Abstract
Reactivated long-term memories can become labile and sensitive to modification. Memories in this destabilized state can be weakened or strengthened, but there is limited research characterizing the mechanisms underlying retrieval-induced qualitative updates (i.e., information integration). We have previously implicated cholinergic transmission in object memory destabilization. Here we present a novel rodent paradigm developed to assess the role of this cholinergic mechanism in qualitative object memory updating. The post-reactivation object memory modification (PROMM) task exposes rats to contextual information following object memory reactivation. Subsequent object exploratory performance suggests that the contextual information is integrated with the original memory in a reactivation- and time-dependent manner. This effect is blocked by interference with M1 muscarinic receptors and several downstream signals in perirhinal cortex. These findings therefore demonstrate a hitherto unacknowledged cognitive function for acetylcholine with important implications for understanding the dynamic nature of long-term memory storage in the normal and aging brain.
Subject terms: Neuroscience, Learning and memory
Introduction
When first acquired, memories exist in a labile state and require protein synthesis-dependent consolidation to stabilize for long-term storage1,2. Following the presentation of reminder cues, however, consolidated long-term memories can be destabilized and again rendered labile, necessitating a second protein synthesis-dependent re-stabilization process referred to as reconsolidation3–5. The process of reconsolidation has been widely posited to play a role in adaptive memory updating, enabling the maintenance of memory accuracy and relevance over time6,7. Indeed, studies in rodents and humans have demonstrated post-reactivation modification by manipulating the strength of fear memory, providing evidence for memory trace weakening or erasure4,8, as well as strengthening through targeted additional training9. However, these studies do not directly assess the integration of new, relevant information presented during the reconsolidation window to update the content of established long-term memories. The distinction between this ‘qualitative’ memory updating and the previously demonstrated ‘quantitative’ changes may seem subtle, but it is likely significant when considering the dynamic nature of long-term storage for different types of material.
As informative as past studies have been, fear conditioning9–14 or other conditioning-based learning (e.g., drug associated cues15) bear little resemblance to human declarative memory, which exerts significant influence over day-to-day behaviour and is subject to regular qualitative modification. Indeed, the modifiability of human declarative memory – beyond merely strengthening and weakening – has long been acknowledged16–19. Perhaps most convincing is the occurrence of false memories, whereby incorrect information integrates into long-term memory and persists over time20. The phenomenon of reconsolidation may provide a link between understanding the cognitive basis of memory modification and its underlying neurobiology21. In order to bridge the gap between these fields, a feasible animal model of declarative memory modification that provides evidence for the incorporation of new information into an existing memory is required.
Object recognition tests have been used extensively to characterize animal models of amnesia22,23 and are widely accepted as standard procedures for studying declarative-like memory in the rodent literature24. Previous studies have adapted spontaneous object recognition tasks to provide evidence for memory updating through reconsolidation mechanisms; typically, at the time of reactivation, the reminder cue is presented with updating information, such as a new object or object location25–27. While these studies have made valuable contributions, the structure of these tasks, whereby the reactivation episode is combined with the presentation of updating information, presents two problems. First, this experience could be encoded as a completely novel learning episode rather than an opportunity to update the original memory; and second, it prevents assessment of the potential for memory modification within the post-reactivation reconsolidation window. A cleaner procedure for studying the neural bases of memory updating would present the updating information following memory reactivation during the labile window. One of the most convincing demonstrations of reconsolidation-mediated constructive memory updating in humans utilized a similar methodology. In this study, information about the contents of a new list of objects was incorporated into the memory of a list memorized earlier, but only if the second list was presented shortly after reactivation of the memory for the first list. Thus, the updating effect was reactivation- and time-dependent, as well as a constructive process28.
Analogous to the study just mentioned, which used objects to study updating of declarative memory in humans, here we present a novel paradigm for use with rodents to investigate the behavioural and neural mechanisms of reconsolidation-based object memory updating. In this post-reactivation object memory modification (PROMM) task, new contextual information appears to be incorporated into a previously acquired object memory when presented while the object memory trace is labile following reactivation.
We also use the PROMM task to investigate the neurobiological mechanisms underlying object memory updating. Previously, our group demonstrated the requirement of cholinergic signaling at M1 muscarinic receptors (mAChRs) in perirhinal cortex (PRh) for destabilization of object memories29,30. This effect appears to be mediated by signaling downstream of the M1 receptor, including the second messenger inositol triphosphate (IP3), stimulation of Ca2+/calmodulin-dependent protein kinase II (CaMKII), and activation of the ubiquitin proteasome system (UPS), which is involved in degrading synaptic proteins and is thought to underlie destabilization at the synaptic level14,30. We hypothesized that this mechanism of memory destabilization is necessary for reconsolidation-mediated object memory updating, but until now there has been no viable rodent model to study this question. Using the PROMM task, the current study presents evidence in support of this hypothesis, implicating cortical acetylcholine (ACh) in a cognitive function that is subtly, but clearly, distinct from its established role in new learning.
Results
Retrieval enables constructive and time-dependent object memory updating in rats
In the PROMM task, rats explore an object during a sample phase, and the object memory is reactivated 24 h later with a brief re-exposure to the identical training objects and context. After the object memory is reactivated, and consequently destabilized, the rat is immediately placed into an empty alternate context (see Methods; Fig. 1). Here, we predict that the alternate contextual information will incorporate into the labile object memory representation. On test day, an additional 24 h later, rats explore the sampled objects in either the same alternate context as seen post-reactivation, or a different alternate context. If the alternate context presented post-reactivation successfully integrates with the reactivated object memory, rats should behave as if this test phase object-context combination is familiar, even though the objects have never been directly experienced in that context. When the objects are presented in combination with an alternate context during test phase that is different than the context explored post-reactivation, rats should behave as if this object-context configuration is unfamiliar, and therefore demonstrate novelty-induced increases in object exploration31. As predicted, rats showed significantly lower total object exploration in the same alternate context condition compared to the different alternate context condition, t(11) = −3.29, p = 0.007 (Fig. 2a).
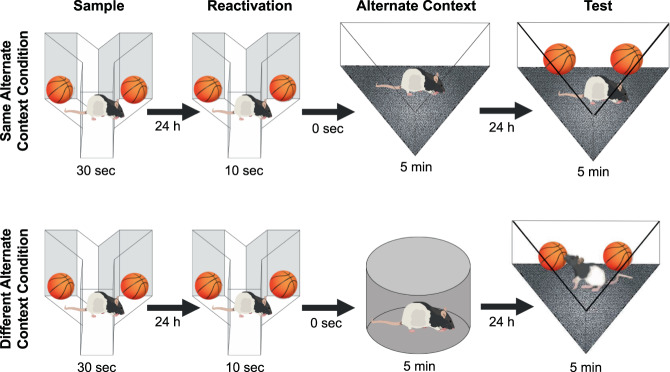
Figure 1.
Illustration of the post-reactivation object memory modification (PROMM) task. The rat samples a pair of identical objects in a Y-apparatus, and the object memory is reactivated 24 h later with a brief re-exposure to the same objects in the same Y-apparatus. After memory reactivation in the Y-apparatus, the rat is immediately placed into an empty alternate context to which it has previously been habituated, but where it has never explored objects. In the test phase, 24 h after memory reactivation, the rat is given 5 min to explore the sample objects in one of two alternate contexts: either the alternate context explored immediately after reactivation (‘same alternate context’ condition; top of figure) or another previously habituated alternate context not presented on that trial (‘different alternate context’ condition; bottom of figure).
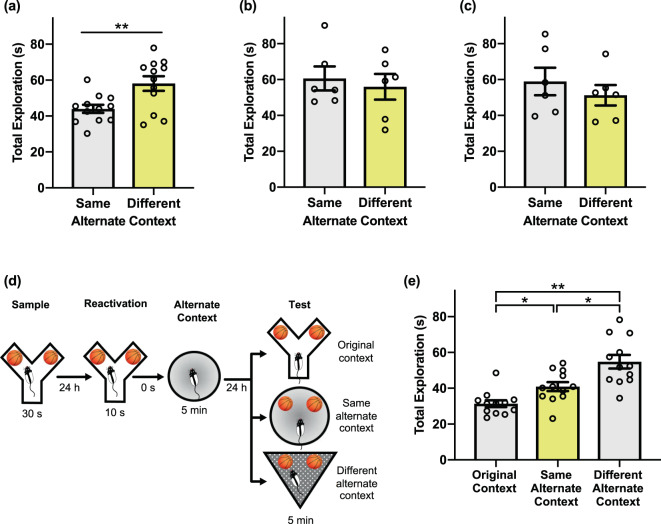
Figure 2.
Behavioural performance in the PROMM task. (a) Total exploration of the sampled objects in the test phase is greater when the objects are presented in a different apparatus than the one explored immediately after memory reactivation (n = 12). This pattern is consistent with the interpretation that rats perceive the same alternate context-object configuration as more familiar than the different alternate context-object configuration. (b) When the reactivation phase is omitted, there is no distinction between test phase exploration of the same alternate context and different alternate context condition (n = 12). (c) There is no difference in test phase object exploration between the alternate context conditions when the alternate arena is presented 6 h after object memory trace reactivation (n = 12). (d) Memory for the original learning was assessed. In the test phase, rats explored the sample objects in either the original context (i.e. the Y-apparatus), the same alternate context as post-reactivation, or a different alternate context (n = 12). (e) Memory for the original object-context configuration appears to be intact when exploration of the sample objects is measured in the Y-apparatus during the test phase. Object exploration in the Y-apparatus during test is lower than in either the different alternate context or the same alternate context, indicating that the original object memory is not detrimentally affected by post-reactivation updating. Object exploration in the same alternate context condition was lower than object exploration in the different alternate context, replicating the effect reported in (a). *p < 0.05, **p < 0.01.
Next, we looked to confirm that this apparent object memory updating is indeed reliant on memory trace reactivation. To this end, we again ran the PROMM task, but with the reactivation phase omitted. In the absence of explicit object memory reactivation, there was no statistical difference between object exploration in the same alternate context condition and the different alternate context condition, t(10) = 0.476, p = 0.645 (Fig. 2b).
It is generally accepted that memory modification must occur within a distinct window of time following reactivation, before the memory trace is reconsolidated and no longer modifiable5. To assess the time-dependency of the updating effect in the PROMM task, we ran the task as above but with the empty alternate context presented 6 h post-reactivation, presumably outside of the reconsolidation window. An independent samples t-test revealed that postponing the presentation of the alternate contextual information abolished the behavioural effect, and there was no statistical difference between test phase object exploration in the same and different alternate context conditions, t(10) = 0.806, p = 0.439 (Fig. 2c).
A key characteristic of the PROMM task is its demonstration of reactivation-based updating, as opposed to reactivation-induced “erasure”. One objective of the PROMM task is to update the original memory with new information while keeping the original memory intact. We therefore aimed to verify that the original object memory established in the sample phase of the PROMM task could still be successfully retrieved after the object memory was modified following the reactivation phase. To investigate this, we measured exploration of the objects on test day in the Y-apparatus, the original context in which the objects were sampled (Fig. 2d,e). A repeated measures one-way analysis of variance (ANOVA) revealed a significant effect of context, F(2,22) = 19.840, p < 0.001. As previously shown, exploration of the sample objects in the same alternate context was significantly lower than exploration of the sample objects in the different alternate context, t(11) = −2.927, p = 0.014. Moreover, total exploration in the original sample context was significantly lower than exploration in both the same alternate context, t(11) = −4.484, p = 0.001, and the different alternate context, t(11) = −5.930, p < 0.001. Thus, the object-context configuration including the original context from the sample phase of the task (i.e., the Y-apparatus) was treated as the most familiar; this is perhaps not surprising because the rats are exposed to this configuration explicitly during both the sample phase and the reactivation phase.
Object memory updating in the PROMM task requires proteasome activity
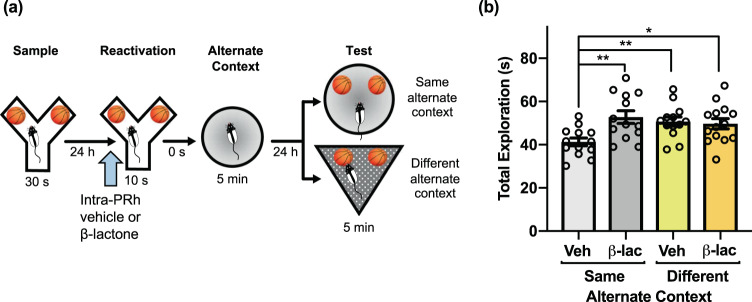
We next aimed to confirm the necessity of UPS activity for the observed memory updating effect. As mentioned, proteasomes of the UPS are suspected to facilitate the synaptic degradation that it posited to underlie memory destabilization14, and we have previously implicated UPS activity in object memory labilization30. In order to verify that UPS activation is similarly important for the object memory updating measured by the PROMM task, we evaluated the effect of blocking 26 S proteasome activity prior to memory reactivation directly within PRh using pre-reactivation intra-PRh microinjections of the proteasome inhibitor clasto-lactacystin β-lactone (β-lac). This prevented the reduction in object exploration typically seen in the same alternate context condition on test day (Fig. 3). A 2 × 2 factorial ANOVA revealed a significant interaction between the alternate context conditions (same, different) and drug conditions (vehicle, β-lac), F(1,49) = 7.025, p = 0.011. Further, a main effect of drug was revealed, F(1,49) = 4.996, p = 0.030. Importantly, rats in the vehicle/same alternate context group had significantly lower test phase object exploration compared to subjects in the β-lac/same alternate context condition, t(20.148) = −3.340, p = 0.003. Rats in the vehicle/same alternate context condition also had lower exploration than rats in the vehicle/different alternate context condition, t(24) = −3.337, p = 0.003, and β-lac/different alternate context condition, t(25) = −2.775, p = 0.010.
Figure 3.
Proteasome inhibition in PRh prevents object memory updating in the PROMM task. (a) Intracranial PRh microinjections (blue arrow) of the proteasome inhibitor β-lactone or its vehicle were administered immediately prior to object re-exposure (n = 53). (b) Pre-reactivation β-lactone reversed the typical reduction in object exploration in the same context test condition. The β-lactone/same alternate context condition had greater object exploration than the vehicle/same alternate context condition. Rats in the different alternate context conditions, regardless of drug, also displayed increased object exploration. *p < 0.05, **p < 0.01.
Systemic mAChR antagonism prevents object memory updating in the PROMM task
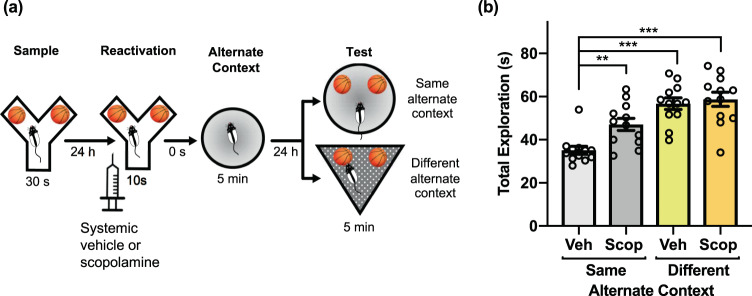
We previously demonstrated that mAChR activity is necessary for object memory destabilization29,30. To investigate the importance of this cholinergic mechanism for reactivation-based object memory modification, we tested the effects of pre-reactivation mAChR antagonism on the apparent object memory updating observed in the PROMM task. Systemic injections of the mAChR antagonist scopolamine hydrobromide prior to the memory reactivation phase prevented the object memory updating effect (Fig. 4). A mixed measures ANOVA, with the drug conditions (vehicle, scopolamine) given within-subjects and the context conditions (same, different) implemented between-subjects, revealed a significant interaction between drug and context conditions, F(1,22) = 5.18, p = 0.033. There was a main effect of drug, F(1,22) = 10.09, p = 0.004, and a main effect of context, F(1,22) = 28.12, p < 001. In the same alternate context condition, rats administered vehicle had significantly less total object exploration in the test phase compared with rats that received scopolamine, t(11) = −4.04, p = 0.002. Further, rats in the vehicle/same alternate context condition also had significantly less test phase total object exploration than the vehicle/different alternate context condition, t(22) = −6.64, p < 0.001, and scopolamine/different alternate context condition, t(22) = −6.23, p < 0.001. There was no statistical interaction in the sample phase exploration, but there was a main effect of context, F(1,22) = 151.87, p < 0.001. However, the different alternate context conditions had greater exploration in sample phase, and yet still explored the objects more in the test phase compared to the same alternate context groups. Also, there was a main effect of drug in reactivation exploration, F(1, 22) = 12.38, p = 0.002. In reactivation, the group means of exploration differed by a fraction of a second, so this difference in reactivation exploration is likely not responsible for differences in test phase performance.
Figure 4.
Muscarinic receptor blockade disrupts object memory updating in the PROMM task. (a) Intraperitoneal injections of scopolamine hydrobromide or saline were given 20 min before the reactivation phase (n = 24). (b) Rats in the same alternate context condition that received pre-reactivation scopolamine displayed greater object exploration in the test phase, similar to the different alternate context conditions, compared to the vehicle/same alternate context condition. **p < 0.01, ***p < 0.001.
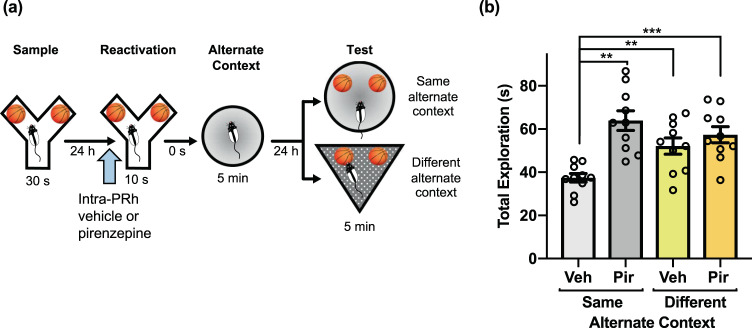
M1 mAChR subtype activation in PRh is necessary for object memory updating
To further evaluate the cholinergic mechanism that facilitates constructive object memory modification, we assessed the effect of M1 mAChR subtype antagonism on object memory updating. Intra-PRh microinjections of pirenzepine, a selective M1 receptor antagonist, administered prior to the reactivation phase of the PROMM task, prevented the updating effect (Fig. 5). A repeated measures ANOVA revealed a significant interaction between drug and context conditions, F(1,9) = 6.69, p = 0.029, as well as a main effect of drug, F(1,9) = 25.85, p = 0.001. Similar to findings with pre-reactivation scopolamine, rats in the same alternate context condition that received microinjections of pirenzepine immediately before object memory reactivation had greater total object exploration in the test phase than rats given vehicle in the same alternate context condition, t(9) = −4.56, p = 0.001. Furthermore, rats in the vehicle/same alternate context condition also had lower test phase object exploration than those in the vehicle/different alternate context, t(9) = −3.39, p = 0.008, and pirenzepine/different alternate context, t(9) = −6.20, p < 0.001, conditions.
Figure 5.
M1 mAChR activation within PRh is necessary for object memory updating in the PROMM task. (a) Intra-PRh microinjections (blue arrow) of the M1 mAChR antagonist pirenzepine or its vehicle were administered immediately prior to object memory reactivation in the Y-apparatus (n = 10). (b) Object exploration in the vehicle/same alternate context condition was lower than object exploration in the pirenzepine/same alternate context condition, vehicle/different alternate context condition, and pirenzepine/different alternate context condition. **p < 0.01, ***p < 0.001.
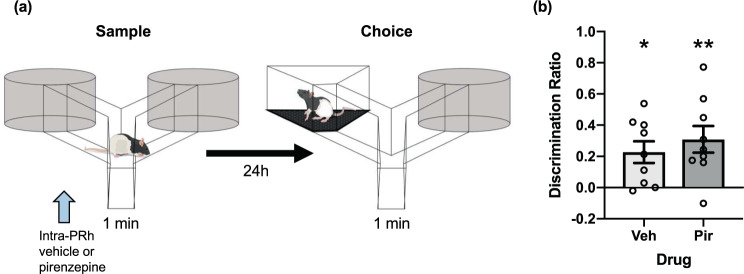
Though it is a well-supported notion that contextual information processing in rats typically depends on the hippocampus rather than PRh32,33, we next performed an experiment to assess the possibility that pre-reactivation intra-PRh M1 mAChR antagonism might interfere with encoding of the post-reactivation alternate context information in the PROMM task. We designed a context recognition task using a procedure similar to the traditional spontaneous object recognition paradigm31. Here, rats explored two identical contexts in the sample phase and then, after a 24-h retention delay, were given a choice between the sampled context and a novel context (see Methods; Fig. 6a). We administered intra-PRh pirenzepine (or vehicle) before the sample phase to determine whether this would interfere with encoding or acquisition processes necessary for subsequent context recognition. Pre-sample intra-PRh M1 mAChR blockade did not impair context recognition memory in the choice phase (Fig. 6b). One sample t-tests revealed that rats explored the novel context at greater than chance levels in the pirenzepine group, t(8) = 3.61, p = 0.007, and the vehicle group, t(8) = 3.28, p = 0.011. Furthermore, an independent samples t-test showed no difference between drug groups, t(16) = −0.74, p = 0.471.
Figure 6.
M1 mAChR blockade within PRh does not disrupt contextual recognition memory. (a) Diagram of the procedure for the context recognition task. Rats study two identical contexts that are attached to arms of a Y-apparatus. After a 24-h delay, rats are presented one of the sample contexts and one novel context. Greater time spent exploring the novel context was considered indicative of intact memory for the sampled context. Intra-PRh microinjections (blue arrow) of pirenzepine or vehicle were administered immediately prior to the sample phase of the context recognition task (n = 18). (b) The choice discrimination ratio of the vehicle group and the pirenzepine group were both significantly greater than zero and did not differ from one another. *p < 0.05, **p < 0.01.
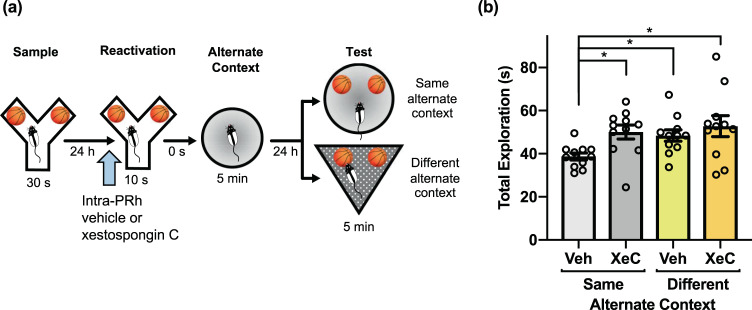
IP3 receptor blockade in PRh prevents object memory updating in the PROMM task
The second messenger IP3, which can be stimulated by M1 receptor activation, binds to receptors on the endoplasmic reticulum to prompt release of intracellular Ca2+ stores32. We have previously provided evidence that this process is necessary for M1 receptor-induced object memory destabilization30, and the resultant rise in Ca2+ (and presumptive CaMKII mobilization) is likely a critical step in the activation of the UPS12,33,34. Accordingly, in the next experiment, intra-PRh microinjections of the IP3R antagonist xestospongin C (XeC) prior to object memory reactivation blocked object memory updating (Fig. 7). A repeated measures ANOVA did not indicate a significant interaction. There were, however, main effects of drug, F(1,10) = 5.33, p = 0.044, and context, F(1,10) = 5.18, p = 0.046. The vehicle/same alternate context condition displayed significantly lower test phase object exploration compared to the XeC/same alternate context condition, t(10) = −3.18, p = 0.01. Further, the vehicle/same alternate context condition had lower test phase exploration than the vehicle/different alternate context, t(10) = −3.30, p = 0.008, and XeC/different alternate context, t(10) = −3.05, p = 0.012, conditions. There were no statistical differences between the sample phase exploration of any condition, but there was a significant main effect of context in reactivation phase exploration, F(1,10) = 5.29, p = 0.044. However, the reactivation phase exploration means differed by less than a second, so this small difference in exploration is likely not critical for test phase performance.
Figure 7.
Activation of intracellular IP3 receptors is required for reactivation-based memory updating in the PROMM task. (a) Microinjections (blue arrow) of the IP3 receptor antagonist XeC or its vehicle were administered into PRh 20 min before memory reactivation in the Y-apparatus (n = 11). (b) Rats administered pre-reactivation XeC displayed greater exploration in the same alternate context condition compared to rats in the vehicle/same alternate context condition. Rats in the different alternate context condition displayed greater object exploration than the vehicle/same alternate context condition, regardless of drug. *p < 0.05.
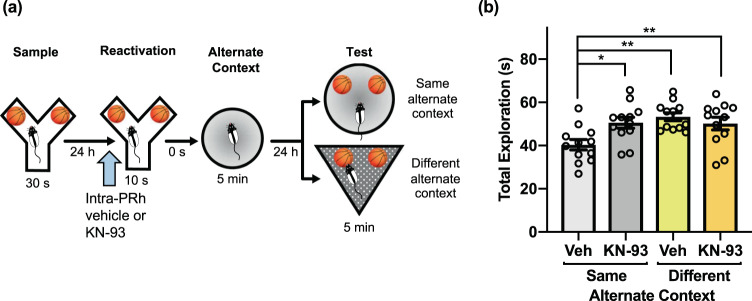
CaMKII activity in PRh is critical for object memory updating in the PROMM task
Elevated intracellular Ca2+ activates CaMKII, which is involved in the phosphorylation and translocation of proteasomes to the synapse12,33,34. To investigate whether CaMKII might be involved in connecting M1 mAChR activation to UPS activity for reactivation-induced object memory updating, we assessed whether inhibition of CaMKII during reactivation would prevent memory updating observed in the PROMM task. Intra-PRh microinjections of the CaMKII inhibitor KN-93 blocked object memory updating (Fig. 8). A repeated measures ANOVA indicated a significant interaction, F(1,11) = 5.19, p = 0.044, and a main effect of context, F(1,11) = 17.67, p = 0.001. Rats in the vehicle/same alternate context condition had lower exploration than the KN-93/same alternate context condition, t(11) = −2.86, p = 0.015. In addition, rats in the vehicle/same alternate context condition had lower test phase object exploration than rats in the vehicle/different alternate context condition, t(11) = −3.90, p = 0.002, and KN-93/different alternate context condition, t(11) = −4.46, p = 0.001.
Figure 8.
Inhibition of intracellular CaMKII within PRh blocks apparent memory updating in the PROMM task. (a) Microinjections (blue arrow) of KN-93, the CaMKII inhibitor, or its vehicle were administered into PRh immediately prior to memory reactivation in the Y-apparatus (n = 12). (b) Rats in the KN-93/same alternate context condition had greater object exploration in test phase compared to rats in the vehicle/same alternate context condition. Rats in the different alternate context condition had elevated test phase object exploration, regardless of drug condition. *p < 0.05, **p < 0.01.
Discussion
Here we present a novel rodent paradigm that can be used to assess post-reactivation, qualitative updating of previously established object memories. Using the PROMM task, we demonstrate that novel contextual information appears to become incorporated into an existing object memory when presented following reactivation of that memory. Similar to analogous work in humans on object memory updating28, this memory modification is constructive and reactivation- and time-dependent. Furthermore, consistent with our previous work on object memory destabilization in rats, the current object memory modification effect requires cholinergic signaling and UPS activation within PRh. To our knowledge, this is the first direct demonstration of cholinergic system involvement in this form of memory updating.
While our group has previously implicated ACh and downstream signaling molecules in destabilization of object memories29,30, designing a new reconsolidation-mediated object memory updating task for rodents was a critical next step to address the involvement of this mechanism in memory updating, specifically. Tasks illustrating retrieval-related amnesia in rodents have contributed significantly to the study of neural mechanisms underlying reconsolidation, and this kind of memory modification likely has relevance to understanding long-term memory interference; however, weakening or ‘erasure’ represent only a sub-set of memory changes. Other forms of memory updating likely involve reactivation-induced integration of relevant information to maintain the adaptiveness of the memory network6. Indeed, there is evidence of reactivation-dependent, time-sensitive, and constructive declarative memory updating in humans28. The present results are highly suggestive of similar memory modification in rodents, and the PROMM task should prove valuable in continuing to uncover the neurobiological mechanisms responsible for this important cognitive function.
We developed the PROMM task to demonstrate information integration into a consolidated memory trace within the transient post-reactivation reconsolidation ‘window’ in rodents. Whereas other recently published memory modification tasks have produced informative results by presenting ‘updating’ information simultaneously with a reminder cue at the time of reactivation25–27, the present study appears to be the first to demonstrate constructive memory modification during the post-reactivation period. A distinct reactivation session, absent of explicit updating information, is a noteworthy adjustment compared to past memory updating tasks, as this procedure enables analysis of reactivation/destabilization mechanisms separately from mechanisms for processing the updating information. Moreover, the current design is better suited to address a central tenet of reconsolidation theory, which specifies a time-dependent window of lability – and hence modifiability – following memory reactivation and is also less likely to support alternative explanations in terms of novel learning episodes merely producing new memories that compete with the original memory35.
Similar to the methodology used by Hupbach and colleagues (2007)28 in humans, the current results appear to demonstrate constructive object memory updating in a rodent model. Importantly, in the PROMM task, rats displayed familiarity for the sample object-context configuration during the test phase, indicating that the original memory trace remained intact after updating the memory trace with new contextual information. Correspondingly, Kwapis et al.27 recently developed a reconsolidation-based object-location memory updating task, demonstrating that both the original object location and an updated object location were treated as familiar compared to a novel object location. Together, the Kwapis et al.27 findings and the current results bode well for use of such rodent models to study the neural mechanisms of long-term memory modification and information integration. Interestingly, in the same study, Kwapis and colleagues27 reported deficits in object-location memory updating in aged mice; this could be explained by age-related decline in cholinergic system functioning36, in line with our present findings that ACh is important for reconsolidation-mediated memory updating.
Although we have taken care here to rule out alternative explanations, important parametric experiments can be done to further investigate the behavioural effects observed in the PROMM task. For example, it is likely that the amount of time in the alternate context following memory reactivation is an important variable. Future work should assess the minimum duration in the alternate context that will produce memory updating. Would the magnitude of this effect grow with additional time in the alternate context? Moreover, although it is possible that alternate context exposure might induce a nonspecific reduction in novel object exploration during the test phase, which could be tested experimentally, there does not appear to be a strong rationale for expecting such an effect. It would be unclear why such a generalized effect would be so reliant on the timing of alternative context exposure, as we show here that object memory updating is restricted to a limited time window following reactivation of the original memory. Finally, data from the ‘original context’ control experiment (Fig. 2e) strongly suggest that the original memory association between the object and the sample phase context remains intact despite post-reactivation updating with new contextual information. As such, the combined results of the control experiments run here speak to a highly specific effect of alternate context exposure on the original memory, the core of which remains nonetheless intact.
The present data are therefore consistent with the notion that the consolidated object memory is updated with the alternate context information presented during the post-reactivation period. Such a process would seem to require the object memory to enter a labile state via memory destabilization. Previously, activation of the UPS, which is involved in degradation of postsynaptic proteins, has been implicated in destabilization of various forms of memory9,14,30,37,38 and in apparent memory updating effects9,26. The current results with proteasome inhibition by ß-lactone, which blocked the object memory updating effect in the PROMM task, are consistent with this speculated mechanism of memory modification that requires reactivation-induced destabilization of the original memory trace. However, as UPS activity has also been implicated in memory acquisition39, additional work will be required to clarify the role of the UPS in the present results.
Consistent with our past work on object memory destabilization, the findings of the current study suggest involvement of an upstream mAChR signaling pathway in recruitment of the UPS for memory trace modification. We previously linked M1 mAChR activation to IP3R stimulation and subsequent UPS activity for memory destabilization in an object recognition reconsolidation task29,30. We proposed that the M1 mAChR second messenger IP3 initiates mobilization of intracellular Ca2+ stores from the endoplasmic reticulum34, and this increase in cytosolic Ca2+ could promote downstream cellular responses to propagate protein degradation. Increases in Ca2+ recruit CaMKII, which has been found to activate and translocate proteasomes to dendritic spines, likely to degrade synaptic proteins involved in memory trace stabilization12,40,41. Accordingly, the present results implicate all of the previously identified components of this pathway for destabilization in object memory updating and extend these findings to include a role for CaMKII in this process, providing a viable signaling link between M1 and IP3 receptor activation and stimulation of the UPS. Thus, the results from the present study strongly suggest that an M1 mAChR-initiated mechanism for memory destabilization is necessary for qualitative modification of cortical memory representations. Although the current findings, as well as our previous results regarding object memory destabilization29,30, strongly implicate M1 mAChRs, including with the use of a highly selective M1 mAChR agonist (CDD-0102A) in the previous studies, the possibility remains that other M1-like receptors are involved. As such, future work should investigate the potential involvement of either M3 or M5 mAChRs in the object memory destabilization/updating process.
The numerous effects we report here on memory updating result from administration of various drugs before the reactivation phase. A natural question therefore arises as to whether these effects could be related to drug effects on either memory retrieval during the reactivation phase and/or acquisition or encoding of the updating information presented in the alternate context. To the former point, we have previously assessed the effects of pre-reactivation scopolamine in the context of an object memory destabilization study29 using the exact behavioural parameters used here. In that experiment, a novel object was presented during the reactivation phase along with the original sample object. Pre-reactivation scopolamine, while appearing to prevent memory destabilization, did not however disrupt preference for the novel object in the reactivation phase, consistent with intact retrieval and recognition of the sample object. Thus, it seems unlikely that scopolamine or pirenzepine in the present study exerted their effects by preventing memory retrieval. Moreover, others have argued, with convincing data from object memory tasks, that memory retrieval is not necessary to initiate the reconsolidation process42.
The context recognition control study (Fig. 6) also addresses an important alternative interpretation. This experiment indicates that intra-PRh pirenzepine, in the dose that blocked memory updating, does not appear to disrupt acquisition of contextual information in a manner sufficient to impair recognition of that context 24 h later. This result is consistent with a large literature indicating that PRh is primarily involved in object, and not spatial or contextual, memory processing24,32,43–46. Furthermore, although mAChRs are widely acknowledged to regulate acquisition of novel information, their antagonism does not typically impair encoding or recognition of previously encountered stimuli47–50. In the current study, all rats were habituated to the alternate contexts at the start of each experiment. Thus, although the association between the alternate context and sample object is ‘novel’ within the structure of a PROMM trial, the specific features of the alternate contexts should not have been novel and therefore not necessarily sensitive to muscarinic antagonism or disruption of the various other mechanistic components targeted by the other drugs used. Thus, it seems unlikely that the effects reported here are due to drug-induced blockade of contextual information acquisition. Rather, it seems that the mechanism we have previously identified as important for object memory destabilization29,30, is in fact necessary for the updating of said object memories.
Furthermore, the manipulations used in the current study have previously been demonstrated to target object memory destabilization specifically, not reconsolidation. In our previous memory destabilization studies, we have used identical parameters, drug doses and infusion protocols, and have no convincing evidence that any of the drugs used in the current study prevent object memory reconsolidation when administered before the reactivation phase29,30. Quite the contrary, when pre-reactivation administration of any of these drugs is combined with post-reactivation vehicle, object recognition is intact 24 h later; however, pairing any of these drugs pre-reactivation with post-reactivation anisomycin prevents the amnesic effects of the latter30. Thus, it seems highly unlikely that the effects reported here resulted from drug-induced reconsolidation blockade. Similarly, although there is strong evidence for state-dependent drug effects explaining apparent reconsolidation phenomena51,52, these same past experiments from our group speak clearly against such an explanation for the present results. In these studies, the same parameters and drugs used here, given pre-reactivation, did not disrupt object memory when rats were tested in a drug-free state 24 h later29,30.
Thus, ACh appears to act as a neuromodulator of object memory updating, but it is likely not the only classical neurotransmitter to be involved in memory trace remodelling. Prediction error and other forms of salient novelty at the time of memory reactivation are strongly implicated in memory destabilization under certain conditions53,54, perhaps to signal the opportunity for memory updating. In addition to past work implicating ACh in novelty-regulated memory destabilization, there appears to be a strong role for dopamine in responding to prediction error in various reconsolidation paradigms55–58. Thus, further investigation is required to delineate the specific roles of various neurotransmitter systems in memory destabilization and modification. The PROMM task should prove highly valuable for such studies. It will also be essential to develop complementary paradigms to investigate whether similar memory updating effects are observed for different forms of learning and memory and whether these changes rely similarly on the cholinergic mechanism implicated for object memory in the current study.
In conclusion, here we present a novel behavioural paradigm that should greatly facilitate the study of the dynamics of long-term memory storage and modification. As this task is based on the object recognition paradigm, it should produce results that could more feasibly apply to human declarative memory processes compared to similar findings that might be reported using fear conditioning or other tasks that predominate in the reconsolidation field. A rodent model with relevance to human declarative memory should prove essential for studying the basis of normal declarative memory change with time, as well as integration of inaccuracies and irrelevant information, processes that likely contribute to development of false and interfering memories. The present study provides a significant first foray into the analysis of the neurobiological basis of memory updating by providing compelling evidence for an important role of cortical cholinergic transmission in integrative object memory updating. This, to our knowledge, represents the first direct demonstration of this cognitive function for ACh, which has long been implicated in other aspects of learning and memory. Should this mechanism generalize to other forms of memory, such as fear conditioning, it could open important avenues for potential treatment of human disorders characterized by intrusive maladaptive memories, as cholinergic drugs are well established and tolerated for various other human disorders. Moreover, the current findings are potentially relevant to other aspects of human cognitive dysfunction more directly related to reduction in cholinergic system efficacy, such as in Alzheimer’s disease and normal aging59,60; both are characterized by severe learning and memory deficits, as well as cognitive inflexibility. The current results suggest that a subtler impairment in the updating of established long-term memories, rather than an exclusive disruption of new learning, could contribute to these symptoms. The data presented here, along with a powerful new tool in the form of the PROMM task, should help to address many of these issues in the coming years.
Methods
Animals
Male Long Evans rats (Charles River, NY) were housed on a 12-h reverse light cycle (lights off 8:00–20:00). Behavioural testing occurred during the dark phase of the cycle. All rats were pair-housed in standard cages with minimal enrichment. Rats were handled regularly during the first week of arrival to the facility. A restricted food regimen was implemented, and behavioural testing commenced once rats reached 85% of their typical body weight (approximately 350–425 g). Rats were allotted 20 g of 18% rodent chow each day, and water was accessible ad libidum. Although object recognition tasks do not necessarily rely on food seeking for their performance, it has been consistently found that object exploratory behavior is more robust when rats are food restricted during testing61. All procedures involving the use of animals were approved by the Animal Care Committee of the University of Guelph and followed the guidelines of the Canadian Council of Animal Care.
Surgery
Bilateral PRh cannulation surgeries occurred when the rats were 250–320 g in body weight. Rats were anaesthetized with inhalant isoflurane throughout the surgery. Medicam (concentration of 5 mg/kg, subcutaneous injection) and lidocaine (20 mg/kg, subcutaneous injection at incision site) were administered prior to surgery. Baytril (5 mg/kg; intramuscular injection) was administered at the start of surgery. Rats were positioned in a stereotaxic frame (incisor bar set to −3.3 mm). A 3–4 cm incision was made across the rat’s head (anterior-to-posterior), and the skin was retracted to expose the skull. Bone screws were inserted into the surface of the skull (2 posterior to coronal suture, 2 posterior to lambdoidal suture) to secure the dental acrylic that stabilizes the guide cannula. Holes were drilled into the skull at the target coordinates for guide cannula implantation. Guide cannula placement was determined according to the following coordinates62 in reference to bregma: anteroposterior −5.5 mm, lateral ±6.6 mm, and dorsoventral −6.5 mm. Dental acrylic was applied to the surface of the skull around the cannulas. Skin was sutured around the dried dental acrylic. Dummy cannulas were inserted into the guides, extending 1.1 mm past the tip of the guide, to prevent blockage. Rats recovered in clean cages over heating pads for 1–2 h following surgery. Rats recovered for 1 week before behavioural experimentation.
Microinjection procedure
Dummy cannulas were removed immediately before the microinjection and placed on a sterilized surface. Drug infusers were connected to the end of tygon tubing that was secured to a glass 1.0 µL Hamilton syringe. Hamilton syringes were guided by a Harvard apparatus syringe pump to administer the drug through the tubing at a constant rate of 0.5 µL/min for 2 min. Drug infusers remained in the guide cannula for an additional 2-min after infusion to ensure adequate diffusion of the drug away from the infuser tips. Dummy cannulas were reinserted following microinjections. Rats were habituated to the microinjection procedure with simulated microinjections (drug infusers were inserted into the guide cannula for the same duration of time as typical microinjections, but no drug was delivered) on two separate occasions the week prior to behavioural testing.
Histology
Cannula guide placement was verified following behavioural testing (Fig. 9). Rats were anesthetized with intraperitoneal injection of 1.2 mL/kg of Euthansol (concentration of 82 mg/mL), and perfused transcardially with phosphate buffered saline (PBS), followed by 4% neutral buffered formalin. Brains were extracted and stored in 4% formalin in a 3 °C fridge for at least 24 h. Brains were transferred to a solution of 20% sucrose in PBS, and left on an orbital shaker until they sank. Brains were sliced with a cryostat to 50 μm in width, and every 3rd slide was mounted onto a gelatin-coated glass microscope slide and thionin stained.
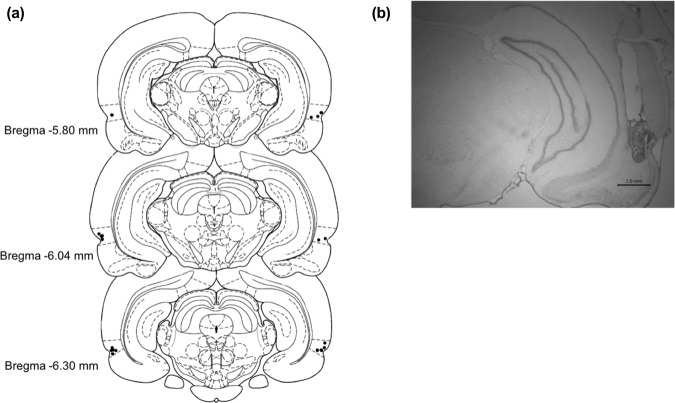
Figure 9.
Schematic representation of cannula tip placements for microinjections targeting PRh from a representative cohort of cannulated rats. (a) Cannula implantation placements of cohort used for the pirenzepine experiment of this study (n = 10). Dotted lines above and below the marked cannula placements (black dots) indicate the dorsal and ventral borders of PRh. (b) Micrograph of a typical cannula guide tract extending to PRh.
Drugs
Systemic drugs
Scopolamine hydrobromide trihydrate (Sigma Aldrich), the mAChR antagonist, was dissolved in 0.9% physiological saline at a dose of 0.8 mg/kg, and was administered with intraperitoneal injections 20-min prior to the reactivation phase in the object memory modification task.
Intracranial drugs
The M1 mAChR antagonist pirenzepine (Sigma Aldrich) was dissolved in 0.9% physiological saline at a concentration of 20 μg/μL. The irreversible 20 S proteasome inhibitor clasto-Lactacystin β-lactone (Sigma-Aldrich) was dissolved in 2% dimethyl sulfoxide (DMSO) and 1 N HCl, neutralized with NaOH and concentrated with 0.9% physiological saline to 32 ng/μL. Xestospongin C (Sigma-Aldrich), the IP3R antagonist, was dissolved in PBS and diluted to 0.3 ng/μL. The CaMKII inhibitor, KN-93 (Sigma Aldrich), was dissolved in 80% DMSO and PBS, and concentrated to 10 μg/μL. All intracranial drugs were microinjected immediately before the reactivation phase on a given PROMM trial, with the exception of XeC, which was administered 20-min prior to the reactivation phase. Vehicle for each experiment corresponded to the solvent used in the preparation of each respective drug.
Statistical analysis
Exploration bouts were manually scored in real-time using customized scoring software. All behavioural testing was video recorded for later offline rescoring. Total object exploration (left + right object exploration) was the measure used for the PROMM task. Discrimination ratio (see below) was the measure of memory for the context recognition task. An object exploration bout was initiated when the rat’s nose was within 2 cm of the object, and the bout terminated once the rat disengaged with the object. Chewing or climbing on the object were not considered object exploration. A context exploration bout started once all four of the rat’s feet entered the context and ended once the rat’s nose was outside of the context. For both tasks, exploration during the sample phases of each group was analyzed to ensure that any differences in test phase exploration were not attributable to exploration differences during acquisition. The results of these analyses are presented only in cases where they were found to be significant.
Analysis of variance (ANOVA) was used to investigate statistical interactions or main effects in 2 × 2 (alternate context condition, drug) experimental designs. One-way ANOVA was used to evaluate group differences in the Y-apparatus control experiment. Planned comparisons established prior to performing an ANOVA were evaluated with two-tailed paired-samples or independent-samples t-tests. Bonferroni correction was used to adjust the α-level accordingly for planned comparisons and post hocs. Two-tailed independent-samples t-tests analyzed differences between alternate context conditions for the PROMM task and its corresponding control experiments. In the context recognition task, one sample t-tests were used to compare choice discrimination ratio to the test value of zero (i.e., ‘chance’). A two-tailed independent-samples t-test was employed to compare the drug groups in the context recognition task. All t-tests in these experiments used α = 0.05, excluding cases employing the Bonferroni correction. All data sets were analyzed using IBM SPSS Statistics 26 software.
Post-reactivation object memory modification (PROMM) task
Rats were habituated to all contexts involved in the PROMM task for 5 min each on two occasions the week before behavioural testing. Figure 1 illustrates the procedure for the PROMM task. The post-reactivation contexts, test phase contexts, and drug conditions were all counterbalanced across trials. Each of the three apparatuses was designated to an individual testing room to ensure contexts were as distinct as possible. Contextual cues (i.e. colourful pictures of various shapes) hung on the walls of the testing rooms. The white plexiglass Y-shaped apparatus was 40 cm tall, and the arms were 27 cm long and 10 cm wide. The triangle apparatus walls were made of white corrugated plastic, with a flat black rubber floor. The posterior wall was 75 cm long and other two walls were 60 cm long, and all of the walls were 60 cm tall. The circle apparatus was made of navy-blue plastic, with a floor made of black fine-grain waterproof sand paper, reaching 48 cm in height with a diameter of 53 cm.
The PROMM task required three consecutive days of testing per trial, as it involves two 24 h delays. On the first day, during the sample phase, rats explored two identical copies of a novel object for a total of 30 s or a 3-min session, whichever occurred first, in the Y-apparatus49. Twenty-four hours later, during the reactivation phase, the object memory was reactivated with a brief re-exposure (maximum 10 s exploration or full 2-min session) to the sampled objects in the same Y-apparatus. Immediately following this object re-exposure, the rat was placed into an empty alternate context (either a circular or triangular apparatus) to freely explore for 5 min. The goal here is to manipulate the contextual information associated with the object memory; the post-reactivation context information should integrate with the labile object memory representation, thereby producing a familiarity-like response when the object and alternate context are subsequently presented together in the test phase. During the test phase, 24 h after the reactivation phase, exploration of the sampled objects was measured in either the same alternate context as the one explored post-reactivation or a different alternate context for 5 min. Drug conditions were run within-subjects for all experiments except for the β-lactone experiment; β-lactone is irreversible, so drug was a between-subjects factor for this experiment. All rats were run on one trial per condition, except in the scopolamine and KN-93 experiments, in which all rats were run on two trials per condition to reduce variability.
Rodents preferentially explore novel stimuli more than familiar stimuli31, so greater test phase object exploration in one condition compared to the other was considered reflective of novelty-induced preferential exploration, and a reduction in this exploration was taken as an index of a familiarity response to the object-context configuration.
Context recognition task
A modified Y-apparatus was constructed with white corrugated plastic, matching dimensions of the Y-apparatus used in the PROMM task. The back wall of each exploratory arm was open, forming an archway leading to an attachable context. Circle and triangle arenas were used as context stimuli, similar to the alternate contexts used for the PROMM task (see Fig. 6). The circle arena had navy-blue plastic walls reaching 45 cm in height with a diameter of 48 cm, and a floor of black fine-grain waterproof sand paper. The triangle arenas had 60 cm high walls made of white corrugated plastic, and a dark rubber mat as the floor. The posterior wall was 68 cm in length, and the two adjacent walls were 53 cm long. Coloured tape was used to add distinct contextual features to the inside walls of the arenas. Rats were habituated to the Y-apparatus for two sessions the week before testing.
A trial in the context recognition task consisted of a sample phase, delay, and choice phase. In the sample phase, rats studied two identical contexts (for 60 s total exploration or the full 3-min session), each attached to an arm of the open-ended Y-apparatus. After a 24-h retention delay, rats were presented one sampled context and one new context in the choice phase (2-min session). Novel side and novel context were counterbalanced. Drug groups were run as a between-subjects condition. Choice discrimination ratio [(novel context exploration - sample context exploration)/total exploration] was the index of recognition memory. Intact context memory was indicated by a discrimination ratio statistically greater than zero (equal novel and sample context exploration generates a discrimination ratio of 0; i.e., ‘chance’). The first minute of exploration in the choice phase was used for discrimination ratio calculation. Rats preferentially explore novel stimuli, so those with intact memory should spend more time in the novel context than the familiar context.
Acknowledgements
Funding for this research was provided by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to B.D.W. (400176), as well as an NSERC Canada Graduate Scholarship-Doctoral award to C.E.W. and a Canada Graduate Scholarship-Master’s award to K.H.J. Images in Figures 1, 2d, 3a, 4a, 6a, 7a, 8a and 9a drawn by K.H.J. for this manuscript.
Author contributions
K.H.J. and C.E.W. contributed equally to the study; K.H.J., C.E.W., C.M., C.S., D.O. and K.A.M. performed experiments; K.H.J., C.E.W. and B.D.W. performed data analysis; K.H.J., C.E.W. and B.D.W. designed the study; K.H.J., C.E.W. and B.D.W. wrote the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kristen H. Jardine and Cassidy E. Wideman.
References
- 1.McGaugh JL. Memory - a century of consolidation. Science (80-.). 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 2.Dudai Y. The Neurobiology of Consolidations, Or, How Stable is the Engram? Annu. Rev. Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 3.Misanin JR, Miller RR, Lewis DJ. Retrograde Amnesia Produced by Electroconvulsive Shock after Reactivation of a Consolidated Memory Trace Author (s): James R. Misanin, Ralph R. Miller and Donald J. Lewis Published by: American Association for the Advancement of Science Stable URL. Science (80-.). 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- 4.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 5.Nader K. Memory Traces Unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 6.Lee JLC. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Nader K, Schiller D. An Update on Memory Reconsolidation Updating. Trends Cogn. Sci. 2017;21:531–545. doi: 10.1016/j.tics.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JLC. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat. Neurosci. 2008;11:1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki A, et al. Memory Reconsolidation and Extinction Have Distinct Temporal and Biochemical Signatures. J. Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamou CB, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat. Neurosci. 2006;9:1237–1239. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- 12.Jarome, T. J., Werner, C. T., Kwapis, J. L. & Helmstetter, F. J. Activity dependent protein degradation is critical for the formation and stability of fear memory in the amygdala. PLoS One6 (2011). [DOI] [PMC free article] [PubMed]
- 13.Lee J, Everitt B, Thomas K. Independent Cellular Processes for Hippocampal Memory Consolidation and Reconsolidation. Science (80-.). 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 14.Lee S-H, et al. Synaptic protein degredation underlies destabilization of retrieved fear memory. Science (80-.). 2008;319:1253–1256. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- 15.Lee JLC, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Loftus EF. The Malleability of Human. Memory. Am. Sci. 1979;67:312–320. [PubMed] [Google Scholar]
- 17.Schacter DL. The Cognitive Neuropsychology of Falso Memories. Cogn. Neuropscyhology. 1999;12:193–195. [Google Scholar]
- 18.Dudai, Y. The Engram Revisited: On the Elusive Permanence of Memory. Mem. Process 29–39, 10.7551/mitpress/9780262014571.003.0002 (2010).
- 19.James, W. The Principles of Psychology. (Holt, 1890).
- 20.Zhu B, et al. Brief Exposure to Misinformation Can Lead to Long-Term False Memories. Appl. Cogn. Psychol. 2012;26:301–307. [Google Scholar]
- 21.Elsey JWB, Van Ast VA, Kindt M. Human memory reconsolidation: A guiding framework and critical review of the evidence. Psychol. Bull. 2018;144:797–848. doi: 10.1037/bul0000152. [DOI] [PubMed] [Google Scholar]
- 22.Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978;273:297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- 23.Mumby, D. G. & Pinel, J. P. J. Rhinal Cortex Lesions and Object Recognition in Rats. 108, 11–18 (1994). [DOI] [PubMed]
- 24.Winters BD, Saksida LM, Bussey TJ. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci. Biobehav. Rev. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Rossato J, et al. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn. Mem. 2007;14:36–46. doi: 10.1101/lm.422607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi J-H, Kim J-E, Kaang B-K. Protein synthesis and degradation are required for the incorporation of modified information into the pre-existing object-location memory. Mol. Brain. 2010;3:1. doi: 10.1186/1756-6606-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwapis, J. L. et al. Aging mice show impaired memory updating in the novel OUL updating paradigm. Neuropsychopharmacology 1–10, 10.1038/s41386-019-0438-0 (2019). [DOI] [PMC free article] [PubMed]
- 28.Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: A subtle reminder triggers integration of new information. Learn. Mem. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stiver ML, et al. Cholinergic manipulations bidirectionally regulate object memory destabilization. Learn. Mem. 2015;22:203–214. doi: 10.1101/lm.037713.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stiver ML, et al. Linking muscarinic receptor activation to UPS-mediated object memory destabilization: Implications for long-term memory modification and storage. Neurobiol. Learn. Mem. 2017;145:151–164. doi: 10.1016/j.nlm.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Ennaceur A, DeLacour J. A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. non-spatial working memory. Behav. Brain Res. 1988;51:83–92. doi: 10.1016/s0166-4328(05)80315-8. [DOI] [PubMed] [Google Scholar]
- 32.Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double Dissociation between the Effects of Peri-Postrhinal Cortex and Hippocampal Lesions on Tests of Object Recognition and Spatial Memory: Heterogeneity of Function within the Temporal Lobe. J. Neurosci. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aggleton JP, Brown MW. Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. Q. J. Exp. Psychol. 2005;58B:218–233. doi: 10.1080/02724990444000131. [DOI] [PubMed] [Google Scholar]
- 34.Felder CC. Muscarini acetylcholine receptors: signal transduction through multiple effectors. Faseb J. 1995;9:619–625. [PubMed] [Google Scholar]
- 35.Duvarci S, Nader K. Characterization of fear memory reconsolidation. J. Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terry AVJ, Buccafusco JJ. The Cholinergic Hypothesis of Age and Alzheimer’s Disease- Related Cognitive Deficits: Recent Challenges and Their Implications for Novel Drug Development. Perspect. Pharmacol. 2003;306:821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- 37.Kaang B-K, Choi J-H. Protein Degradation during Reconsolidation as a Mechanism for Memory Reorganization. Front. Behav. Neurosci. 2011;5:2. doi: 10.3389/fnbeh.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fustiñana, M. S., De La Fuente, V., Federman, N., Freudenthal, R. & Romano, A. Erratum: Protein degradation by ubiquitin-proteasome system in formation and labilization of contextual conditioning memory. Learn. Mem. 21, 478–487 (2014). [DOI] [PMC free article] [PubMed]
- 39.Jarome TJ, Helmstetter FJ. The ubiquitin-proteasome system as a critical regulator of synaptic plasticity and long-term memory formation. Neurobiol. Learn. Mem. 2013;105:107–116. doi: 10.1016/j.nlm.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarome TJ, Ferrara NC, Kwapis JL, Helmstetter FJ. CaMKII regulates proteasome phosphorylation and activity and promotes memory destabilization following retrieval. Neurobiol. Learn. Mem. 2016;128:103–109. doi: 10.1016/j.nlm.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bingol B, et al. Autophosphorylated CaMKIIα Acts as a Scaffold to Recruit Proteasomes to Dendritic Spines. Cell. 2010;140:567–578. doi: 10.1016/j.cell.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 42.Balderas I, Rodriguez-Ortiz CJ, Bermudez-Rattoni F. Retrieval and reconsolidation of object recognition memory are independent processes in the perirhinal cortex. Neuroscience. 2013;253:398–405. doi: 10.1016/j.neuroscience.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 44.Brown MW, Barker GRI, Aggleton JP, Warburton EC. What pharmacological interventions indicate concerning the role of the perirhinal cortex in recognition memory. Neuropsychologia. 2012;50:3122–3140. doi: 10.1016/j.neuropsychologia.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray EA, Bussey TJ, Saksida LM. Visual Perception and Memory: A New View of Medial Temporal Lobe Function in Primates and Rodents. Annu. Rev. Neurosci. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- 46.Lee ACH, et al. Differentiating the roles of the hippocampus and perirhinal cortex in processes beyond long-term declarative memory: A double dissociation in dementia. J. Neurosci. 2006;26:5198–5203. doi: 10.1523/JNEUROSCI.3157-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang YI, Mishkin M, Aigner TG. Effects of muscarinic blockade in perirhinal cortex during visual recognition. Proc. Natl. Acad. Sci. USA. 1997;94:12667–12669. doi: 10.1073/pnas.94.23.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warburton E, et al. Cholinergic Neurotransmission is Essential for Perirhinal Cortical Plasticity and Recognition Memory. Neuron. 2003;38:987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- 49.Winters BD, Saksida LM, Bussey TJ. Paradoxical Facilitation of Object Recognition Memory after Infusion of Scopolamine into Perirhinal Cortex: Implications for Cholinergic System Function. J. Neurosci. 2006;26:9520–9529. doi: 10.1523/JNEUROSCI.2319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aigner TG, Walker DL, Mishkin M. Comparison of the effects of scopolamine administered before and after acquisition in a test of visual recognition memory in monkeys. Behav. Neural Biol. 1991;55:61–67. doi: 10.1016/0163-1047(91)80127-z. [DOI] [PubMed] [Google Scholar]
- 51.Gisquet-Verrier P, et al. Integration of New Information with Active Memory Accounts for Retrograde Amnesia: A Challenge to the Consolidation/Reconsolidation Hypothesis? J. Neurosci. 2015;35:11623–11633. doi: 10.1523/JNEUROSCI.1386-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Osorio-Gómez D, Saldivar-Mares KS, Perera-López A, McGaugh JL, Bermúdez-Rattoni F. Early memory consolidation window enables drug induced state-dependent memory. Neuropharmacology. 2019;146:84–94. doi: 10.1016/j.neuropharm.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 53.Exton-McGuinness MTJ, Lee JLC, Reichelt AC. Updating memories-The role of prediction errors in memory reconsolidation. Behav. Brain Res. 2015;278:375–384. doi: 10.1016/j.bbr.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Winters BD, Tucci MC, DaCosta-Furtado M. Older and stronger object memories are selectively destabilized by reactivation in the presence of new information. Learn. Mem. 2009;16:545–553. doi: 10.1101/lm.1509909. [DOI] [PubMed] [Google Scholar]
- 55.Rossato JI, et al. State-dependent effect of dopamine D1/D5receptors inactivation on memory destabilization and reconsolidation. Behav. Brain Res. 2015;285:194–199. doi: 10.1016/j.bbr.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Wideman, C. E., Jardine, K. H. & Winters, B. D. Involvement of classical neurotransmitter systems in memory reconsolidation: Focus on destabilization. Neurobiol. Learn. Mem. 156 (2018). [DOI] [PubMed]
- 57.Reichelt AC, Exton-McGuinness MT, Lee JLC. Ventral Tegmental Dopamine Dysregulation Prevents Appetitive Memory Destabilization. J. Neurosci. 2013;33:14205–14210. doi: 10.1523/JNEUROSCI.1614-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flavell CR, Lee JLC. Dopaminergic D1 receptor signalling is necessary, but not sufficient for cued fear memory destabilisation. Psychopharmacology (Berl). 2019;236:3667–3676. doi: 10.1007/s00213-019-05338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bartus RT. On neurodegenerative diseases, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 60.Perry E, et al. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Int. J. Geriatr. Psychiatry. 1996;11:765–771. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ennaceur A. One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav. Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 62.Paxinos, G. & Charles, W. The Rat Brain in Stereotaxic Coordinates, 10.1017/CBO9781107415324.004 (Academic Press, 1998).