Abstract
To develop a high throughput colorimetric biosensor for detection of Staphylococcus aureus (SA) based on specific aptamer and catalysis of dsDNA-SYBR Green I (SG I) complex. SA specific aptamer was immobilized on a 96-well plate by hybridization with the capture probe anchored on the plate surface through streptavidin-biotin binding. In presence of SA, the aptamer was dissociated from the capture probe-aptamer duplex due to the stronger interaction between the aptamer and SA. The consequent single-strand capture probe could be hybridized with a three-way junction (TWJ) probe. With the presence of SG I, the dsDNA-SG I complex catalyze the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) under photo-irradiation, producing sensitive photo-catalyzed colorimetric response to SA. Under the optimal conditions, the proposed method could directly detect SA with the limit of detection (LOD) at 81 CFU mL−1 in PBS buffer in 5.5 hours, which demonstrated the sensitive and fast quantification of target pathogenic bacteria. The method showed weak colorimetric signal to Escherichia coli and Pseudomonas aeruginosa, indicating the high specificity for SA. In addition, the method can simultaneously detect 96 samples which can be used for high throughput analysis. The designed method may become a powerful tool for pathogenic microorganisms screening in clinical diagnostics, food safety and environmental monitoring.
Subject terms: Biochemistry, Biotechnology
Introduction
Staphylococcus aureus (SA) is the most common pathogen that causes a wide range of human infections. It is the major cause of bacteremia, infective endocarditis as well as skin and soft tissue infection and device-related infections1. Rapid identification of SA in the early stages of infection is important for reducing high mortality. However, the conventional culture method, known as “gold standard” for bacterial detection, usually requires 3–5 days incubation. It also needs at least 12 hours of growth on solid media to complete the identification2. Time consuming and insensitive are common problems with these methods.
Various methods which can shorten the detection time and increase the sensitivity have been used for bacterial detection, including enzyme-linked immunosorbent assay (ELISA)3, polymerase chain reaction (PCR)4, surface plasmon resonance biosensor5, electrochemical biosensor6 and so on. Despite improvements, these methods still require sophisticated equipment, complex sample preparation and long-term blood culture, which limit their use in clinical applications7. Besides, false positive results often occur in PCR detection8. Therefore, the development of a new platform that can distinguish SA in a short time is highly desired.
In this study, an aptamer-based high throughput colorimetric biosensor for detection of SA was devised. The SA specific aptamer, reported by Y.S. Xu et al.2 has strong binding affinity to the bacteria. The TWJ DNA nanostructure used in the assay for signal amplification is another high light of the study. In recent years, as an enzyme-free amplification method, more and more attention has been paid to the self-assembly of nanostructures, which are molecules spontaneously combine into stable, well-defined aggregates under equilibrium conditions9. Among them, TWJ composed of three complementary oligonucleotide branches has become an extremely important building block for constructing DNA structure and dynamic assembly. Meanwhile, the TWJ strategy has obvious advantages, including simple probe design, economical biosensor manufacturing and excellent signal amplification. Therefore, it opens broad prospects for applications in biosensing and bioanalysis10,11.
X. F. Zhang etal reported a new discovery that the dsDNA-SG I complex possessed photocatalytic activity, which could catalyze the oxidation of oxidase substrates TMB with dissolved oxygen under photo-irradiation12. Compared with G-quadruplex-based DNAzyme13,14, peroxidase mimicking nanozyme15, and HRP16,17, which also have similar catalytic activity for TMB oxidation, the dsDNA-SG I proposed here offers several distinct advantages, such as simple and universal, label-free, visual sensing, suitable for convenient biosensor designs, etc.
In this study, for the first time, we designed an aptamer-based photo-irradiation colorimetric biosensor for detection of SA.By using this method, a new platform which can distinguish SA in a short time with selectivity and sensitivity has been achieved. The TWJ DNA nanostructure exhibited excellent signal amplification effect through self-assembly, and the dsDNA-SG I complex possessed photocatalytic activity, which made the method more specific and simpler. In addition, this analysis is a high throughput analysis which can be used to detect 96 samples at once. Compared with other reported methods, our biosensor has such advantages, include fast speed and more sensitive and specific. So, it may be a powerful tool for SA screening in clinical diagnostics.
Experimental
Reagents and apparatus
DNA oligonucleotides were synthesized and purified by Sangon Inc. (Shanghai, China). Their sequences are listed in Table 1. Streptavidin, bovine serum albumin (BSA), SYBR Green I (SG I), TMB, citric acid, disodium hydrogen phosphate, TE buffer, 0.1 M PBS buffer, 2 × SSC hybridization buffer and transparent ELISA plate were also purchased from Sangon Inc. (Shanghai, China). Colorimetric bioassay was carried out on a PHOMO Automatic enzyme immunoassay analyzer (Zhengzhou Antu Instruments Co. Ltd., China)
Table 1.
Oligonucleotides used in the present work.
| Oligonucleotides | Sequences (5′-3′) |
|---|---|
| SA aptamer: | TCCCTACGGCGCTAACCCCCCCAGTCCGTCCTCCCAGCCTCACACCGCCACCGTGCTACAAC |
| Capture probe: | bio-TTTTTGTTGTAGCAC |
| detection probe P1: | CTCATGGAGAGAGAATTTGGGTGCGAGACGTGCTACAA |
| detection probe P2: | CAGCGATCAGTTCAACTCTCTCCATGAG |
| detection probe P3: | GTCTCGCACCCAAAGAACTGATCGCTG |
Preparation ofstaphylococcus aureussample
The strains of SA were cultured with Luria-Bertani medium at 37 °C for 24 hours, and then we diluted the SA suspension using 0.1 M PBS buffer to obtain an appropriate optical density value of about 0.5 at 600 nm. After that, the bacterial solution was serially diluted and inoculated onto the solid medium for 24 hours at 37 °C to quantify the CFU mL−1. The concentration was estimated by calculating the average number of CFU.
Streptavidin coated transparent ELISA plate
Streptavidin was diluted in the coating buffer (100 mM Na2HPO4, 50 mM citric acid, pH 5.0) to the final concentration 5.0 µg mL−1. Then add 200 µL of the coating solution to each well. The plates were closed in a humidified box and incubated at 35 °C overnight.
Then the plates were washed with 0.01 M PBS buffer containing 0.05% Tween-20. After washing, 200 µL 0.1% bovine serum albumin PBS solution was added per well. The plates were saturated overnight at room temperature, then washed with 0.01 M PBS buffer containing 0.05% Tween-20 and dried at room temperature. Finally, the plate was filled with moisture absorbent and dried at 4 °C for storage18.
Preparation of colorimetric biosensor
100 μL of 10 μM biotin labeled capture probe was dropped on the streptavidin-coated plate surface and incubated 30 minutes at room. After washed with the SSC buffer, 100 μL of 10 μM SA aptamer was added in the plates and incubated at 37 °C for 30 minutes. The plates were further rinsed with the washing buffer and different concentrations of SA were added in the reaction system, and then incubated for 2 hours at 37 °C. After SSC buffer washed the plates, three detection probes P1, P2, P3 (50 μL, 20 μM) were added to combine with the capture probe. After incubated at 37 °C for 90 minutes, 30 μL SYBR Green I (1:100) was embed in the dsDNA for 30 minutes, and then washed with PBS buffer. At last, 50 μL TMB (200 mg/L) and 20 μL 10% H2O2 were dropped in the plates. The plates were irradiated with white LED for 30 minutes. 50 μL 2 M H2SO4 were added to stop the solution. The absorbance were measured by PHOMO Automatic enzyme immunoassay analyzer in 450 nm and 630 nm
Results and discussion
Design of the colorimetric biosensor
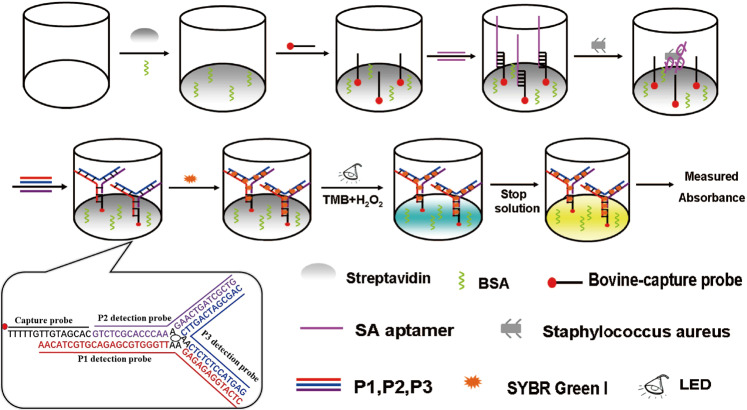
An overview of the high throughput colorimetric biosensor for detection of SA based on TWJ DNA nanostructure and the catalysis of dsDNA-SG was illustrated in Fig. 1. The 96-well plate was coated with streptavidin. Then, BSA was used to avoid nonspecific adsorption on the plate surface. Biotin labeled capture probe anchored on the plate surface through streptavidin-biotin system. SA specific aptamer was immobilized on the 96-well plate by hybridization with the capture probe. In the presence of SA, for the stronger interaction between aptamer and SA, the aptamer was dissociated from the capture probe-aptamer duplex. Then the consequent single-strand capture probe could be hybridized with a TWJ DNA nanostructure which composed by three detection probes (P1, P2 and P3). After the addition of SG I, it formed dsDNA-SG I complex, which can catalyze the oxidation of TMB under LED photo-irradiation. The catalytic color is proportional to the added bacteria concentration. The designed biosensor showed acceptable precision and reproducibility. It provided a pragmatic tool for convenient detection of SA in clinical diagnosis.
Figure 1.
Schematic illustration of the principle for detection of staphylococcus aureus with aptamer-high throughput colorimetric biosensor based on photocatalytic activity of dsDNA-SG I complex.
Signal amplification performance of designed biosensor
Compared to a single oligonucleotide branch, the TWJ nanostructure composed of three mutually complementary oligonucleotide branches (P1, P2, P3) can carry more SG I. Then the dsDNA-SG I complex catalyze oxidation of TMB under LED photo-irradiation. The catalytic color will further increase and the sensitivity will be improved. Meanwhile, the TWJ strategy also simplified probe design and biosensor fabrication, as well as excellent signal amplification. Thus, it opens a promising avenue for applications in biosensing and bioanalysis10.
Optimization of experimental conditions
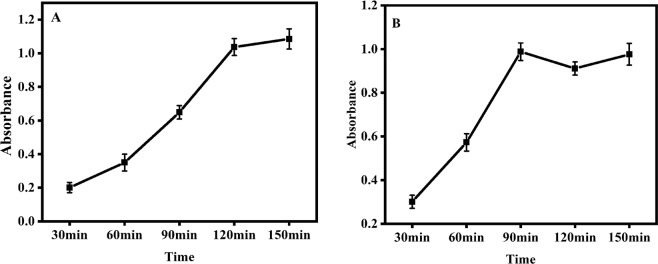
In order to achieve the perfect assay performance, aptamer-SA incubation time and TWJ hybridization time were selectively optimized as the most valuable influence factors for the detection. Figure 2A showed the effect of aptamer-SA incubation time on the absorbance. The concentration of the test SA was 104 CFU mL−1. With the increasing incubation time, the absorbance increased and tended to a steady value at 120 minutes. Prolonged incubation time did not increase the signal response obviously. So, 120 minutes was identified as the optimal incubation time. The effect of TWJ hybridization time for capture probe and P1/P2/P3 probe was also studied in the time range from 30 to 120 minutes. (Fig. 2B). The absorbance increased obviously within 90 minutes and then reached a platform. Thus, 90 minutes was selected as the appropriate time for the following experiments.
Figure 2.
Optimizations of experimental parameters: (A) evaluation of binding time for specific aptamer and SA (B) evaluation of TWJ hybridization time for capture probe and P1/P2/P3 probe. (SA concentration: 104 CFU mL−1).
Analytical performance of designed biosensor
The analytical performance of biosensor was performed under optimal experimental conditions. Serial dilution of SA in PBS buffer were to the final concentration of 102, 103, 104, 105, 106, 107 CFU mL−1. The absorbance increased with the increasing SA concentration. In the range of 102 to 107 CFU mL−1, the relationship between absorbance value and logarithm of SA concentration showed linear, and the correlation coefficient was 0.996 (Fig. 3). The detection limit was estimated to be 81 CFU mL−1 in PBS buffer. The biosensor proposed in this research can complete the detection of SA in 5.5 hours. Compared with conventional culture methods, this method is significantly time-saving and easier to operate19,20.
Figure 3.
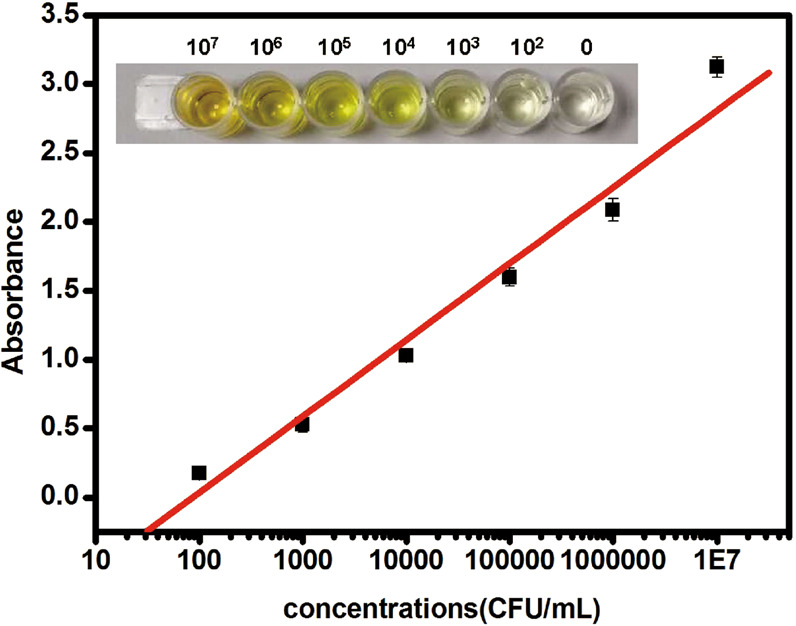
Absorbance responses to 102, 103, 104, 105, 106, and 107 CFU/mL Staphylococcus aureus.
In order to further highlight the advantages of the designed biosensor in detecting SA, the analytical properties were compared with those of other biosensors2,21,22. Characteristics including the detection limit and dynamic range are summarized in Table 2. It proved that this method not only had great improvement in detection limit but also possessed the advantage of wide dynamic range.
Table 2.
Comparison of analytical performance of the proposed method with other reported bioassays for the detection of Staphylococcus aureus.
Specificity of the strategy
To prove the specificity of the biosensor, two bacterias, Escherichia coli (E. coli) and Pseudomonas aeruginosa (PA) were tested under the same experimental conditions as those for SA. The concentrations of the test bacteria were 104 CFU mL−1. To further prove the identification ability of this method, mixtures of microorganisms (SA, PA, E. coli, 104 CFU mL−1) were also tested in the meantime. Each bacterium and the mixtures of microorganisms were tested for three times. The results were shown in Fig. 4. The absorbance of these two bacteria (PA and E. coli) were only as low as the blank, but the SA and microorganisms’ mixtures had obvious absorbance responses, indicating that the colorimetric biosensor had a good specificity and identification ability for the detection of SA.
Figure 4.
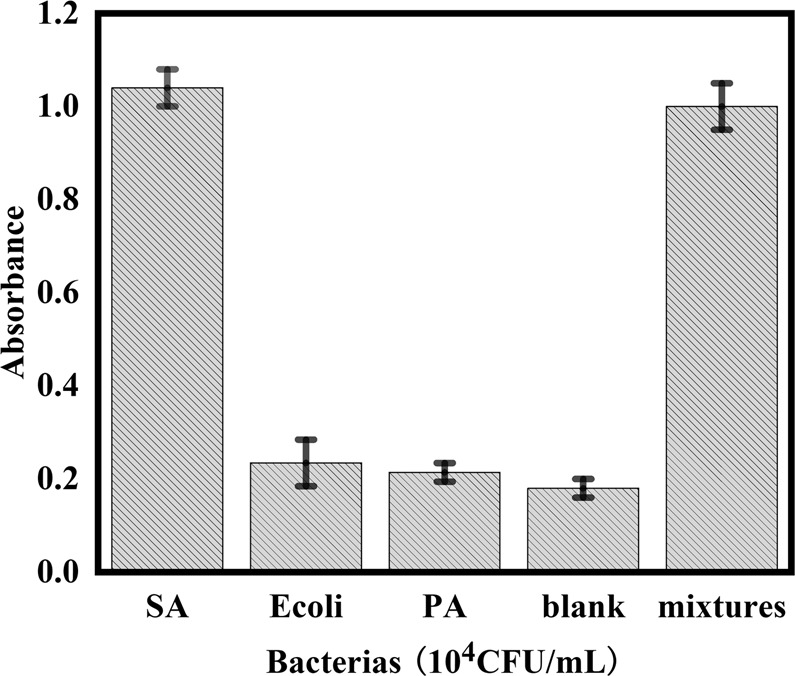
Absorbance responses of the colorimetric biosensor to 104 CFU/mL Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, microorganisms mixtures and blank.
Detection of SA in milk samples
In order to prove the proposed colorimetric biosensor can detect SA in actual samples sensitively and specifically, the cultured SA were inoculated into milk at a concentration of 0 to 107 CFU mL−1. Each concentration was tested for three times. The absorbance responses of the biosensor to different SA concentrations were shown in Fig. 5. The responses increased with the increasing SA concentration. The fabricated bioassay method can detect SA concentration down to 100 CFU mL−1 in real milk samples. In addition, the method can complete the detection in 5.5 hours. Compared with traditional culture methods (3–5 days), this method is simple, fast, with higher sensitivity and specificity, showing the potential as a pragmatic tool for SA detection in real samples.
Figure 5.
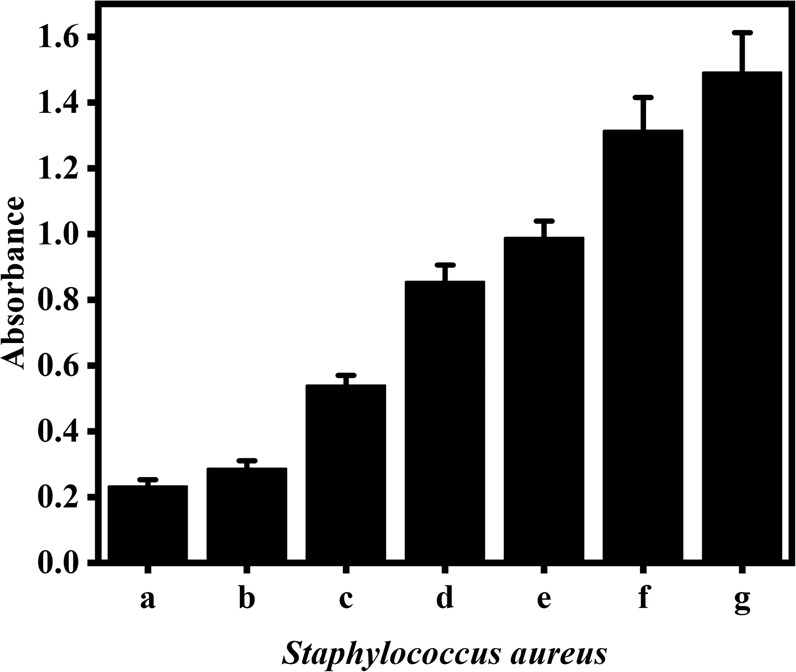
Absorbance responses to SA in the milk. 0 (a); 102 (b); 103 (c); 104 (d); 105 (e); 106 (f); 107 (g) CFU/mL SA.
Conclusions
Aptamer-based high throughput colorimetric biosensor for direct detection of Staphylococcus aureus has been successfully developed. The biosensor shows wide linear range, low detection limit, high specificity, and could be used for detection of SA in real samples. Importantly, the application of TWJ DNA nanostructure and photo catalytic activity of dsDNA–SG I complex not only amplifying the detection signal, but also shortening the detection time. The method provided a direct sensing platform for detection of SA and the whole analytical process can be finished in 5.5 hours. The bioassay strategy could be used to develop other assay method for pathogenic bacteria and would become a powerful tool for pathogenic microorganism screening in clinical diagnostics, food safety, biothreat detection and environmental monitoring.
Acknowledgements
This work was funded by key program self-financing project in Hebei province (No. 172777192), technology support project of Shijiazhuang (No. 171792173) and technology support project of Shijiazhuang (No. 151460713).
Author contributions
Conceptualization: Tianxiao Yu. Data curation: Tianxiao Yu, Yao Zhang, Jingrui Zhang. Funding acquisition: Tianxiao Yu, Hong Xu, Jun Ge. Methodology: Tianxiao Yu, Yan Zhao, Yanjie Han, Caihong Xu. Project administration: Wenju Wang, Qing Guo. Writing – original draft: Tianxiao Yu. Writing – review & editing: Tianxiao Yu, Hong Xu, Jun Ge.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wenju Wang, Email: wenjuwang@163.com.
Qing Guo, Email: qingguo123@126.com.
Jun Ge, Email: 404828162@qq.com.
References
- 1.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu YS, et al. Aptamer-based hydrogel barcodes for the capture and detection of multiple types of pathogenic bacteria. Biosens. Bioelectron. 2018;100:404–410. doi: 10.1016/j.bios.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Albritton HL, Kozlowski PA, Lillis RA, Mcgowin CL, Siren JD. A novel whole-bacterial enzyme linked-immunosorbant assay to quantify Chlamydia trachomatis specific antibodies reveals distinct differences between systemic and genital compartments. PLoS One. 2017;12:e0183101. doi: 10.1371/journal.pone.0183101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roisin S, Huang TD, De RM, Nonhoff C, Bogaerts P. Prospective evaluation of a high multiplexing real-time polymerase chain reaction array for the rapid identification and characterization of bacteria causative of nosocomial pneumonia from clinical specimens: a proof-of-concept study. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:109–116. doi: 10.1007/s10096-017-3108-3. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Zhu J, Zhang H, Liu W, Ge J. High sensitivity gram-negative bacteria biosensor based on a small-molecule modified surface plasmon resonance chip studied using a laser scanning confocal imaging-surface plasmon resonance system. Sens. Actuators B Chemical. 2018;259:492–497. doi: 10.1016/j.snb.2017.12.042. [DOI] [Google Scholar]
- 6.Luo CH, et al. A sensitive electrochemical DNA biosensor for specific detection of Enterobacteriaceae bacteria by Exonuclease III-assisted signal amplification. Biosens. Bioelectron. 2013;48:132–137. doi: 10.1016/j.bios.2013.03.084. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, et al. Coupling a universal DNA circuit with graphene sheets/polyaniline/AuNPs nanocomposites for the detection of BCR/ABL fusion gene. Analytica Chim. Acta. 2015;889:90–97. doi: 10.1016/j.aca.2015.06.050. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Luo C, Zhong L, Ding SJ. Sensitive detection of enteropathogenic E. coli using a bfpA gene-based;electrochemical sensor. Microchim. Acta. 2013;180:1233–1240. doi: 10.1007/s00604-013-1061-0. [DOI] [Google Scholar]
- 9.Wang C, Wang ZQ, Zhang X. Amphiphilic building blocks for self-assembly: from amphiphiles to supra-amphiphiles. Acc. Chem. Res. 2012;45:608–618. doi: 10.1021/ar200226d. [DOI] [PubMed] [Google Scholar]
- 10.Hu R, et al. An electrochemical biosensor for highly sensitive detection of microRNA-377 based on strand displacement amplification coupled with three-way junction. J. Electroanal. Chem. 2017;789:160–166. doi: 10.1016/j.jelechem.2017.02.038. [DOI] [Google Scholar]
- 11.Xu Y, et al. Ultrasensitive and rapid detection of miRNA with three-way junction structure-based trigger-assisted exponential enzymatic amplification. Biosens. Bioelectron. 2016;81:236–241. doi: 10.1016/j.bios.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XF, et al. Photocatalytic oxidation of TMB with the double stranded DNA-SYBR Green I complex for label-free and universal colorimetric bioassay. ChenComm. 2015;51:14465. doi: 10.1039/c5cc06105a. [DOI] [PubMed] [Google Scholar]
- 13.Orbach R, Willner B, Willner I. Catalytic nucleic acids (DNAzymes) as functional units for logic gates and computing circuits: from basic principles to practical applications.Chem. Commun. 2015;51:4144–4160. doi: 10.1039/c4cc09874a. [DOI] [PubMed] [Google Scholar]
- 14.Gong L, et al. DNAzyme-based biosensors and nanodevices. Chem. Commun. 2015;51:979–995. doi: 10.1039/C4CC06855F. [DOI] [PubMed] [Google Scholar]
- 15.Lin YH, Ren JS, Qu XG. Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc. Chem. Res. 2014;47:1097–1105. doi: 10.1021/ar400250z. [DOI] [PubMed] [Google Scholar]
- 16.Yang S, Ding S, Li L, Ding S, Cao Q. One-Step Preparation of Direct Electrochemistry HRP Biosensor via Electrodeposition. J. Electrochem. Soc, 2017;164:B710–B714. doi: 10.1149/2.2001713jes. [DOI] [Google Scholar]
- 17.Xu F, Ren S, Gu Y. A Novel Conductive Poly(3,4-ethylenedioxythiophene) BSA Film for the Construction of a Durable HRP Biosensor Modified with NanoAu Particles. Sensors. 2016;16:374–384. doi: 10.3390/s16030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valimaa L, Pettersson K, Vehniainen M, Karp M, Lovgren T. A high-capacity streptavidin-coated microtitration plate. Bioconjugate Chem. 2003;14:103–111. doi: 10.1021/bc020058y. [DOI] [PubMed] [Google Scholar]
- 19.Luo CH, et al. A Rapid and Sensitive Aptamer-Based Electrochemical Biosensor for Direct Detection of Escherichia Coli O111. Electroanalysis. 2012;24:1186–1191. doi: 10.1002/elan.201100700. [DOI] [Google Scholar]
- 20.Li Q, et al. Rapid and Sensitive Strategy for Salmonella Detection Using an InvA Gene-Based Electrochemical DNA Sensor. Int. J. Electrochem. Sci. 2012;7:844–856. [Google Scholar]
- 21.He X, et al. Aptamer-Fluorescent Silica Nanoparticles Bioconjugates Based Dual-Color Flow Cytometry for Specific Detection of Staphylococcus aureus. J. Biomed. Nanotechnol. 2014;10:1359–1368. doi: 10.1166/jbn.2014.1828. [DOI] [PubMed] [Google Scholar]
- 22.Gustavo A, Zelada G, José LSA, Blondeau P. Label-free detection of Staphylococcus aureus in skin using real-time potentiometric biosensors based on carbon nanotubes and aptamers. Biosens. Bioelectron. 2012;31:226–232. doi: 10.1016/j.bios.2011.10.021. [DOI] [PubMed] [Google Scholar]