Inanici F, et al. Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 2018;26:1272–1278. doi: 10.1109/TNSRE.2018.2834339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Slutzky MW. Brain-machine interfaces: powerful tools for clinical treatment and neuroscientific investigations. Neuroscientist. 2019;25:139–154. doi: 10.1177/1073858418775355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Andersen RA, Aflalo T, Kellis S. From thought to action: The brain-machine interface in posterior parietal cortex. Proc. Natl Acad. Sci. USA. 2019;23:201902276. doi: 10.1073/pnas.1902276116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Orsborn AL, Pesaran B. Parsing learning in networks using brain-machine interfaces. Curr. Opin. Neurobiol. 2017;46:76–83. doi: 10.1016/j.conb.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rosenfeld JV, Wong YT. Neurobionics and the brain-computer interface: current applications and future horizons. Med J. Aust. 2017;206:363–368. doi: 10.5694/mja16.01011. [DOI] [PubMed] [Google Scholar]
- 134.Hochberg LR, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Benabid AL, et al. An exoskeleton controlled by an epidural wireless brain-machine interface in a tetraplegic patient: a proof-of-concept demonstration. Lancet Neurol. 2019;18:1112–1122. doi: 10.1016/S1474-4422(19)30321-7. [DOI] [PubMed] [Google Scholar]
- 136.Bouton CE, et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;533:247–250. doi: 10.1038/nature17435. [DOI] [PubMed] [Google Scholar]
- 137.Capogrosso M, et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;539:284–288. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ajiboye AB, et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet. 2017;389:1821–1830. doi: 10.1016/S0140-6736(17)30601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Flesher SN, et al. Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 2016;8:361ra141. doi: 10.1126/scitranslmed.aaf8083. [DOI] [PubMed] [Google Scholar]
- 140.O’Doherty JE, et al. Active tactile exploration using a brain-machine-brain interface. Nature. 2011;479:228–231. doi: 10.1038/nature10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O’Doherty JE, Shokur S, Medina LE, Lebedev MA, Nicolelis MAL. Creating a neuroprosthesis for active tactile exploration of textures. Proc. Natl Acad. Sci. USA. 2019;116:21821–21827. doi: 10.1073/pnas.1908008116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun L, et al. Co-transplantation of human umbilical cord mesenchymal stem cells and human neural stem cells improves the outcome in rats with spinal cord injury. Cell Transplant. 2019;28:893–906. doi: 10.1177/0963689719844525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ma K, et al. Insulin-like growth factor-1 enhances neuroprotective effects of neural stem cell exosomes after spinal cord injury via an miR-219a-2-3p/YY1 mechanism. Aging (Albany NY) 2019;11:12278–12294. doi: 10.18632/aging.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li G, et al. Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials. 2016;83:233–248. doi: 10.1016/j.biomaterials.2015.11.059. [DOI] [PubMed] [Google Scholar]
- 145.Li L, et al. A MnO2 nanoparticle-dotted hydrogel promotes spinal cord repair via regulating reactive oxygen species microenvironment and synergizing with mesenchymal stem cells. ACS Nano. 2019;13:14283–14293. doi: 10.1021/acsnano.9b07598. [DOI] [PubMed] [Google Scholar]
- 146.Lai BQ, et al. Tissue-engineered neural network graft relays excitatory signal in the completely transected canine spinal cord. Adv. Sci. 2019;6:19. doi: 10.1002/advs.201901240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Massoto TB, et al. Mesenchymal stem cells and treadmill training enhance function and promote tissue preservation after spinal cord injury. Brain Res. 2020;1726:11. doi: 10.1016/j.brainres.2019.146494. [DOI] [PubMed] [Google Scholar]
- 148.Angeli CA, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N. Engl. J. Med. 2018;379:1244–1250. doi: 10.1056/NEJMoa1803588. [DOI] [PubMed] [Google Scholar]
- 149.Shokur S, et al. Training with brain-machine interfaces, visuo-tactile feedback and assisted locomotion improves sensorimotor, visceral, and psychological signs in chronic paraplegic patients. PloS ONE. 2018;13:e0206464. doi: 10.1371/journal.pone.0206464. [DOI] [PMC free article] [PubMed] [Google Scholar]