Abstract
Summit Lake, Nevada (USA) is the last high-desert terminal lake to have a native self-sustaining population of threatened Lahontan cutthroat trout (Oncorhynchus clarkii henshawi). From spring 2015 to fall 2017, we quantified adult abundance and survival and the total annual spawning run. Abundance and survival were estimated with mark-recapture using PIT tags, and the annual spawning run was estimated with PIT tag detections and counts of spawners. Adult abundance fluctuated from 830 (95% CI 559–1248) to 1085 (95% CI 747–1614), with no overall temporal trend, as a decrease in male abundance was generally offset by an equal increase in female abundance. Estimated mean adult survival was 0.51 (95% CI 0.44–0.58). The spawning run increased from 645 (2015) to 868 (2016), but then decreased slightly to 824 (2017, mean = 789 ± 118). Female spawners increased in 2016 but decreased slightly in 2017, whereas male spawners decreased each year. In addition, the proportion of adults that spawned each year increased overall. Our study suggests that the adult population remained stable although most of the study period included the recent, severe regional drought in the western United States (2012–2016).
Subject terms: Conservation biology, Population dynamics, Ecology
Introduction
Amid the backdrop of global biodiversity decline, North American freshwater fauna is declining five times faster than terrestrial fauna, including current extinction rates of freshwater fish 877 times greater than background rates1–3. Since the mid-1800s, habitat loss, overfishing and invasive species have severely altered western United States (US) freshwater fish communities4. Today climate change predictions for the large expanse of mountain ranges in the western US (increased climatic variability that will increase drought frequency, duration, and severity, and shift precipitation to more rain and less snow) threaten to compound the above disturbances5–8. These legacy, current and future disturbances combine into a formidable challenge for conserving western US freshwater fish biodiversity, often necessitating active management of fisheries that are susceptible to further decline and localized extinctions9.
Cutthroat trout (Oncorhynchus clarkii spp.) are salmonids native to the coastal and inland waters of western North America10. Consisting originally of approximately 14 subspecies, the historic distribution of cutthroat species ranged from Alaska to southern Texas and the Pacific coast to the Rocky Mountains10,11. Distribution and abundance of many subspecies have declined over the past century. Two subspecies are extinct and three subspecies are on the US endangered species list10,12. Cutthroat trout population dynamics research has been concentrated in the rivers and streams of the Rocky Mountains, the eastern side of the Intermountain Region (area between the Sierra Nevada/Cascade Mountains and the Rocky Mountains), and the Sierra Nevada Mountains13–24. Little is known about the population dynamics of cutthroat trout in lakes across the western US, especially the desert terminal mountain lakes of the Great Basin.
Lahontan cutthroat trout (Oncorhynchus clarkii henshawi, Lahontan cutthroat) was the top fish predator in ancient Lake Lahontan, the large inland sea during the Pleistocene that covered much of northwestern Nevada and small portions of northeastern California and southeastern Oregon. As Lake Lahontan desiccated over subsequent millennia, Lahontan cutthroat were restricted to remnant streams, rivers and lakes. By the mid-1800s, Lahontan cutthroat occupied 11 lakes, which ranged from the northwestern corner of Nevada to the middle portion of the Sierra Nevada Mountains in California, with six lakes in the Sierra Nevada Mountains and five lakes eastward in the high desert sagebrush steppe of the western Great Basin. Over the next one hundred years, Lahontan cutthroat were extirpated from 9 lakes. The mountain ecosystems of Independence Lake (California) and Summit Lake (Nevada), representing only 0.4% of the historic lake habitat, contain the last native self-sustaining adfluvial populations25. The precipitous decline of the adfluvial populations was a major reason the US Fish and Wildlife Service added Lahontan cutthroat to the US endangered species list in 197026.
Summit Lake (Fig. 1a,b) is a desert terminal mountain lake in the Black Rock Range of the Black Rock Desert in remote northwestern Nevada. Approximately 8,000 years ago, a landslide blocked an area north of Pleistocene Lake Lahontan that had southward drainage, creating Summit Lake and potentially isolating its Lahontan cutthroat population25,27. The Lahontan cutthroat in Summit Lake (Fig. 1c) have been recognized as a unique subpopulation with significant cultural heritage for the region’s indigenous people10,25,26,28,29. The inextricable cultural connection between the Summit Lake Paiute Tribe (SLPT) and the fishery is reflected in the tribe’s original name, Agai Panina Ticutta, which translates as the Summit Lake Fish Eaters. Summit Lake (up to the high-water mark) is entirely contained within the Summit Lake Paiute Reservation, established in 191329. The lake’s proximity to the Black Rock Desert, the population’s adaptation to the lake’s warm eutrophic alkaline waters, and the population’s genetic divergence from other Lahontan cutthroat populations warranted the population’s inclusion in the Northwestern Lahontan basin distinct population segment in the Lahontan cutthroat recovery plan25,26,30–34. In addition, the lake’s remote location and the restricted access to the watershed by the SLPT has kept the ecosystem and the Lahontan cutthroat population relatively undisturbed, buffering it from major disturbances (e.g., invasive salmonids, watershed development, public or commercial harvest) that have caused the decline of other Lahontan cutthroat populations35.
Figure 1.

(a) Southwestern side of Summit Lake looking northeast; Summit Lake Mountain and watershed in the distance, (b) Northwestern side of Summit Lake looking southeast; high-desert sagebrush (Artemisia tridentata) steppe ecosystem - photo courtesy of Elizabeth Sisson, (c) Lahontan cutthroat caught during the lake mark-recapture effort of this study.
In this study, we addressed the following conservation questions about the population dynamics of the lake population: (1) what are the total, female and male adult abundances?, (2) what are the overall, female, and male adult survival rates?, and (3) what is the total number of spawners in the primary spawning tributary and what is their proportion of the adult population? We estimated abundance and survival with a robust design mark-recapture approach using passive integrated transponder (PIT) tags. The number of spawners was estimated by the detection of tagged individuals by a PIT antenna and the capture of tagged and non-tagged individuals in the spawning tributary during the spawning run.
Results
The lake mark-recapture effort resulted in 1501 captures, with total captures per primary period ranging from 81 (post-spawn 2016) to 279 (fall 2015) (mean = 188 ± 70). We tagged 1168 individuals and 274 were recaptured (Table 1; Supplementary Datasets S1, S2).
Table 1.
Summary counts of adfluvial Lahontan cutthroat captured during the lake mark-recapture effort at Summit Lake, Nevada, USA, 2015–2017.
| Year | Primary Period | Number of secondary periods | New captures | Recaptures | Individual recaptures | Total captures |
|---|---|---|---|---|---|---|
| 2015 | Pre-spawn | 8 | 212 | 47 | 33 | 259 |
| Fall | 6 | 237 | 42 | 37 | 279 | |
| 2016 | Pre-spawn | 2 | 171 | 68 | 37 | 239 |
| Post-spawn | 4 | 48 | 33 | 30 | 81 | |
| Fall | 4 | 119 | 33 | 33 | 152 | |
| 2017 | Pre-spawn | 2 | 153 | 52 | 52 | 205 |
| Post-spawn | 4 | 87 | 30 | 25 | 117 | |
| Fall | 5 | 141 | 28 | 27 | 169 | |
| Total | — | 35 | 1168 | 333 | 274 | 1501 |
| Mean | — | 4 | 146 | 42 | 34 | 188 |
| SD | — | 2 | 62 | 14 | 8 | 70 |
The New captures category is the number of new (without a Passive Integrated Transponder - PIT tag) individuals captured. The Recaptures category is the number of captures of PIT tagged individuals, including each capture of individuals recaptured more than once. The Individual recaptures category is the number of tagged individuals recaptured, not including the additional recaptures of individuals recaptured more than once. SD = standard deviation.
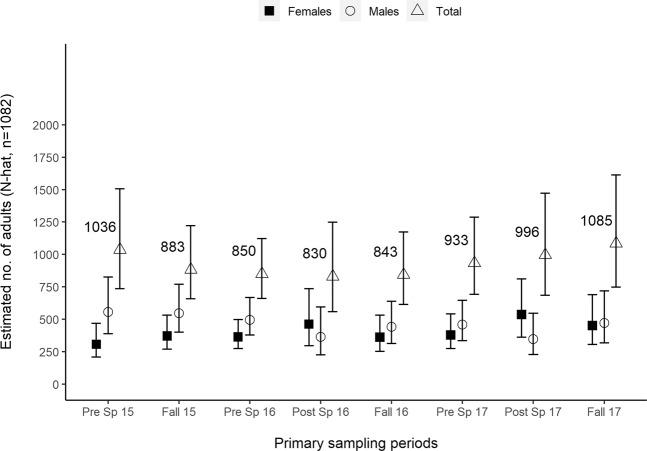
The top model (ΔAICc = 0) for all adults (n = 1082, male, female, unknown sex) had time-invariant survival and lambda, and time-varying capture (p) and recapture (c) rates. In addition, lambda and recapture were modeled as a function of sex; and survival, lambda and recapture were modeled as a function of fork length (Table 2). From pre-spawn 2015 to fall 2017, estimated abundance was 1036 (95% CI 735–1507), 883 (95% CI 658–1223), 850 (95% CI 661–1122), 830 (95% CI 559–1248), 843 (95% CI 615–1173), 933 (95% CI 691–1288), 996 (95% CI 685–1473), and 1085 (95% CI 747–1614) (Fig. 2, Supplementary Table S1). Female abundance was estimated at 307 (95% CI 209–469), 371 (95% CI 269–531), 364 (95% CI 275–498), 462 (95% CI 295–735), 362 (95% CI 252–532), 379 (95% CI 274–541), 536 (95% CI 361–812), and 452 (95% CI 305–691) (Fig. 2, Supplementary Table S2). Male abundance was estimated at 556 (95% CI 388–826), 546 (95% CI 401–770), 495 (95% CI 378–667), 364 (95% CI 226–596), 442 (95% CI 312–639), 458 (95% CI 334–647), 348 (95% CI 227–547), 472 (95% CI 319–719) (Fig. 2, Supplementary Table S3). Annual survival (SA) was 0.51 (95% CI 0.44–0.58). The sex coefficients (logit) for lambda and recapture were −0.04 (95% CI −0.08 – −0.005) and 0.55 (95% CI 0.05–1.05), respectively. The fork length coefficients (logit) for survival, lambda, and recapture were −0.004 (95% CI −0.006 – −0.002), −0.002 (95% CI −0.002 – −0.002), and 0.006 (95% CI 0.003–0.009), respectively.
Table 2.
Model selection results for seasonal survival (Ss), lambda (λ), and capture (p)/recapture (c) rates of adult (male, female, and unknown sex, ≥ 300 mm, n = 1082) adfluvial Lahontan cutthroat captured during the lake mark-recapture effort at Summit Lake, Nevada, USA, 2015–2017.
| Model | AICca | ΔAICcb | AIC weight | Model likelihood | Kc | Deviance |
|---|---|---|---|---|---|---|
| Ss(.d,fle)-λ(.,sex,fl),p(tf),c(t,sex,fl) | 9550.44 | 0.00 | 0.71 | 1.00 | 25 | 9499.45 |
| Ss(.,fl)-λ(.,sex,fl),p(t,fl),c(t,sex,fl) | 9552.22 | 1.78 | 0.29 | 0.41 | 26 | 9499.16 |
| Ss-λ(ag,fl),p-c(t,fl) | 9570.87 | 20.44 | 0.00 | 0.00 | 26 | 9517.81 |
| Ss-λ(.,fl),p-c(t,fl) | 9618.56 | 68.12 | 0.00 | 0.00 | 22 | 9573.79 |
| Ss(a),λ(a,sex),p(t),c(t,sex) | 9742.46 | 192.02 | 0.00 | 0.00 | 26 | 9689.39 |
| Ss-λ(.,sex),p-c(t,sex) | 9779.37 | 228.94 | 0.00 | 0.00 | 25 | 9728.39 |
| Ss-λ(a),p-c(t) | 9795.72 | 245.29 | 0.00 | 0.00 | 22 | 9750.96 |
| Ss(.), p-c(t) | 9856.58 | 306.14 | 0.00 | 0.00 | 18 | 9820.06 |
| Ss-p-c(.) | 10290.48 | 4740.05 | 0.00 | 0.00 | 4 | 10282.45 |
aAIC (Akaike Information Criterion) for small sample size.
bDifference between model AICc and model with the lowest AICc.
cNo. of model parameters.
d(.) = rate constant across primary sampling periods.
efl = fork length.
ft = parameter varies across primary sampling periods.
ga = parameter varies annually.
AIC (Akaike Information Criterion) model selection was performed. Sex and fork length (fl) are covariates.
Figure 2.
Adult abundance estimates derived from the adult (male, female, and unknown sex, ≥300 mm, n = 1082) adfluvial Lahontan cutthroat captured during the lake mark-recapture effort at Summit Lake, Nevada, USA, 2015–2017. The abundance () estimates and primary sampling periods are located on the x and y axes, respectively. The abundance, female, and male estimates were derived from the top model of AIC (Akaike Information Criterion) model selection performed with the adult lake mark-recapture data (Table 2, Supplementary Tables S1–3). The error bars represent 95% confidence intervals.
From 2015–2017, respectively, the PIT antenna detected 238, 337, 344 spawning trout (mean = 306 ± 59), of which 77, 133, 159 were female (mean = 123 ± 41); 129, 191, 162 were males (mean = 160 ± 31); and 32, 13, 23 were unknown sex (mean = 22 ± 9). Concurrently at the fish weir, 268, 465, 433 spawning trout were caught (mean = 388 ± 105), of which 92, 171, 190 were female (mean = 150 ± 51); 169, 294, 239 were male (mean = 234 ± 63); and 7, 0, 4 unknown sex (mean = 3 ± 3). The proportion of tagged individuals was 0.38, 0.40, and 0.43 (mean = 0.40 ± 0.03). Thus, from 2015–2017 we estimated total spawning trout as 645, 868, 824 (mean = 789 ± 124), respectively, with total female spawners of 206, 338, 379 (mean = 307 ± 90), male spawners of 348, 494, 387 (mean = 410 ± 76), and unknown sex spawners of 91, 36, 58 (mean = 61 ± 27). The estimated proportion of adults that participated in the spawning run was 0.62, 1.0, and 0.88 (mean = 0.73 ± 0.23; with values above 1.0 restricted to 1.0).
Discussion
Overall, the Summit Lake population was stable during our two year and 9-month study period. Population growth rate (lambda) differed by sex, with females exhibiting a slightly higher growth rate than males. However, this sex-specific difference in abundance trend did not appear to result in a severe sex imbalance. In addition, fork length had a negative effect on the population growth rate.
The apparent reduction in total adult abundance we observed during the first two-thirds of our study may have resulted from low snowpacks prior to and during this portion of our study period. From 2012 to 2014, Summit Lake snowpacks (measured in snow water equivalent or SWE, the equivalent depth of liquid water) decreased by approximately 40% (Supplementary Table S4)36. Cutthroat fry recruitment has been shown to be positively correlated to the lower third approximately (negatively correlated for the upper two - thirds) of the range of snow - water content (via streamflow) in a spawning tributary’s watershed37. Summit Lake fry in 2012 would have started becoming adults in 2015. Consequently, the apparent decline in abundance during this period may be partially attributed to decreased fry recruitment from 2012 to 2014. However, the reason(s) is unclear for the seemingly opposite abundance trends for males and females. With no difference in male and female survival, differential recruitment between the sexes was probably the main driver, with skewed embryo survival a possible mechanism. Morán et al. found that brown trout (Salmo trutta) families with high embryo mortality tended to produce female-biased clutches of alevin38. With lower streamflow and higher water temperatures likely to have increased embryo mortality in Mahogany Creek from 2012–2016, adult recruitment may have been skewed toward females via this mechanism of skewed embryo survival39,40.
Sex did not seem to have a significant effect on total, female, and male survival. Cutthroat trout survival in this mountain lake is low compared to adult (total) or adult female survival from three other Lahontan cutthroat studies14,41,42. The Truckee River (Nevada) study had the lowest survival (0.36). The estimate was probably biased low by apparent survival (which is always lower than true survival), hatchery Lahontan cutthroat (which can exhibit lower fitness than wild conspecifics), small size (large juveniles or first year adults were used, which could have been subject to high competition and predation pressure from the more abundant wild populations of brown trout (Salmo trutta) and rainbow trout (Oncorhynchus mykiss)), or drought25,41,43–46. The Walker Lake (another mountain desert terminal lake in the region, Nevada) study estimated adult survival at 0.44 in 1999 (highest rate of their study)42. True survival was estimated, but hatchery Lahontan cutthroat were used; and at approximately 11 g/L, the total dissolved solid level in Walker Lake significantly decreased Lahontan cutthroat survival47,48. In contrast, the natural cutthroat trout population of mountain Independence Lake had the highest survival rate (0.68), but survival was estimated for females only14. Though wild invasive salmonids (primarily kokanee salmon, Oncorhynchus nerka) inhabit Independence Lake, the Lahontan cutthroat population is wild and had favorable snowpack during the study49. Accordingly, given the study designs and stressors that lowered survival in above Lahontan cutthroat studies, it is plausible that our estimate can be considered low and driven by a stressor. In addition, Summit Lake survival is in the lower half of a survey of annual survival rates from other trout studies14,15,19,50–52.
The female and male spawning numbers and proportions followed their respective abundance trends, but the total spawning numbers and proportions generally increased while total abundance first declined then increased to remain relatively stable for the study period. Like with abundance, snowpack size and timing and the potentially skewed recruitment (increase in females) may have driven the total spawning run numbers and proportions. Spawning runs have been shown to be positively correlated to the lower third approximately (negatively correlated for the upper two-thirds thereafter) of the range of spring water flow (via snow-water content) in the spawning tributary37,53. The Summit Lake spawning runs and proportions increased in 2016 and 2017 with consecutively larger snowpacks, and the spawning runs generally matched the snowpack trend from 2012 to 2017 (Supplementary Table S4)36. Moreover, the March - April snowpacks of 2015, which were the smallest March - April snowpacks during 2012–2017, probably contributed to the historically low spawning run at the fish weir in 2015. The small decline (from 2016) of the spawning run in 2017 may have been partially due to high SWE and water flows that year53. Finally, the spawning proportion for 2016 (1.00) is not realistic but can be viewed in the context of the confidence interval of the abundance estimate for the period, suggesting the proportion was higher than 2015 but like 2017.
Drought (along with invasive salmonids and agricultural dewatering) has been implicated in the decline of various species of river - or stream - dwelling cutthroat trout populations, but not adfluvial cutthroat populations13–15,20,21,24. However, taken together, our observations at Summit Lake from 2015–2017 (e.g., potential decline in adult abundance from 2015 to 2016, opposite female and male abundance trends, low annual survival, larger proportion of spawners the last two years, smallest spawning run on record at the fish weir) were likely driven by the severe sustained regional drought from 2012–201654. Considering also the potential negative legacy effects of drought, what is the prognosis for the Summit Lake adfluvial Lahontan cutthroat population55–57? Populations naturally fluctuate with changing abiotic or biotic conditions or drift processes43. Thus, declining abundance during normal drought cycles and conditions may not be a concern. Other trout populations have comeback from precipitous declines and drought58. For the Summit Lake Lahontan cutthroat population in the lake, the increase of 2016 and 2017 spawning runs may signal a comeback, and in the short run, continued average to above average snowpacks and low anthropogenic stressors may promote a rebound. But the long - term prognosis is uncertain. Like cutthroat populations across the western US, this population faces unknown impacts from climate change6. Declining abundance and diverging male and female abundance under changing drought cycles and conditions may have negative long - term consequences. The prediction of increased frequency, severity, and duration of drought, and an increased percentage of rain, may decrease abundance, reduce the effective population size, and skew the sex ratio5,8,38,55–57,59. These dynamics may expose this small and isolated population to disproportionately large drift processes (e.g. genetic drift) that could threaten its long-term viability59.
Opportunities for future research
Intertwined, cascading and compounding drought mechanisms in the lake and Mahogany Creek probably caused low survival and recruitment, with evidence at Summit Lake and other trout systems highlighting potential mechanisms that should be investigated. In the lake, evapoconcentration (increased solute concentration and water temperature, and decreased oxygen) may have increased chemical and thermal stress that decreased survival or reproductive readiness, quality, or frequency30,47,60–63. In Mahogany Creek, decreased habitat, predation refugia, and post-spawning body condition may have decreased optimal spawning habitat, increased overall spawning mortality, or caused differential spawning mortality between the sexes7,40,55–57,64–70. In addition, low fry or juvenile recruitment may have been driven by disturbed stream morphology and ecology that diverted significant streamflow from the main creek channel, which may have physically impeded lakeward migration or decreased instream survival or movement via low streamflows and increased thermal stress7,24,71,72.
Utilizing additional or different sampling or modeling techniques should offer additional population dynamics insights by refining estimates and capturing dynamics and mechanisms not addressed in this study. Different sampling techniques are needed to capture juveniles, and sample the heavily vegetated littoral zone and areas further from shore or that are deep. Models that use live/dead or movement/occupancy data could be used to improve abundance and survival estimates73. Additionally, a two-sex (to address skewed sex ratios) integrated population model should be considered to perform a population viability analysis. Integrated population models are a relatively new and popular class of population models because they incorporate data from multiple sources to reduce parameter uncertainty74. Therefore, future research should focus on the complete life history of the population across time, so that the biotic and abiotic mechanisms driving long - term viability can be understood6,7,61–63,75.
Methods
Study area and trout population
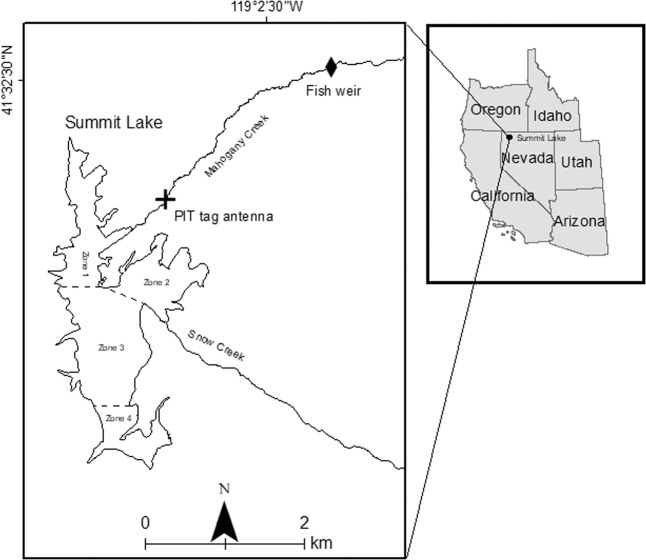
Located within the Black Rock Range, Summit Lake (Figs. 1a,b, 3; 41.515 N −119.063 W) is in the northwestern corner of Nevada (Humboldt County), US, at the base of Summit Lake Mountain. Locally, the climate is typical of the Great Basin (warm summers, cold winters, low annual precipitation), sagebrush (Artemisia tridentata) is the dominant vegetation, and the geology is tuff, basalt, rhyolite, and pyroclastics27,76. The lake and its tributaries are a closed system (i.e., not connected to other surface aquatic systems)27,28,77. Because of the lake’s high surface elevation (1780 m) and small surface area (2.8 km2), the lake freezes in the winter, stratifies in the summer (surface temperatures >22 °C), and mixes in the spring and fall (i.e., dimictic). The lake is eutrophic, alkaline, and rich in invertebrates and macrophytes77. From 1981–1983, surface pH ranged from 8.36–8.54, total dissolved solids (TDS) ranged from 242.4–293.6, specific conductance (μ mho/cm @ 25 °C) ranged from 281–355, and total alkalinity (as CaCO3) ranged from 128.0–159.2. The lake has a mean depth of 6 m, but the southern half of the lake is generally deeper and contains the maximum depth of approximately 15 m77. The lake elevation decreased approximately 4 m (27%) during the recent severe drought in the western US (2012–2016)54,78.
Figure 3.
Summit Lake, Nevada, USA, including Mahogany Creek, the sole, perennial spawning tributary. The dashed lines indicate the four sampling zone boundaries, and the cross and diamond represent the PIT tag antenna and fish weir locations, respectively.
Mahogany Creek (Fig. 3), which flows into the northeast shoreline, is the sole perennial spawning tributary and thus serves as the primary source of Lahontan cutthroat recruitment for the lake population28. The creek is relatively small, with a mean bankfull width of 2.25 m and mean maximum bankfull depth of 0.42 m40. Though spawners utilize most of the creek and its tributaries when conditions permit, most spawning occurs in the lower stretch (approximately 9 km)78. Depending on environmental conditions, the spawning run and fry migration can occur from March to June and April to November, respectively28. The Summit Lake watershed contains additional creeks that are not perennial, do not have significant spawning runs, or are tributaries to Mahogany Creek.
Public access and fishing have never been allowed on the Reservation. Subsistence fishing is allowed for tribal members but is highly regulated79. Invasive salmonids have never been established in the lake or the creeks28,77. Egg take and hatchery supplementation of Lahontan cutthroat fingerlings derived from the Summit Lake population were discontinued after 198480. Grazing on the federal and Reservation portions of Mahogany Creek was discontinued in 1974 and 1991, respectively, and in the remainder of the Reservation was minimal from 1990–2004 and henceforth discontinued28,81. From 1978 to 2017, the spawning run at the fish weir averaged 1198 ± 583, with a maximum and minimum of 2400 (1999) and 269 (2015), respectively; and from 1997–2017, the spawning run decreased 77% (Supplementary Table S5)78.
Field methods
We conducted this study of Lahontan cutthroat in accordance with the approved procedures in US Fish and Wildlife Permit #TE-17827A-4, and Protocol #00679 of the Institutional Animal Care and Use Committee of the University of Nevada, Reno, US. Lahontan cutthroat are listed as threatened under the US Endangered Species Act. The Summit Lake Paiute Tribe approved the research and protocols and granted access to the Summit Lake Paiute Reservation.
Lake mark-recapture effort
We used trap nets for the lake sampling for three primary reasons. Most importantly, as Lahontan cutthroat are threatened, trap nets exert less stress, harm or mortality than other passive methods such as gill nets82. Next, trap nets are generally used in shallow water (but can be used up to 15 m) to intercept fish travelling close and parallel to the shoreline, which is ideal because adfluvial Lahontan cutthroat utilize the littoral zone to varying degrees during daily or seasonal cycles82–85. In addition, trap nets allowed us to standardize our effort more easily by using the same equipment and length of time repeatedly82. Our nets were made of nylon mesh (mesh sizes 13 or 25 mm) with a single leader (1.2 m height, 30.5 m length) in the center of the rectangular throat (1.2 m height × 1.8 m width × 0.6 m length).
We employed a spatially stratified, random sampling plan for this small lake to minimize bias from potential spatial differences in population density (suggested from initial sampling) throughout the lake. Within each primary sampling period (defined below), sampling was performed multiple days a week for consecutive weeks. Each day we deployed between five and ten nets, which were evenly distributed across the four sampling zones (Fig. 3). Primarily, the nets were set perpendicular to shoreline, and most sets were for 20–24 hours. After pulling the nets and processing all captures each day, the nets were moved to a different location within their assigned zone or to another zone to achieve even distribution of effort. For each net set we recorded zone assignment, GPS location, date/time of set and pull, and depth (m).
We anesthetized with CO2 (g) and marked (i.e., tagged) new adult individuals by implanting a Biomark 12 mm full duplex Passive Integrated Transponder (PIT) tag in the pelvic girdle, according to Biomark guidelines86. Individuals ≥300 mm were defined as adults based on size data from recent research on the Summit Lake population (Chandra, unpublished data) and literature on Lahontan cutthroat life history25. After processing, the fish recuperated in net pens alongside the boat in the lake before release. We recorded the following data from each capture: fork length (mm), mass (kg), new capture or recapture, PIT tag number, and sex (male, female, or unknown).
We determined sex by gamete expression or morphology. During the pre-spawn sampling periods, the anus was gently squeezed from posterior to anterior to express gametes. When gametes were not expressed, and for the other sampling periods, we used visual inspection of head shape/length, overall body shape, and coloration to assign sex, based on the sexual morphology of salmon, brook and bull trout, and Lahontan cutthroat87–90. Sex was assigned independently each time an individual was captured. Each capture occasion was reviewed by at minimum two researchers, and unanimous decision was required to assign male or female, otherwise unknown was assigned. At the end of the study, recaptured individuals were coded the sex assigned most frequently. We quantified misidentification by calculating the percentage of instances in which recaptured individuals with known gamete expression had been misidentified. The overall success rate was 79% but with misidentification heavily biased toward females. 35% of female assignments were male, and zero male assignments were female. We used this information to correct any potential influence of misidentification on abundance, survival and spawning estimates (described below)91.
To enable precise estimation of abundance and survival rates, our sampling methods followed a standard ‘robust design’ analytical approach; ‘primary periods’ were spaced far enough in time such that births and/or deaths (ignoring migration) were likely to affect population dynamics, and each primary period consisted of two or more ‘secondary periods’ occurring within a short enough time interval to justify a closed population assumption (no births/deaths)92. We recognized that the spawning run is a high emigration and mortality event that would violate the assumptions of a closed population model (to estimate abundance, ) with respect to the lake sampling43. Thus, we chose primary periods before and after the spawning run to prevent sampling during the spawning run. Our three annual primary periods were based on meteorological seasons and consisted of ‘pre - spawn’ (March – April, spring), ‘post - spawn’ (June – July, summer), and ‘fall’ (October – November, fall). The daily capture data was consolidated into weekly capture histories. Thus, the secondary periods were consecutive weeks within these primary periods.
We used the CloseTest program, which analyzes a capture history to detect additions or losses, to test that each pre - spawn period was closed93,94. The 2015 pre - spawn data indicated losses but no additions, which is in line with the brief overlap of sampling with the beginning of the run. The spawning run had a delayed start likely due to the smallest snowpack (measured in SWE) and lowest streamflow of the study (and drought) period (Supplementary Table S4)36,54. Most of the run occurred in late April and May (due to two late precipitation events)69. By reviewing the detection record of the PIT antenna, we estimated that seven percent of PIT tagged spawners had migrated into Mahogany Creek during the lake pre - spawn sampling. Thus, we believe the closed population assumption is still reasonable despite a slight potential for positive bias in resulting abundance estimate for the first primary period95. In addition, we adjusted (described below) the abundance and confidence interval estimates to account for the bias. The CloseTest results for pre - spawn 2016 and 2017 indicated no additions nor losses. Therefore, no adjustments were made to the estimates for those periods.
The mark-recapture effort was conducted from March 2015 to November 2017 (approximately two years and nine months) for a total of eight primary and 35 secondary (weeks) periods. The number of sampling weeks per primary period ranged from two (pre-spawn 2016 and 2017) to 8 (spring 2015) (mean = 4 ± 2) (Table 1, Supplementary Table S6). We deployed 597 net sets, ranging from 31 (pre-spawn 2016) to 121 (fall 2015) per primary period (mean = 75 ± 38) (Supplementary Datasets S3, S4).
Spawning run
A permanent fish weir facility, located approximately 3.5 km upstream from the mouth, captured all spawning trout migrating further upstream. Depending on environmental conditions and the duration of the run, the SLPT generally performed daily checks of the weir from March to June. The following data was collected from each capture: sex (male, female, or unknown), mass (kg), fork length (mm), and PIT number (if present). After processing, the fish were released upstream to continue their migration. Since 1978, the SLPT has enumerated the spawning run at this location78.
Because the fish weir is not located at the mouth of the creek, the fish weir counts do not represent the total spawners that participate in the run. Thus, the annual spawner estimates in Mahogany Creek were derived from the annual counts of PIT - tagged spawners detected by a PIT antenna and spawners captured at the fish weir. To generate the counts, we assumed adfluvial Lahontan cutthroat populations have an annual spawning run with a subset of adults, are obligate tributary spawners, and infrequently enter or reside in tributaries outside of the spawning run25,40. We classified all detected or captured individuals during the run as a spawner, although actual spawning could not be confirmed. PIT - tagged spawners were detected by a single, stationary PIT antenna (Biomark pass-through antenna with a Biomark IS1001 transceiver) located approximately 750 m upstream from the mouth. The PIT antenna system is automated and records detections 24 hours/day and 365 days/year. The approximate stream width and depth at the location are 74 and 41 cm, and the approximate antenna width and height are 71 and 71 cm, respectively. The detection rate of the antenna was approximately 97%, based on the proportion of PIT - tagged spawners captured at the fish weir that were detected by the PIT antenna40.
Analyses
Abundance and survival
We used Program Mark version 9.0 to estimate abundance (), seasonal survival (Ss), and lambda (λ) for each primary period96. We selected the Pradel ‘robust design survival and lambda’ model because we did not need to estimate the additional parameters (e.g., temporary emigration rates) in the standard robust design model97. The ‘Huggins’ p and c’ data type (p = capture, c = recapture) was chosen so that individual covariates (sex, fork length) could be used to model survival, lambda, and capture/recapture97–99. In addition, unequal time periods between the primary sampling periods were accounted for in the model setup. The model uses a Horvitz - Thompson estimator to estimate abundance for each primary period97. Summit Lake is closed system, and because we avoided sampling during the temporary emigration of the spawning run, our survival estimates represent true survival (S) rather than apparent survival43.
We processed models with combinations of constant, seasonal, or annual survival, lambda, or capture/recapture rates, and linear, quadratic, or interactive relationships of sex or fork length. We excluded models with non - identifiable parameters. Akaike information criterion (AICc, small sample sizes) model selection was applied to identify the top models100,101. We estimated annual survival (SA) as the product of the primary period survival estimates, and used the delta method to estimate standard error for SA97.
To examine the sensitivity of our results to sex misidentification, we randomly removed 35% (estimated from our sex misidentification analysis) of the original female capture histories, added them to the original male capture histories (assignment was not changed for individuals of unknown sex), and performed AICc model selection separately on these adjusted female and male capture histories91. We stopped this process after ten iterations because the sex-specific survival and abundance estimates did not change substantially across iterations. We randomly selected one each of the ten alternative male and female capture history databases and combined them with the unknown sex capture history database to estimate total, female and male abundance and survival. Further, sex (male, female, or unknown) was assigned to every individual in this analysis and did not change in the individual’s capture history.
We applied a post-hoc bias correction to our abundance estimate and confidence interval bounds for the first primary period to correct for the estimated 7% of individuals that migrated out of the study population to spawn during this primary period (see above). To do this, we first computed true capture probability (ptrue) as ptrue = 1.07 * p1, where p1 represents the mean estimated probability of capturing an individual ≥ 1x during the first primary period. We then computed the ‘true’, or bias-corrected, abundance estimate for this primary period by multiplying the abundance estimate N1 (estimated abundance for the first primary period) and the corresponding lower and upper confidence bound estimates by the ratio p1/ptrue [Ntrue = N1 ∗ (p1/ptrue)].
Spawning run metrics
We accounted for sex misidentification by subtracting 35% of the female counts and adding them to the male counts before performing the below calculations. We estimated the annual count of total, female, and male spawners by dividing the number of tagged spawners detected by the antenna by the proportion of tagged vs. total spawners captured at the fish weir, and then dividing by 0.97 (the detection probability of the PIT antenna)40. In addition, total female, male and unknown spawners were estimated by multiplying the total tagged spawners detected by the antenna by the proportion female, male and unknown spawners detected by the antenna. Last, the annual totals were divided by their respective ‘pre-spawn’ primary period abundance estimates to derive the proportion of the adult population that spawned each year.
Supplementary information
Acknowledgements
Thank you to the Summit Lake Paiute Tribe for their partnership in this project. We thank the United States Fish and Wildlife Service’s Tribal Wildlife Grant Program (Grant #F13AP00375), the National Fish and Wildlife Foundation’s Desert Terminal Lakes Restoration Fund (Grant #40577), the Bureau of Reclamation’s Desert Terminal Lakes Program, the Bureau of Indian Affairs Fisheries Management Services Contract (#A10AV00358), and the University of Nevada College of Science and Global Water Center for funding this project. Additional thanks to Kyle Mathews, Rachel Youmans, Nicholas Rzyska-Filipek, Bonnie Teglas, Geoffrey Kibler, Andrew Reckers, Sabrina Heitmann, Jason Schumacher, Scott Hoffman, Jason Barnes, and Doug Ouellette.
Author contributions
Conceptualization: Sudeep Chandra, Zeb Hogan, William Cowan, Christopher L. Jerde.Data Curation: James B. Simmons.Formal Analysis: James B. Simmons, Christopher L. Jerde, Kevin Shoemaker.Funding Acquisition: Sudeep Chandra, Zeb Hogan, William Cowan.Investigation: James B. Simmons, Teresa Campbell, William Cowan, Jessica Saenz.Methodology: James B. Simmons, Christopher L. Jerde, Sudeep Chandra, Zeb Hogan, William Cowan, Jessica Saenz.Project Administration: James B. Simmons, Sudeep Chandra, Zeb Hogan, William Cowan, Jessica Saenz.Resources: Sudeep Chandra, Zeb Hogan, William Cowan.Software: James B. Simmons.Supervision: James B. Simmons, Christopher L. Jerde, Sudeep Chandra, Zeb Hogan, William Cowan.Validation: James B. Simmons, Christopher L. Jerde, Kevin Shoemaker.Visualization: James B. Simmons.Writing – Original Draft Preparation: James B. Simmons.Writing – Review & Editing: James B. Simmons, Teresa Campbell, Christopher L. Jerde, Sudeep Chandra, Zeb Hogan, William Cowan, Jessica Saenz, Kevin Shoemaker.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Competing interests
Will Cowan is a member of the Summit Lake Paiute Tribe and was an employee of the United States Fish and Wildlife Service who served as the Summit Lake Paiute Tribe’s Natural Resources Department Director (per an Intergovernmental Personnel Agreement) and as Principal Officer on the United States Fish and Wildlife Service and Nevada Department of Wildlife permits. He retired from United States Fish and Wildlife Service on February 28, 2019. In addition, Will Cowan received funding from the United States Fish and Wildlife and National Fish and Wildlife Foundation.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-65992-0.
References
- 1.Ricciardi A, Rasmussen JB. Extinction rates of North American freshwater fauna. Conserv. Biol. 1999;13:1220–1222. [Google Scholar]
- 2.Butchart SH, et al. Global biodiversity: indicators of recent declines. Science. 2010;328(5982):1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- 3.Burkhead NM. Extinction rates in North American freshwater fishes, 1900-2010. Bioscience. 2012;62(9):798–808. [Google Scholar]
- 4.Brouder, M. J. & Scheurer, J. A. Status, Distribution, and Conservation of Native Freshwater Fishes of Western North America: A Symposium Proceedings (American Fisheries Society, 2007).
- 5.Cayan, D. R. et al. Natural variability, anthropogenic climate change, and impacts on water availability and flood extremes in the western United States In Water Policy and Planning in a Variable Changing Climate (ed. Miller, K.A et al.). 17-42 (CRC Press, 2016).
- 6.Lynch AJ, et al. Climate change effects on North American inland fish populations and assemblages. Fisheries. 2016;41(7):346–361. [Google Scholar]
- 7.Whitney JE, et al. Physiological basis of climate change impacts on North American inland fishes. Fisheries. 2016;41(7):332–345. [Google Scholar]
- 8.Harpold, A. A., Dettinger, M. & Rajagopal, S. Defining snow drought and why it matters. Eos. 98, 10.1029/2017EO068775 (2017).
- 9.Paukert C, et al. Adapting inland fisheries management to a changing climate. Fisheries. 2016;41(7):374–384. [Google Scholar]
- 10.Trotter, P. Cutthroat: Native Trout of the West, 2nd edn (University of California Press, 2008).
- 11.Loxterman JL, Keeley ER. Watershed boundaries and geographic isolation: patterns of diversification in cutthroat trout from western North America. BMC Evol. Biol. 2012;12(1):38. doi: 10.1186/1471-2148-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.USFWS. ECOS Environmental conservation online system. https://ecos.fws.gov/ecp0/pub/SpeciesReport.do?groups=E&listingType=L&mapstatus=1, accessed 7 May 2018 (2017).
- 13.Stapp P, Hayward GD. Effects of an introduced piscivore on native trout: insights from a demographic model. Biol. Invasions. 2002;4:299–316. [Google Scholar]
- 14.Rissler, P. H., Scoppettone, G. G. & Shea S. Life history, ecology and population viability analysis of the Independence Lake strain Lahontan cutthroat trout (Oncorhynchus clarkii henshawi) (U.S. Geological Survey, 2006).
- 15.Budy P, Thiede GP, McHugh P. Quantification of the vital rates, abundance, and status of a critical, endemic population of Bonneville cutthroat trout. N. Am. J. Fish. Manag. 2007;27:593–604. [Google Scholar]
- 16.Johnston FD, et al. The demography of recovery of an overexploited bull trout, Salvelinus confluentus, population. Can. J. Fish. Aquat. Sci. 2007;64(1):113–126. [Google Scholar]
- 17.High B, Meyer K, Schill DJ, Mamer ERJ. Distribution, abundance, and population trends of bull trout in Idaho. N. Am. J. Fish. Manag. 2008;28:1687–1701. [Google Scholar]
- 18.Brenden TO, Bence JR, Lantry BF, Lantry JR, Schaner T. Population dynamics of Lake Ontario lake trout during 1985-2007. N. Am. J. Fish. Manag. 2011;31:962–979. [Google Scholar]
- 19.Cox BS, Guy CS, Fredenberg WA, Rosenthal LR. Baseline demographics of a non-native lake trout population and inferences for suppression from sensitivity-elasticity analyses. Fish. Manag. Ecol. 2013;20:390–400. [Google Scholar]
- 20.Meyer KA, Larson EI, Sullivan CL, High B. Trends in the distribution and abundance of Yellowstone cutthroat trout and nonnative trout in Idaho. J. Fish. Wildl. Manag. 2014;5(2):227–242. [Google Scholar]
- 21.Kennedy P, Meyer K. Trends in abundance and the influence of bioclimatic factors on Westslope cutthroat trout in Idaho. J. Fish. Wildl. Manag. 2015;6(2):305–317. [Google Scholar]
- 22.Ng EL, Fredericks JP, Quist MC. Population dynamics and evaluation of alternative management strategies for nonnative lake trout in Priest Lake, Idaho. N. Am. J. Fish. Manag. 2016;36(1):40–54. [Google Scholar]
- 23.Budy PE, Bowerman T, Al-Chokhachy R, Conner M, Schaller H. Quantifying long-term population growth rates of threatened bull trout: challenges, lessons learned, and opportunities. Can. J. Fish. Aquat. Sci. 2017;74:2131–2143. [Google Scholar]
- 24.Carim KJ, Vindenes Y, Eby LA, Barfoot C, Vøllestad LA. Life history, population viability, and the potential for local adaptation in isolated trout populations. Glob. Ecol. Conserv. 2017;10:93–102. [Google Scholar]
- 25.Gerstung, E. R. Status, life history, and management of the Lahontan cutthroat trout In Status and Management of Interior Stocks of Cutthroat Trout (ed. Gresswell, R.E.) 93-106 (American Fisheries Society, 1988).
- 26.Coffin, P. D. & Cowan, W. F. Lahontan cutthroat trout (Oncorhynchus clarki henshawi) recovery plan (U.S. Fish and Wildlife Service, 1995).
- 27.Curry BB, Melhorn WN. Summit Lake landslide and geomorphic history of Summit Lake basin, northwestern Nevada. Geomorphology. 1990;4:1–17. [Google Scholar]
- 28.Vinyard GL, Winzeler A. Lahontan cutthroat trout (Oncorhynchus clarki henshawi) spawning and downstream migration of juveniles into Summit Lake, Nevada. West. N. Am. Nat. 2000;60(3):333–341. [Google Scholar]
- 29.SLPT. Our Story. Summit Lake Paiute Tribe. http://www.summitlaketribe.org/about-us.html, accessed 22 Jan 2018.
- 30.Vigg S, Koch DL. Upper lethal temperature range of Lahontan cutthroat trout in waters of different ionic concentration. Trans. Am. Fish. Soc. 1980;109:336–339. [Google Scholar]
- 31.Gall, G. A. E. & Loudenslager, E. J. Biochemical genetics and systematics of Nevada trout populations, Final Report to Nevada Department of Wildlife (University of California, 1981).
- 32.Wilkie MP, Wood C. The adaptations of fish to extremely alkaline environments. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996;113(4):665–673. [Google Scholar]
- 33.Dickerson BR, Vinyard GL. Effects of high chronic temperatures and diel temperature cycles on the survival and growth of Lahontan cutthroat trout. Trans. Am. Fish. Soc. 1999;128:516–521. [Google Scholar]
- 34.Nielsen JL, Sage GK. Population genetic structure in Lahontan cutthroat trout. Trans. Am. Fish. Soc. 2002;131:376–388. [Google Scholar]
- 35.La Rivers, I. Fishes and fisheries of Nevada (University of Nevada Press, 1994).
- 36.NRCS. Snow Telemetry (SNOTEL) and Snow Course Data and Products. https://www.wcc.nrcs.usda.gov/snow, accessed 1 August 2019.
- 37.Drummond RA, McKinney TD. Predicting the recruitment of cutthroat trout fry in Trappers Lake, Colorado. Trans. Am. Fish. Soc. 1965;94(4):389–393. [Google Scholar]
- 38.Morán P, Labbé L, Garcia de Leaniz C. The male handicap: male-biased mortality explains skewed sex ratios in brown trout embryos. Biol. Lett. 2016;12(12):20160693. doi: 10.1098/rsbl.2016.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Recsetar M, Bonar S. Survival of Apache trout eggs and alevins under static and fluctuating temperature regimes. Trans. Am. Fish. Soc. 2013;142:373–379. [Google Scholar]
- 40.Campbell T, et al. Population connectivity of adfluvial and stream-resident Lahontan cutthroat trout: Implications for resilience, management, and restoration. Can. J. Fish. Aquat. Sci. 2019;76(3):426–437. [Google Scholar]
- 41.Alexiades AV, Peacock MM, Al-Chokhachy R. Movement patterns, habitat use, and survival of Lahontan cutthroat trout in the Truckee River. N. Am. J. Fish. Manag. 2012;32:974–83. [Google Scholar]
- 42.Sedinger JS, Blomberg EJ, VanDellen AW, Byers S. Environmental and population strain effects on survival of Lahontan cutthroat trout in Walker Lake, Nevada: a Bayesian approach. N. Am. J. Fish. Manag. 2012;32:515–522. [Google Scholar]
- 43.Williams, B. K., Nichols, J. D. & Conley, M. J. Analysis and Management of Animal Populations (Academic Press, 2002).
- 44.Kostow K. Differences in juvenile phenotypes and survival between hatchery stocks and a natural population provide evidence for modified selection due to captive breeding. Can. J. Fish. Aquat. Sci. 2004;61:577–589. [Google Scholar]
- 45.Araki H, Berejikian BA, Ford MJ, Blouin MS. Fitness of hatchery-reared salmonids in the wild. Evol. Appl. 2008;1(2):342–355. doi: 10.1111/j.1752-4571.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones, J. California’s most significant droughts: comparing historical and recent conditions (California Natural Resources Agency, 2015).
- 47.Dickerson BR, Vinyard GL. Effects of high levels of total dissolved solids in Walker Lake, Nevada, on survival and growth of Lahontan cutthroat trout. Trans. Am. Fish. Soc. 1999;128:507–515. [Google Scholar]
- 48.Marioni, N. Effects of declining lake levels on fish populations in Walker Lake, NV (Master’s thesis, University of Nevada Reno, 2007).
- 49.Margulis SA, Cortés G, Girotto M, Durand M. A Landsat-era Sierra Nevada snow reanalysis (1985–2015) J. Hydrometeorol. 2016;17:1203–1221. [Google Scholar]
- 50.Mills KH, Chalanchuk SM, Allan DJ. Abundance, annual survival, and recruitment of unexploited and exploited lake charr, Salvelinus namaycush, populations at the Experimental Lakes Area, northwestern Ontario. Environ. Biol. Fish. 2002;64:281–292. [Google Scholar]
- 51.Al-Chokhachy R, Budy P. Demographic characteristics, population structure, and vital rates of a fluvial population of bull trout in Oregon. Trans. Am. Fish. Soc. 2008;137(6):1709–1722. [Google Scholar]
- 52.Syslo JM, et al. Response of non-native lake trout (Salvelinus namaycush) to 15 years of harvest in Yellowstone Lake, Yellowstone National Park. Can. J. Fish. Aquat. Sci. 2011;68:2132–2145. [Google Scholar]
- 53.Jonsson B, Jonsson N, Hansen LP. Factors affecting river entry of adult Atlantic salmon in a small river. J. Fish. Biol. 2007;71:943–956. [Google Scholar]
- 54.Wise E. Five centuries of US West Coast drought: occurrence, spatial distribution, and associated atmospheric circulation patterns. Geophys. Res. Lett. 2016;43:4539–4546. doi: 10.1002/2016GL068487. [DOI] [Google Scholar]
- 55.Elliott JM, Hurley MA, Elliott JA. Variable effects of droughts on the density of a seat-trout Salmo trutta population over 30 years. J. Appl. Ecol. 1997;34:1229–1238. [Google Scholar]
- 56.Humphries P, Baldwin D. Drought and aquatic ecosystems: an introduction. Freshw. Biol. 2003;48:1141–1146. [Google Scholar]
- 57.Matthews WJ, Marsh-Matthews E. Effects of drought on fish across axes of space, time, and ecological complexity. Freshw. Biol. 2003;48:1232–1253. [Google Scholar]
- 58.Lobon-Cervia J. Why, when and how do fish populations decline, collapse and recover? The example of brown trout (Salmo trutta) in Rio Chaballos (northwestern Spain) Freshw. Biol. 2009;54:1149–1162. [Google Scholar]
- 59.Groom, M. J., Meffe, G. K. & Carroll, C. R. Principles of Conservation Biology, 3rd edn (Sinauer Associates, Inc., 2006).
- 60.Horne, A. & Goldman, C. Limnology, 2nd edn (McGraw-Hill, Inc., 1994).
- 61.Schreck C, Contreras-Sanchez W, Fitzpatrick M. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture. 2001;197:3–24. [Google Scholar]
- 62.Rideout RM, Rose GA, Burton MP. Skipped spawning in female iteroparous fishes. Fish. 2005;6:50–72. [Google Scholar]
- 63.McBride RS, et al. Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish. 2015;16:23–57. [Google Scholar]
- 64.Magoulick DD, Kobza RM. The role of refugia for fishes during drought: a review and synthesis. Freshw. Biol. 2003;48:1186–1198. [Google Scholar]
- 65.Parker, R., Youmans, R. & Cowan, W. 2012 Annual Narrative Report, Fisheries Management Services Contract Number CTH61T655U2 (Summit Lake Paiute Tribe, 2013).
- 66.Parker, R., Youmans, R. & Cowan, W. 2013 Annual Narrative Report, Fisheries Management Services Contract Number A10AV00358 (Summit Lake Paiute Tribe, 2014).
- 67.Saenz, J., Zeyer, R. & Cowan, W. 2014 Annual Narrative Report, Fisheries Management Services Contract Number A10AV00358 (Summit Lake Paiute Tribe, 2015).
- 68.Penaluna BE, Dunham JB, Noakes DG. Instream cover and shade mediate avian predation on trout in semi-natural streams. Ecol. Freshw. Fish. 2016;25:405–411. [Google Scholar]
- 69.Zeyer, R., Sáenz, J. & Cowan, W. 2015 Annual Narrative Report, Fisheries Management Services Contract Number A10AV00358 (Summit Lake Paiute Tribe, 2016).
- 70.Youmans, R., Zeyer, R., Mathews, K. & Cowan, W. 2016 Annual Narrative Report, Report No.: Fisheries Management Services Contract Number A10AV00358 (Summit Lake Paiute Tribe, 2017).
- 71.Walters AW, Bartz KK, McClure MM. Interactive effects of water diversion and climate change for juvenile chinook salmon in the Lemhi River Basin (USA) Conserv. Biol. 2013;27(6):1179–1189. doi: 10.1111/cobi.12170. [DOI] [PubMed] [Google Scholar]
- 72.Fenkes M, Shiels HA, Fitzpatrick JL, Nudds RL. The potential impacts of migratory difficulty, including warmer waters and altered flow conditions, on the reproductive success of salmonid fishes. Comp. Biochem. Physiol., Part A. 2016;193:11–21. doi: 10.1016/j.cbpa.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White, G. C., Kendall, W. L., & Barker, R. J. Multistate survival models and their extensions in Program MARK. J. Wildl. Manage. 70(6), 1521–1529 (2006).
- 74.Tenan S, et al. Evaluating mortality rates with a novel integrated framework for nonmonogamous species. Conserv. Biol. 2016;30(6):1307–1319. doi: 10.1111/cobi.12736. [DOI] [PubMed] [Google Scholar]
- 75.Johnston FD, Post JR. Density-dependent life-history compensation of an iteroparous salmonid. Ecol. Appl. 2009;19(2):449–467. doi: 10.1890/07-1507.1. [DOI] [PubMed] [Google Scholar]
- 76.Xue T, et al. Long-term trends in precipitation and precipitation extremes and underlying mechanisms in the U.S. Great Basin during 1951–2013. J. Geophys. Res. Atmos. 2017;122:6152–6169. [Google Scholar]
- 77.Vigg, S. Limnological survey of Summit Lake, Nevada (Desert Research Institute, 1983).
- 78.Rzyska-Filipek, N., Youmans, R., Mathews, K. & Cowan, W. 2017 Annual Narrative Report, Fisheries Management Services Contract Number A10AV00358 (Summit Lake Paiute Tribe, 2018).
- 79.SLPT. Minutes-Resolutions. http://www.summitlaketribe.org/minutes-resolutions.html, accessed 7 May 2018.
- 80.Cowan, W. F. Interpretation of pertinent statistics concerning the Summit Lake fisheries management program, 1968-1990 (Summit Lake Paiute Tribe, 1991).
- 81.Novak-Echenique, P. & Youmans, R. Summit Lake Indian Reservation Range Management Plan (United States Department of Agriculture and Summit Lake Paiute Tribe, 2013).
- 82.Hubert, W. A., Pope, K. L. & Dettmers, J. M. Passive Capture Techniques In Fisheries Techniques, 3rd edn (ed. Zale, A. V., et al.) 223–265 (American Fisheries Society, 2012).
- 83.Sigler WF, Helm WT, Kucera PA, Vigg S, Workman GW. Life history of the Lahontan cutthroat trout, Salmo clarki henshawi, in Pyramid Lake, NV. Great Basin Nat. 1983;43(1):1–29. [Google Scholar]
- 84.Beauchamp DA, Vecht SA, Thomas GL. Temporal, spatial, and size-related foraging of wild cutthroat trout in Lake Washington. Northwest Sci. 1992;66(3):149–159. [Google Scholar]
- 85.Baldwin CS, Beauchamp DA, Gubala CP. Seasonal and diel distribution and movement of cutthroat trout from ultrasonic telemetry. Trans. Am. Fish. Soc. 2002;131:143–158. [Google Scholar]
- 86.Gibbons JW, Andrews KM. PIT tagging: simple technology at its best. Bioscience. 2004;54(5):447–454. [Google Scholar]
- 87.Benke, R. J. Trout and salmon of North America, 1st edn (The Free Press, 2002).
- 88.Merz JE, Merz WR. Morphological features used to identify Chinook salmon sex during fish passage. The Southwest Nat. 2004;49(2):197–202. [Google Scholar]
- 89.Kazyak DC, Hildebrand RH, Holloway AE. Rapid visual assessment to determine sex in brook trout. N. Am. J. Fish. Manag. 2013;33:665–668. [Google Scholar]
- 90.Nitychoruk JM, et al. Sexual and seasonal dimorphism in adult adfluvial bull trout (Salvelinus confluentus) Can. J. Fish. Aquat. Sci. 2013;91:480–488. [Google Scholar]
- 91.Nichols JD, Kendall WL, Hines JE, Spendelow JA. Estimation of sex-specific survival from capture-recapture data when sex is not always known. Ecology. 2004;85(12):3192–3201. [Google Scholar]
- 92.Kendall, W. L. & Pollock, K. H. The robust design in capture-recapture studies: a review and evaluation by Monte Carlo simulation In Wildlife 2001: Populations (ed. McCullough, D. R. et al.) 31-43 (Elsevier, 1992).
- 93.Stanley TR, Richards JD. Software review: a program for testing capture-recapture data for closure. Wildl. Soc. Bull. 2005;33(2):782–785. [Google Scholar]
- 94.Stanley, T. R. & Richards, J. D. CloseTest. USGS Fort Collins Science Center. https://www.sciencebase.gov/catalog/item/53c5b442e4b0b58d96eeb76e, accessed 8 Nov 2019 (2011)
- 95.Kendall WL. Robustness of closed capture-recapture methods to violations of the closure assumption. Ecology. 1999;80(8):2517–2525. [Google Scholar]
- 96.White GC, Burnham KP. Program MARK: survival estimation from populations of marked animals. Bird Stud. 1999;46:120–139. [Google Scholar]
- 97.Pradel, R. Utilization of capture–mark–recapture for the study of recruitment and population growth rate. Biometrics. 52(2), 703–709 (1996).
- 98.Huggins RM. On the statistical analysis of capture experiments. Biometrika. 1989;76:133–140. [Google Scholar]
- 99.Huggins RM. Some practical aspects of a conditional likelihood approach to capture experiments. Biometrics. 1991;47:725–732. [Google Scholar]
- 100.Akaike, H. Information theory as an extension of the maximum likelihood principle In Second International Symposium on Information Theory (ed. Petrov, B. N. et al.) 267–281 (Akademiai Kiado, 1973).
- 101.Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-theoretic Approach, 2nd edn (Springer, 2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].