Abstract
Advances in the treatment of bulimia nervosa and binge-eating disorder (BN/BED) have been marred by our limited understanding of the underpinning neurobiology. Here we measured regional cerebral blood flow (rCBF) to map resting perfusion abnormalities in women with BN/BED compared with healthy controls and investigate whether intranasal oxytocin (OT), proposed as a potential treatment, can restore perfusion in disorder-related brain circuits. Twenty-four women with BN/BED and 23 healthy women participated in a randomized, double-blind, crossover, placebo-controlled study. We used arterial spin labelling MRI to measure rCBF and the effects of an acute dose of intranasal OT (40 IU) or placebo over 18–26 min post dosing, as we have previously shown robust OT-induced changes in resting rCBF in men in a similar time-window (15–36 min post dosing). We tested for effects of treatment, diagnosis and their interaction on extracted rCBF values in anatomical regions-of-interest previously implicated in BN/BED by other neuroimaging modalities, and conducted exploratory whole-brain analyses to investigate previously unidentified brain regions. We demonstrated that women with BN/BED presented increased resting rCBF in the medial prefrontal and orbitofrontal cortices, anterior cingulate gyrus, posterior insula and middle/inferior temporal gyri bilaterally. Hyperperfusion in these areas specifically correlated with eating symptoms severity in patients. Our data did not support a normalizing effect of intranasal OT on perfusion abnormalities in these patients, at least for the specific dose (40 IU) and post-dosing interval (18–26 min) examined. Our findings enhance our understanding of resting brain abnormalities in BN/BED and identify resting rCBF as a non-invasive potential biomarker for disease-related changes and treatment monitoring. They also highlight the need for a comprehensive investigation of intranasal OT pharmacodynamics in women before we can fully ascertain its therapeutic value in disorders affecting predominantly this gender, such as BN/BED.
Subject terms: Psychiatric disorders, Biomarkers, Pharmacology, Psychiatric disorders, Biomarkers
Introduction
Bulimia nervosa (BN) and binge-eating disorder (BED) are psychiatric disorders characterized by recurrent binge eating1. In BN, binge eating is accompanied by excessive compensatory behaviours, which are absent in BED2. As recovery rates after standard treatment remain poor in both disorders3, developing new effective treatments that address binge eating are needed; however, progress is impeded by our limited understanding of the neurobiology of BN/BED.
BN/BED have been conceptualized as impulsive/compulsive eating disorders4 with altered reward sensitivity5 and food-related attentional biases6. Consistent with this model of BN/BED, neuroimaging studies in these patients have highlighted changes in the function, structure and neurochemistry in brain areas involved in incentive processing (such as the orbitofrontal cortex (OFC), ventral tegmental area (VTA), substantia nigra (SN), nucleus accumbens and the amygdala), inhibitory control (such as the medial prefrontal cortex (PFC) and the anterior cingulate (ACG)) and habitual behaviour (such as the dorsal striatum) (see refs. 7,8 for detailed reviews). Most of these studies focused on morphological differences between BN/BED patients and healthy controls. Some studies investigated functional abnormalities using task-based BOLD functional magnetic resonance imaging (fMRI), allowing for inferences that are restricted to the neural circuits employed by the specific tasks. Other studies investigated alterations in spontaneous fluctuations of the BOLD signal at rest, suggesting aberrant functional connectivity in brain networks involved in salience attribution, self-referential processing and cognitive control in people with BN/BED9–13). However, BOLD fMRI (whether focusing on fluctuations at rest or task-evoked changes) typically yields only relative metrics and is prone to several artefacts, including motion, low-frequency physiological noise and baseline drift14. Three other studies, all with small sample sizes, used single-photon emission computerized tomography (SPECT) to measure regional cerebral blood flow (rCBF), which provides a proxy of brain metabolism and neural activity15. These studies reported rCBF increases in the temporal lobes and/or the frontal cortex of patients with BN and BED in response to anticipation or exposure to food/body shape-related stimuli16–18. However, SPECT is a high-cost technique that requires the injection of a radionuclide tracer, making it suboptimal as a routine screening tool in non-specialized centres or for evaluating short-term responses19.
Recently, arterial spin labelling (ASL) MRI has allowed the non-invasive quantification of rCBF with high reproducibility and spatial resolution20. ASL is a quantitative technique and has been used widely to quantify changes in brain physiology associated with various neuropsychiatric disorders21 (e.g., autism spectrum disorder22, schizophrenia23, depression24) and in response to pharmacological treatments25,26. Hence, ASL provides a non-invasive and widely available tool to investigate abnormalities in resting brain physiology of BN/BED and the effects of potential treatments.
One such potential treatment is intranasal oxytocin (OT)27–29. The implication of OT in binge eating is supported by a wide range of evidence. Candidate gene studies have found associations between polymorphisms in the OT receptor gene and binge-purge tendencies in women (rs53576/rs2254298)30, bulimia (rs53576)31 and overeating (rs2268493/rs2268494)32. Similarly, Prader–Willi syndrome patients present with a reduction in OT-synthesizing neurons in the paraventricular nucleus of the hypothalamus33, which is accompanied by compulsive overeating behaviour from early childhood34. However, the only two studies conducted to compare the levels of OT in the cerebrospinal fluid of patients with BN and healthy controls could not find any differences35,36. Pharmacological animal37 and human studies38,39 have demonstrated that OT suppresses eating, including hedonic eating in men39. Although the exact mechanisms underlying the suppressive effects of OT on eating remain to be fully uncovered27,28, current understanding supports a combination of both metabolic and cognitive effects40, including modulation of reward-related signalling41,42. In addition, some small clinical studies starting to explore the potential of intranasal OT in improving disordered eating in patients with BN have shown that intranasal OT (40 IU) reduces caloric intake43 and decreases vigilance towards angry faces44 in women with BN. We have recently shown that a divided dose of intranasal OT (64 IU) modulates risk-taking behaviour in women with BN/BED45 and increases vigilance towards food, vs. neutral, images in a dot probe task in both women with and without BN/BED46. However, in the same cohort of women with BN/BED, we failed to find any effect of the same divided dose of intranasal OT (64 IU) on eating behaviour or stress response47. Apart from the lack of clarity regarding the effectiveness of intranasal OT to improve eating symptomatology in patients with BN/BED, it is also not clear which brain circuits intranasal OT targets in BN/BED patients.
In this study, we investigate abnormalities in resting brain physiology in women with BN/BED using ASL. Studying the brain at rest allows us to investigate baseline alterations in brain physiology that are not restrained to the specific neural circuits engaged by tasks. We further investigate whether an acute dose of 40IU of intranasal OT can restore alterations in resting brain physiology in BN/BED 18–26 min post dosing. We have previously shown that ASL captures OT-induced changes as early as 15–36 min post dosing in resting rCBF after a single acute intranasal administration (40 IU) in both healthy men48,49 and men at clinical high-risk for psychosis (CHR-P)50 (earlier time intervals have simply never been studied). We specifically address two questions: (1) Do women with BN/BED, compared with healthy women, present alterations in resting brain perfusion, as measured using ASL MRI? (2) Can 40 IU intranasal OT restore or attenuate these resting rCBF alterations in women with BN/BED 18–26 min post doing?
Methods
Participants
We recruited 25 women meeting the DSM-5 criteria for either BN (n = 20) or BED (n = 5) and 27 women with no current or prior eating disorder. We excluded three participants (two healthy controls and one BN patient) due to a large discrepancy in the time post dosing that we sampled rCBF in the OT and placebo visits (it exceeded the duration of one rCBF scan, i.e., >8 min). We further excluded two healthy women due to corruption of the data files. Our final sample included 23 healthy and 24 women with BN/BED. Ethical approval for the study was granted by the London—Camberwell St Giles Research Ethics Committee (Reference: 14/LO/2115). All participants provided written informed consent and participated voluntarily. See Supplementary Materials for further details regarding inclusion/exclusion criteria. Our previous work has demonstrated that n = 16 per group is sufficient to quantify OT-induced rCBF changes in men using a between-subjects49 or within-subjects design48.
Study design and procedure
We employed a double-blind, placebo-controlled crossover design. Participants visited our centre on three occasions: one screening and two experimental visits. During screening, we measured height, weight, and collected basic demographics. Participants then completed the Eating Disorder Examination—Questionnaire (EDEQ) Version51 and Depression, Anxiety, and Stress Scales52 online in their own time between the screening and the first experiment visits, to minimize burden during the visits. Both questionnaires have been validated for online application, with online responses matching well the responses of participants in the controlled environment of the lab53,54. To habituate all participants to the scanner environment and minimize its potential distressing impact, all participants were first trained in a mock-scanner.
The experimental visits were conducted 2 days apart, ensuring participants were tested in the same phase of the oestrous cycle for both treatment conditions (OT and placebo). Each participant was asked to report the first day of their last menstrual period and any hormonal contraception they were currently taking. Participants were asked to eat 2.5 h prior to each experimental visit, to control for baseline hunger. All participants were tested at approximately the same time in the early evening (5–7 pm) for both the OT and placebo treatments, to minimize potential circadian variability in resting brain activity55 or OT levels56. Fifty minutes after arrival, participants self-administered 40 IU intranasal OT (Syntocinon, 40 IU/ml; Novartis, Basel, Switzerland) in ten puffs, one puff every 30 s, each puff containing 0.1 ml Syntocinon (4 IU) or placebo (same excipients as Syntocinon except for OT) and alternating between nostrils, over a period of 5 min. Participants were randomly allocated to a treatment order (OT/placebo or placebo/OT). After drug administration, participants were guided to the MRI scanner, where a single pulsed continuous ASL scan (8:20 min) was acquired 18–26 min (±4 mins) post dosing. Our choice of OT dose and post dosing interval was driven by our previous work, whereby we have shown that 40 IU intranasal OT induce robust changes in rCBF in both healthy men (as early as 15–32 min post dosing)48,49 and men at CHR-P (at 22–36 min)50. Furthermore, previous clinical studies have shown beneficial effects of a dose of 40 IU intranasal OT in women with BN43,44.
MRI data acquisition
We used a three-dimensional (3D) pseudo-continuous ASL (3D-pCASL) sequence to measure changes in rCBF over 18–26 min post dosing. Participants were instructed to lie still, maintain their gaze on a centrally placed fixation cross during scanning and to let their mind flow (as per current practice for the acquisition of open-eyes resting-state data57). Labelling of arterial blood was achieved with a 1825 ms train of Hanning-shaped RF pulses in the presence of a net magnetic field gradient along the flow direction (the z-axis of the magnet). After a post-labelling delay of 2025 ms, a whole-brain volume was read using a 3D interleaved “stack-of-spirals” Fast Spin Echo readout58, consisting of 8 interleaved spiral arms in the in-plane direction, with 512 points per spiral interleave. Echo time (TE) was 11.088 ms and repetition time (TR) was 5135 ms. Fifty-six slice partitions of 3 mm thickness were defined in the 3D readout. The in-plane field of view (FOV) was 240 × 240 mm. The spiral sampling of k-space was re-gridded to a rectangular matrix with an approximate in-plane resolution of 3.6 mm. Each sequence used five control-label (C-L) pairs. Individual CBF maps were computed for each of the perfusion weighted difference images derived from every C-L pair, by scaling the difference images against a proton density image acquired at the end of the sequence, using identical readout parameters. This computation was done according to the formula suggested in the recent ASL consensus article59. The sequence uses four background suppression pulses to minimize static tissue signal at the time of image acquisition. We acquired eight 3D-pCASL sequences, with the duration of the entire acquisition time of each sequence being 8:20 min.
A 3D high-spatial-resolution, magnetization prepared rapid acquisition T1-weighted scan was also acquired (FOV of 270 mm, TR/TE/TI = 7.328/3.024/400 ms). The final resolution of the T1-weighted image was 1.1 × 1.1 × 1.2 mm.
MRI data pre-processing
A multi-step approach was performed for the spatial normalization of the CBF maps to Montreal Neurological Institute (MNI) space: (1) co-registration of the proton density image from each sequence to the participant’s T1 image after resetting the origin of both images to the anterior commissure. The transformation matrix of this co-registration step was then applied to the CBF map, to transform the CBF map to the space of the T1-image; (2) unified segmentation of the T1 image; (3) elimination of extra-cerebral signal from the CBF map, by multiplication of the “brain only” binary mask obtained in step (2), with each co-registered CBF map; (4) normalization of the subject’s T1 image and the skull-stripped CBF maps to the MNI152 space using the normalization parameters obtained in step (2). Finally, we spatially smoothed each normalized CBF map using an 8 mm Gaussian smoothing kernel. All of these steps were implemented using the ASAP (Automatic Software for ASL processing) toolbox (version 2.0)60. The resulting smoothed CBF maps were then entered into Statistical Parametric Mapping (SPM) 12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) for group-level statistical analysis, as described below.
Statistical analyses
Diagnosis, treatment and diagnosis × treatment effects on resting rCBF
To investigate the effects of diagnosis and treatment on global CBF, we first extracted mean global CBF values with an explicit binary mask for grey matter using the fslmeants command (FSL suite, http://www.fmrib.ox.a.c.uk/fsl). The binary mask was derived from a standard T1-based probabilistic map of grey-matter distribution by thresholding all voxels with a probability > 0.20. We tested for the main effects of treatment or diagnosis and for the treatment × diagnosis interaction on global grey-matter CBF signal using mixed analysis of variance and the Greenhouse-Geisser correction against violations of sphericity.
We then tested the effects of diagnosis, treatment and diagnosis × treatment on mean rCBF values extracted using fslmeants from 14 regions-of-interest (ROIs) corresponding to anatomical areas shown to be affected in BN/BED in previous structural, functional or neurochemical studies7,8. A detailed description of these ROIs can be found in Supplementary Fig. S1. The VTA, SN and hypothalamus masks were retrieved from a recently published high-resolution probabilistic atlas of human subcortical brain nuclei61. The OFC mask included the areas 14 and 11 m, and the medial PFC included area FPm from the connectivity-based parcellation map in Franz-Xaver Neubert et al.62. All the remaining masks were retrieved from the Harvard-Oxford Atlas distributed with FSL. To create the dorsal striatum masks, we pooled together in one single mask the caudate and putamen masks from the Harvard-Oxford Atlas. For the insula, amygdala, accumbens and dorsal striatum, we decided to consider right and left homologous structures separately, as we have previously described some degree of left lateralization of the effects of intranasal OT on rCBF in men49,63. We used a full factorial linear mixed model, including diagnosis, treatment and diagnosis × treatment as fixed effects, participants as a random effect and global grey-matter CBF as a nuisance variable. All analyses were implemented in SPSS24 (http://www-01.ibm.com/software/uk/analytics/spss/). Results are reported at a level of significance α = 0.05. For the ROI analyses, we contained the false discovery rate for the number of ROIs tested at α = 0.05 using the Benjamini–Hochberg procedure64.
Finally, we conducted a whole-brain exploratory investigation of treatment, diagnosis and treatment × diagnosis effects on rCBF, using global grey-matter CBF as a covariate (see Supplementary Materials for details). We used cluster-level inference at α = 0.05 using family-wise error correction for multiple comparisons and a cluster-forming threshold of P = 0.005 (uncorrected). These statistical thresholds had been determined a priori based on our own work investigating the effects of intranasal OT on rCBF in humans48,49 and are standardly applied in ASL studies measuring rCBF65–70.
Associations between clinical measures and rCBF in women with BN/BED and healthy controls
To investigate whether changes in rCBF in women with BD/BED were related to the severity of clinical symptomatology, we correlated the mean rCBF in each of the four anatomical ROIs where we found significant differences between the BN/BED and healthy groups with clinical measures. We focused our analyses on mean rCBF values extracted from anatomical ROIs to avoid potential issues of selection bias that might have emerged if we had based our analyses on rCBF extracted from clusters showing significant diagnostic group differences in the whole-brain analyses. As a measure of eating symptom severity, we used the global EDEQ scores. As patients with BN/BED score highly on scales measuring anxiety, stress and depression, and scores on these scales were highly correlated with each other, we used within-group principal component analysis (PCA) on these three measures to obtain a single score reflecting affective/stress symptom severity. The first principal component accounted for 70.81% and 83.47% of the total variance in the patients and healthy controls, respectively, and was used in our analyses to examine the specificity of the association of rCBF with eating symptom severity.
We estimated partial Pearson’s correlation coefficients (with bootstrapping—1000 samples) between mean rCBF and global EDEQ or the first component of the stress, anxiety and depression scores in each ROI, adjusting for global CBF and body mass index (BMI). To examine the specificity of the association between mean rCBF and eating symptomatology, we re-estimated the partial correlations with global EDEQ after including scores on the first principal component from the PCA on the stress, anxiety and depression measures. We estimated these correlations separately in patients and healthy controls, because group differences in mean scores on these measures might result in illusory correlations if the two samples were pooled together71. For completeness, we then compared these correlations between the patient and healthy control groups using the Fisher r-to-z transformation.
Post-hoc analyses
BMI
As BMI influences perfusion in the brain72 and patients presented high variability in BMI, we repeated all our analyses including BMI as a nuisance variable to account for BMI-related variability in rCBF.
Hormonal contraception
Hormonal contraception can reduce intranasal OT-induced effects on brain physiology, at least in response to social stimuli73. As 37.5% of our women were under hormonal contraception, we repeated all analyses including hormonal contraception as a nuisance variable.
Diagnostic category, psychiatric comorbidities and current pharmacological treatment
To disentangle whether the main effect of diagnosis was driven specifically by BED status, current pharmacological treatment or the presence of other psychiatric conditions, we repeated our analyses for the main effect of diagnosis either excluding the five BED patients or including diagnostic category and comorbidities/current treatment as nuisance variables.
Grey-matter volume
Variations in grey-matter volume (GMV) can be associated with local variations in brain tissue metabolic demand, thereby influencing rCBF74,75. For this reason, we explored whether the main effects of diagnosis were related to differences in GMV. For each participant, we used GM volume probability maps obtained after segmentation of the T1-weighted structural image and the fslmeants command to estimate mean GMV values in ROIs based on the clusters where BN/BED patients showed significant increases in rCBF compared with controls in the whole-brain analyses (details provided in Supplementary Materials). We then repeated the whole-brain analysis testing for the main effects of diagnosis on rCBF including GMV as a covariate. For completeness, we also ran an exploratory whole-brain analysis testing for the main effect of diagnosis on GMV (further details in Supplementary Material). Our main motivation was to investigate if clusters showing significant effects of diagnosis in rCBF mapped onto brain areas showing significant GMV abnormalities, giving us insight in the interpretation of rCBF abnormalities in BN/BED.
Results
Sample characteristics
Women with BN/BED and healthy controls did not differ in age, height, BMI, educational level, or hormonal status (see Table 1). Women with BN/BED had significantly higher weight, anxiety, depression and stress scores, and EDEQ global and subscale scores compared with healthy women. Women with BN/BED had an average duration of eating disorder of 10.15 years and the average age of onset was 15.90 years old. Of the 25 women with BN/BED, 7 women had a comorbid psychiatric disorder. Specifically, five women had comorbid depression, four women had comorbid generalized anxiety disorder, four women had borderline personality disorder, one woman had social anxiety, one woman had obsessive-compulsive disorder, and one woman had an autism spectrum disorder. At the time of the study, seven women were taking an antidepressant, one woman was taking a mood stabilizer, and one woman was taking an antipsychotic drug. Participants with BN/BED reported an average binge-eating frequency of 14.14 episodes over the past 28 days (SD = 9.88). The women with BN endorsed an average frequency of self-induced vomiting equal to 10.40 occasions over the past 28 days (SD = 13.61), an average laxative abuse frequency of 5.13 occasions over the past 28 days (SD = 8.35), an average frequency of “hard exercise intended to control weight or shape” equal to 7.31 occasions over the past 28 days (SD = 8.57), and 1 participant reported using diuretic pills on 4 occasions over the past 28 days.
Table 1.
Sociodemographic characteristics.
| Variable | Healthy controls | BN/BED | Statistic | p |
|---|---|---|---|---|
| Number | 23 | 25 (20 BN; 5 BED) | - | - |
| Age (years) | 23.6 (3.79) | 25.58 (6.31) | T(42) = 1.231 | 0.225 |
| Height (cm) | 162.85 (5.95) | 166.38 (7.64) | T(46) = 1.684 | 0.099 |
| Weight (kg) | 59.19 (5.51) | 67.13 (15.22) | T(46) = 2.160 | 0.037* |
| BMI (kg/cm2) | 22.57 (1.89) | 24.19 (5.21) | T(46) = 1.407 | 0.166 |
| RQF (education) | 5.31 (1.67) | 4.875 (1.75) | T(42) = 0.836 | 0.408 |
| Hormonal status | ||||
| Contraception | 41.7% | 32.0% | ||
| Follicular phase | 29.2% | 28.0% | χ2 (3) = 5.090 | 0.165 |
| Luteal phase | 8.3% | 32.0% | ||
| Non-available | 20.8% | 8% | ||
| Duration ED | - | 10.15 (5.80) | - | - |
| Age onset ED | - | 15.90 (5.07) | - | - |
| Comorbidities | 0% | 28% | - | - |
| Medication use | 0% | 20% | - | - |
| Frequency (last 28 days): | ||||
| Binge eating | 14.14 (9.88) | |||
| Self-induced vomiting | 10.40 (13.61) | |||
| Laxative abuse | - | 5.13 (8.35) | - | - |
| Excessive exercising to control shape | 7.31 (8.57) | |||
| Eating disorder questionnaire (EQED) | ||||
| Global EQED | 1.17 (1.12) | 3.91 (1.30) | T(43) = 7.794 | <0.0001* |
| Restraint | 0.91 (0.90) | 3.05 (1.76) | T(43) = 5.240 | <0.0001* |
| Eating concern | 0.64 (0.86) | 3.51 (1.39) | T(43) = 8.521 | <0.0001* |
| Weight concern | 1.35 (1.52) | 4.32 (1.40) | T(43) = 7.048 | <0.0001* |
| Shape concern | 1.77 (1.57) | 4.76 (1.39) | T(43) = 6.994 | <0.0001* |
| Depression score | 3.05 (4.49) | 20.39 (12.31) | Mann–Whitney U = 55 | <0.0001* |
| Anxiety score | 1.16 (2.52) | 11.83 (9.56) | Mann–Whitney U = 37 | <0.0001* |
| Stress score | 3.68 (5.04) | 20.33 (11.69) | Mann–Whitney U = 14 | <0.0001* |
This table shows the sociodemographic characteristics of our cohort of healthy and BN/BED women. Data are presented as mean (SD). The fourth and fifth columns summarize the statistics corresponding to groups comparison for each variable, when applicable. Statistical significance was set to p < 0.05 and is highlighted with the symbol *.
Drug blinding
Participants guessed the drug condition correctly on 57 out of the total 104 visits (54.8% of visits), which was not significantly different from chance (one-sample binomial test, z (103) = 57.00, p = 0.377).
Diagnosis, treatment and diagnosis × treatment effects on resting global CBF
There were no effects of diagnosis, treatment or diagnosis × treatment on global grey-matter CBF (Supplementary Fig. S2).
Diagnosis, treatment and diagnosis × treatment effects on resting rCBF
ROI analyses
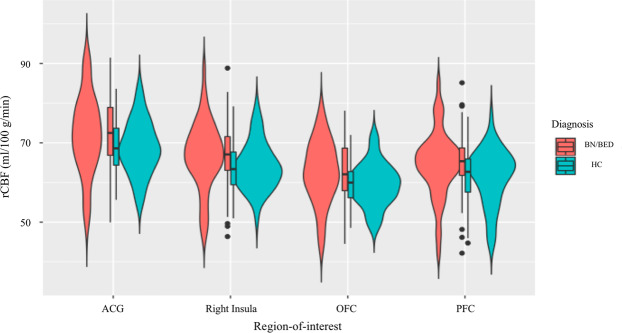
Women with BN/BED showed significant rCBF increases in the medial PFC and OFC ROIs compared with healthy controls (Supplementary Table S1). These differences did not survive correction for the number of total ROIs tested. Accounting for BMI, women with BN/BED showed significant rCBF increases in the medial PFC, OFC, right insula and ACG gyrus ROIs (Table 2 and Fig. 1), which remained significant after correcting for multiple testing.
Table 2.
Effects of diagnosis, treatment and diagnosis × treatment on resting regional cerebral blood flow (rCBF) within neural circuits relevant for BN/BED (hypothesis-driven analysis).
| Region-of-interest | Main effect of treatment | Main effect of diagnosis | Interaction treatment × diagnosis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | P (uncorrected) | P (adjusted) | F | P (uncorrected) | P (adjusted) | F | P (uncorrected) | P (adjusted) | |
| VTA | 0.727 | 0.396 | 1.386 | 0.296 | 0.588 | 0.915 | 0.003 | 0.960 | 0.960 |
| SN | 1.400 | 0.240 | 1.680 | 0.027 | 0.871 | 1.016 | 0.006 | 0.939 | 1.095 |
| Right Amy | 0.154 | 0.696 | 1.624 | 4.231 | 0.043 | 0.120 | 0.008 | 0.927 | 1.179 |
| Left amy | 0.122 | 0.727 | 1.272 | 3.71×10-4 | 0.985 | 0.985 | 0.282 | 0.596 | 1.192 |
| PFC | 1.979 | 0.163 | 2.282 | 7.462 | 0.008 | 0.028 | 0.107 | 0.744 | 1.302 |
| Orbitofrontal | 0.105 | 0.746 | 1.160 | 9.100 | 0.003 | 0.042 | 0.005 | 0.944 | 1.016 |
| Insula right | 0.058 | 0.811 | 1.032 | 7.854 | 0.006 | 0.028 | 0.970 | 0.327 | 1,526 |
| Insula left | 0.038 | 0.846 | 0.846 | 1.264 | 0.264 | 0.528 | 0.038 | 0.846 | 1.184 |
| HPT | 1.315 | 0.254 | 1.185 | 0.254 | 0.616 | 0.862 | 0.076 | 0.784 | 1.219 |
| ACG | 0.134 | 0.715 | 1.430 | 8.347 | 0.005 | 0.035 | 0.596 | 0.442 | 1.031 |
| Dorsal striatum right | 0.080 | 0.778 | 1.089 | 2.983 | 0.088 | 0.205 | 0.965 | 0.329 | 1.152 |
| Dorsal striatum left | 0.054 | 0.817 | 0.953 | 0.019 | 0.892 | 0.961 | 0.985 | 0.324 | 2.268 |
| Acc right | 0.304 | 0.583 | 1.632 | 0.657 | 0.420 | 0.735 | 0.802 | 0.373 | 1.044 |
| Acc left | 0.040 | 0.842 | 0.907 | 0.042 | 0.838 | 1.067 | 1.200 | 0.276 | 3.864 |
This table shows the results of a hypothesis-driven investigation of the effects of diagnosis, treatment and diagnosis × treatment on rCBF within 14 anatomical regions-of-interest suggested to be involved in BN/BED. We tested these effects in a liner mixed model, controlling for global grey-matter cerebral blood flow and BMI. Statistical significance was set to p < 0.05, after correction for multiple testing with the Benjamini–Hochberg procedure.
Acc accumbens, ACG anterior cingulate gyrus, Amy amygdala, PFC prefrontal cortext, HPT hypothalamus, SN substantia nigra, VTA ventral tegmental area.
Fig. 1. Increases in mean resting regional cerebral blood flow (rCBF) in the anterior cingulate, right insula, orbitofrontal and medial prefrontal cortices in BN/BED women compared with healthy women (hypothesis-driven analysis).
These graphs illustrate the changes in mean rCBF in BN/BED patients compared with healthy controls for the regions-of-interest where we identified a significant main effect of diagnosis. For all of these four regions-of-interest, BN/BED women presented higher mean rCBF than healthy women. ACG anterior cingulate gyrus, OFC orbitofrontal cortex, PFC medial prefrontal cortex. Box plots and violin plots depicting mean rCBF (marginal means) on each region-of-interest for each diagnosis/treatment groups; middle horizontal lines represent the median; boxes indicate the 25th and 75th percentiles.
Whole-brain analysis
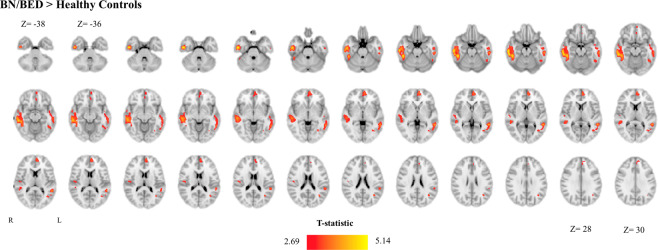
Exploratory whole-brain analysis revealed rCBF increases in BN/BED patients in three clusters (Supplementary Fig. S3 and Supplementary Table S2). Two clusters spanned the inferior/middle temporal gyrus bilaterally (with a bigger extent at the right lobe) and one cluster the PFC, medial OFC and ACG bilaterally (extending more into the left hemisphere). Accounting for BMI extended the right inferior/middle temporal gyrus cluster to include the right posterior insula (Fig. 2 and Supplementary Table S3).
Fig. 2. Increases in resting regional cerebral blood flow (rCBF) in the brain of BN/BED patients (whole-brain analysis).
This figure shows the results of a directed T-contrast analysis at the whole-brain level where we tested for increases (BN/BED > Controls) or decreases (Controls > BN/BED) in rCBF in patients compared with controls, accounting for global grey-matter cerebral blood flow and BMI. Whole-brain cluster-level inference was applied at α = 0.05 using family-wise error (FWE) correction for multiple comparisons and a cluster-forming threshold of p = 0.005 (uncorrected). Images are shown as T-statistic in radiological convention. We did not find any significant cluster BN/BED patients presented lower rCBF than healthy controls.
We did not observe any treatment or treatment × diagnosis effects.
Associations between clinical symptoms and rCBF in women with BN/BED and healthy controls
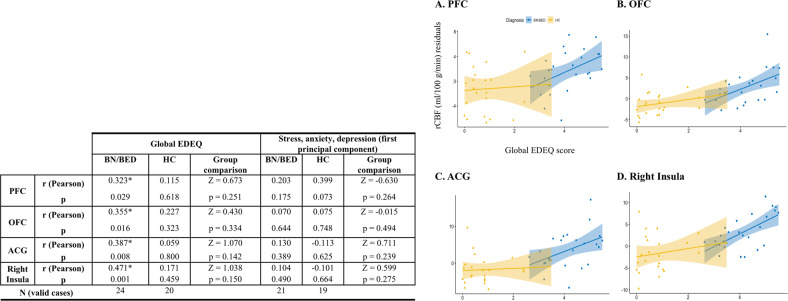
We observed significant positive partial correlations (adjusting for BMI and global CBF) between mean rCBF extracted from the medial PFC, OFC, ACG and right insula ROIs and global EDEQ scores, in patients but not in controls (Fig. 3). Partial correlations between mean rCBF in these ROIs and global EDEQ were still significant after additionally accounting for stress, anxiety and depression (Supplementary Table S4), except for the medial PFC ROI for which the correlation was at trend-level (p = 0.051). However, direct comparisons of these correlations between the two groups yielded no statistically significant differences (Fig. 3 and Supplementary Table S4).
Fig. 3. Increases in resting regional cerebral blood flow (rCBF) in the brain of BN/BED patients correlate positively with eating disorder symptom severity.
Left panel: partial Pearson correlations between mean rCBF in each of the four anatomical regions-of-interest where we found significant differences between the BN/BED and healthy groups, and clinical symptomatology. We used the global EDEQ scores as a measure of eating disorder symptom severity and the first principal component of the anxiety, stress and depression scores. Partial Pearson correlations were calculated with bootstrapping (1000 samples) adjusting for global CBF and BMI, separately for controls and patients. In the last columns of each sub-section, we present the result of the statistical comparison of the correlations between the two groups, as assessed by Fisher’s r-to-z transformation. Right panel, we present scatter plots depicting the relationship between mean rCBF (marginal scores after regressing out the effects of global CBF and BMI in the y-axis) and global EDEQ scores (in the x-axis) in each region-of-interest. Statistical significance was set to p < 0.05 and is highlighted with the symbol *. PFC medial prefrontal cortex, OFC orbitofrontal cortex; ACG anterior cingulate gyrus.
Post-hoc analyses
Hormonal contraception
Accounting for use of hormonal contraception produced no change in our findings (Supplementary Table S5 and Supplementary Fig. S4).
Diagnostic category, psychiatric comorbidities and current pharmacological treatment
Excluding the BED patients (Supplementary Fig. S5) or accounting for diagnostic category (Supplementary Fig. S6) as a covariate in our model produced did not substantially change our results. We also did not find any substantial changes in our results when we accounted for comorbidities/current drug treatment (Supplementary Fig. S7).
Is regional GMV associated with the effect of diagnosis in rCBF?
The main effect of diagnosis in the whole-brain analysis remained virtually unaltered after accounting for GMV values in the ROIs based on the clusters where BN/BED patients showed significant increases in rCBF compared with controls in the whole-brain analyses (Supplementary Fig. S8). Furthermore, women with BN/BED showed lower GMV than controls in two clusters, one spanning the postcentral and precentral gyri and another the inferior/middle temporal gyrus, both in the right hemisphere (Supplementary Fig. S9). Interestingly, the right temporal gyrus cluster partially overlapped with the cluster showing rCBF increases in women with BD/BED compared with controls (Supplementary Fig. S9). In the right temporal gyrus, rCBF and GMV correlated negatively in patients but not in controls (Supplementary Fig. S10) (BN/BED: r = −0.496, p = 0.012; healthy controls: r = −0.024, p = 0.913; Groups comparison: z = −1.66, p = 0.048). We did not identify any area in the brain where patients presented higher GMV than controls.
Discussion
Using ASL, a highly reproducible and widely available quantitative MRI technique, we demonstrated, for the first time, resting hyperperfusion abnormalities in women with BN/BED, compared with healthy controls, in key neural circuits implicated in BN/BED psychopathology. Hyperperfusion abnormalities in the PFC, medial OFC, ACG and the right posterior insula were positively and specifically associated with symptom severity as reflected on the global EDEQ scale in women with BN/BED. However, a single dose of 40 IU intranasal OT did not restore or attenuate these perfusion abnormalities at 18–26 min post dosing. Our findings enhance our understanding of resting brain abnormalities in BN/BED and identify resting rCBF as a non-invasive potential biomarker for disease-related changes and treatment monitoring. We discuss each of our main findings below in turn.
Abnormalities in resting brain perfusion in women with BN/BED compared with healthy controls
Our hypothesis-driven analyses showed that women with BN/BED, compared with healthy controls, presented with increased resting rCBF in the medial PFC, medial OFC, ACG and the right posterior insula. Exploratory whole-brain analysis showed further increases in the inferior/middle/superior temporal cortices in women with BN/BED. Sensitivity analyses confirmed that these findings were not driven by the inclusion of BED patients and did not reflect current medication or other psychiatric comorbidities. In women with BN/BED, estimates of rCBF in three key areas where we found women with BN/BED and healthy women to differ in rCBF (medial PFC, ACG and right insula) correlated positively with global scores on the EDEQ, even after adjusting for anxiety, depression and stress, supporting a specific association between rCBF abnormalities in these regions and the underlying eating disorder psychopathology. Given the high reliability and ease of acquisition, our findings suggest that measuring resting rCBF at BN/BED patients with ASL MRI can offer a non-invasive in-vivo biomarker of potential value for understanding disease-related functional changes, posing minimal demand and no risk on patients. In addition, future large-scale studies should characterize the sensitivity and specificity of changes in resting rCBF in the identified brain regions in terms of distinguishing between patients and controls, or distinguishing between patients who respond to specific treatments from those who do not.
We are aware of only three studies examining rCBF in BN/BED, using SPECT and small sample sizes (7 < n < 14). One study found increased rCBF in the right temporal lobe in response to own body images when a pooled sample of patients with DSM-IV BN-purging type and anorexia nervosa-restrictive type were compared with a pooled sample of healthy controls, patients with DSM-IV BN-non-purging type and patients with anorexia nervosa-purging type17. Another study reported increased rCBF in the left temporal lobe and inferior frontal region bilaterally in patients with DSM-III BN before eating16. Finally, the only study examining patients with BED reported that obese BED patients, compared with non-obese BED patients and healthy controls, presented increased rCBF responses in the frontal and prefrontal cortices in response to food visual stimuli18. These studies did not report on rCBF changes at rest, which precludes direct comparisons between these studies and ours. Nevertheless, we note that the changes in rCBF reported in these studies are in the same direction and largely overlap with regions where we found resting hyperperfusion during BN/BED in our study. Therefore, it is possible that hyperperfusion in these areas in women with BN/BED marks a disease-relevant state, which can be captured at rest and without the need of a specific experimental manipulation.
The mechanisms behind these increases in resting rCBF in BN/BED remain unknown. Increases in resting rCBF are likely to reflect basal regional hypermetabolism, potentially associated with increases in resting neural activity15. We note however that the few small-scale studies evaluating resting brain glucose metabolism using PET during BN have generally reported decreases in global and regional glucose metabolism, including parietal and anterior frontal hypometabolism76–80. The only exception is one study that found increased glucose metabolism in the temporal lobes of patients with BN, which matches our findings of increased perfusion in these areas78. BN patients have been reported to present higher GMV in some areas such as the medial OFC8, which could account for the increases in rCBF we report herein; however, we found that this is unlikely to be the case in our sample. Exploratory whole-brain analysis showed decreased GMV in the right temporal lobe in BN/BED compared with controls. This cluster partially overlapped with one cluster showing increased rCBF in patients. GMV was negatively correlated with rCBF in this area in patients, but not in controls. We speculate that two possible mechanisms could account for this paradoxical relationship between rCBF and GMV in the temporal lobe of patients with BN/BED. One possibility is that the increases in rCBF we observed in the right temporal lobe might reflect a mechanism of local functional plasticity in response to GMV loss in this region, which ultimately may help to maintain temporal lobe function in patients with BN/BED81,82. Alternatively, it is also conceivable that enduring neural hyperactivity, accompanied by increases in rCBF, could drive GMV loss due to excitotoxicity83. Future longitudinal studies examining how changes in GMV and rCBF in these brain regions develop may elucidate these hypotheses.
As our data were acquired at rest, we can only speculate regarding the specific contribution of the perfusion and structural abnormalities observed in this study to core behavioural manifestations in BN/BED. We note that BN/BED patients have been reported to present with enhanced sensitivity to the anticipation of food reward/hedonic value5, which matches the increases in rCBF we observed in the medial OFC (a key area involved in reward/outcome value processing84, including the hedonic value of food85). BN, in particular, and, to some extent, BED have also been associated with continuous feelings of monitoring of binge-activating stimuli in the environment and body shape/weight self-judgement, which match our findings of increased rCBF in the medial PFC and ACC (two key areas involved in self-monitoring and control regulation86) in patients. Evidence from lesion studies have supported a direct implication of the temporal lobe (predominantly the right lobe) in eating disorders87. Although the exact mechanisms by which temporal lobe dysfunction may contribute to eating dysregulation remain elusive, some studies have implicated the superior/middle temporal gyri in the brain responses to palatable food88 and in inhibitory control, including cognitive control of appetite89—two core elements of binge eating. Importantly, previous studies have reported attenuated recruitment of these areas during response inhibition in both BN90,91 and BED92.
In contrast to our hypotheses, we could not find any resting rCBF alterations in the ventral or dorsal striatum in patients with BN/BED. This is surprising, given the pivotal role of the striatal circuits in cognitive processes known to be disrupted in BN/BED (such as incentive and habitual behaviours93, impulsivity94 and self-regulation95) and previous neuroimaging studies demonstrating functional and structural abnormalities in these areas (see refs. 7,8 for detailed reviews). It is possible that while disrupted rCBF in the striatum in BN/BED may not manifest at rest, alterations may become evident in condition engaging this region, such as the anticipation or valuation of hedonic stimuli (e.g., food).
Does intranasal OT restore rCBF abnormalities in women with BN/BED?
The lack of treatment or treatment × diagnosis effects does not support a normalizing effect of 40 IU intranasal OT on resting rCBF abnormalities in women with BN/BED 18–26 min post dosing. A number of reasons might explain the lack of effects of intranasal OT on rCBF in this study. First, it is possible that we may have missed the active time-window for intranasal OT treatment effects in women with BN/BED. We have previously mapped intranasal OT-induced effects on brain perfusion in men and demonstrated that while 40 IU intranasal OT can induce changes in resting perfusion as early as 15 min post dosing (earlier intervals have not been sampled), the effects do vary as a function of method of administration, dose and the latency of the sampling interval post dosing48. However, we are still lacking an in-depth pharmacodynamics investigation of OT-induced changes in rCBF in women. Previous studies have shown that the effects of the administration of the same dose of intranasal OT to men and women can result in different effects on behaviour and brain function96–100. A recent study has shown that differences in the effects of a range of doses of intranasal OT on brain responses to happy and fearful faces between men and women do not simply reflect differences in dose sensitivity, but rather gender-specific effects101. Therefore, although we have informed this current study based on our in-depth characterizations of the pharmacodynamics of intranasal OT in men, it is possible that crucial differences exist in the spatiotemporal pattern of rCBF changes after intranasal OT between genders. Future studies should systematically investigate the potential effects of these factors, including a comparison of acute vs. chronic regimens of administration, characterization of rCBF changes over an extended period of time and dose–response studies in women. Second, hormonal contraception has been shown to blunt responses to intranasal OT in women73. Although accounting for contraception in our analyses produced virtually no change in our results, it is plausible that OT might have exerted an effect on rCBF in our sample in the absence of hormonal contraception. Finally, while we could not detect any significant treatment effects on rCBF in women with or without BN/BED at rest in this study, we cannot exclude that significant effects of intranasal OT might emerge during targeted experimental challenges, such as exposure to food cues102 or stress103. Indeed, we have recently shown that a divided dose of intranasal OT (64 IU) modulates risk-taking behaviour in women with BN/BED45 and increases vigilance towards food, vs. neutral, images in a dot probe task in both women with and without BN/BED46 (but does not affect eating behaviour or stress response47), using the same sample.
Limitations
One limitation of our study is that we only included women. Our findings should thus not be extrapolated to men with BN/BED. Second, we could only enrol a small number of BED patients, which did not allow us to isolate effects related to diagnostic category. Third, although we have asked subjects to eat 2.5 h before our experimental sessions in order to minimize the effects of baseline hunger, we did not standardized the amount or type of food consumed, which may have introduced some noise in our data. Lastly, 20% of our BN/BED sample had other psychiatric comorbidities and 28% were under current pharmacological treatment, respectively, which may have confounded our results. We have investigated the impact of such factors in a sensitivity analysis where we repeated our main analyses including current treatment and comorbidities as a nuisance variable. Although our findings remained largely unaltered, future replication in samples of women with BN/BED and without these confounds would be beneficial.
Conclusions
BN/BED in women is accompanied by increased resting perfusion in key brain areas potentially associated with the underlying eating psychopathology. Intranasal OT did not attenuate or restore these abnormalities, at least for the specific dose and post-dosing interval examined. Future studies examining a more comprehensive range of doses, time-windows and schemes of administration will be needed before we can ascertain the therapeutic value of intranasal OT in improving resting rCBF disturbances in women with BN/BED. Given the high reliability and ease of acquisition, measuring resting rCBF at BN/BED patients with ASL MRI offers a promising non-invasive in-vivo biomarker of functional changes in these patients, with potential implications for diagnosis and treatment monitoring.
Supplementary information
Acknowledgements
The authors wish to thank the study volunteers for their participation. This study was partly funded by a Swiss Fund for Anorexia Nervosa grant (43-14) to J.T., a Guy’s and St. Thomas’ NHS Foundation Trust grant to J.T., a King’s Health Partners Challenge Fund to J.T. and an Economic and Social Research Council Grant (ES/K009400/1) to Y.P.
Author contributions
Y.P. and J.T. designed the study. M.L. collected the data. D.M. performed data analysis. F.Z. contributed to the pulse sequence implementation. S.R. and F.Z. contributed to data analysis. D.M. and Y.P. interpreted the data and drafted the first version of the manuscript. All authors (D.M., M.L., S.R., F.Z., J.T., Y.P.) provided critical revisions and approved the final version of the manuscript to be published.
Data availability
Data can be provided upon request.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Daniel Martins, Monica Leslie
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-020-00871-w).
References
- 1.Smith KE, et al. The validity of DSM-5 severity specifiers for anorexia nervosa, bulimia nervosa, and binge-eating disorder. Int. J. Eat. Disord. 2017;50:1109–1113. doi: 10.1002/eat.22739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarenga MS, et al. Eating attitudes of anorexia nervosa, bulimia nervosa, binge eating disorder and obesity without eating disorder female patients: differences and similarities. Physiol. Behav. 2014;131:99–104. doi: 10.1016/j.physbeh.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 3.Aguera Z, et al. Short-term treatment outcomes and dropout risk in men and women with eating disorders. Eur. Eat. Disord. Rev. 2017;25:293–301. doi: 10.1002/erv.2519. [DOI] [PubMed] [Google Scholar]
- 4.Turton R, Chami R, Treasure J. Emotional eating, binge eating and animal models of binge-type eating disorders. Curr. Obes. Rep. 2017;6:217–228. doi: 10.1007/s13679-017-0265-8. [DOI] [PubMed] [Google Scholar]
- 5.Schienle A, Schafer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol. Psychiatry. 2009;65:654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Stojek M, et al. A systematic review of attentional biases in disorders involving binge eating. Appetite. 2018;123:367–389. doi: 10.1016/j.appet.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessler RM, Hutson PH, Herman BK, Potenza MN. The neurobiological basis of binge-eating disorder. Neurosci. Biobehav. Rev. 2016;63:223–238. doi: 10.1016/j.neubiorev.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly B, et al. Neuroimaging in bulimia nervosa and binge eating disorder: a systematic review. J. Eat. Disord. 2018;6:3. doi: 10.1186/s40337-018-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domakonda MJ, He X, Lee S, Cyr M, Marsh R. Increased functional connectivity between ventral attention and default mode networks in adolescents with bulimia nervosa. J. Am. Acad. Child Adolesc. Psychiatry. 2019;58:232–241. doi: 10.1016/j.jaac.2018.09.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amianto F, et al. Intrinsic connectivity networks within cerebellum and beyond in eating disorders. Cerebellum. 2013;12:623–631. doi: 10.1007/s12311-013-0471-1. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, et al. Altered intrinsic functional brain architecture in female patients with bulimia nervosa. J. Psychiatry Neurosci. 2017;42:414–423. doi: 10.1503/jpn.160183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunlop K, et al. Increases in frontostriatal connectivity are associated with response to dorsomedial repetitive transcranial magnetic stimulation in refractory binge/purge behaviors. Neuroimage Clin. 2015;8:611–618. doi: 10.1016/j.nicl.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, et al. Resting-state synchrony between anterior cingulate cortex and precuneus relates to body shape concern in anorexia nervosa and bulimia nervosa. Psychiatry Res. 2014;221:43–48. doi: 10.1016/j.pscychresns.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Turner, R. Uses, misuses, new uses and fundamental limitations of magnetic resonance imaging in cognitive science. Phil. Trans. R. Soc. Lond. B Biol. Sci.371, 10.1098/rstb.2015.0349 (2016). [DOI] [PMC free article] [PubMed]
- 15.Paulson OB, Hasselbalch SG, Rostrup E, Knudsen GM, Pelligrino D. Cerebral blood flow response to functional activation. J. Cereb. Blood Flow. Metab. 2010;30:2–14. doi: 10.1038/jcbfm.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nozoe S, et al. Comparison of regional cerebral blood flow in patients with eating disorders. Brain Res. Bull. 1995;36:251–255. doi: 10.1016/0361-9230(94)00199-b. [DOI] [PubMed] [Google Scholar]
- 17.Beato-Fernandez L, Rodriguez-Cano T, Garcia-Vilches I. Psychopathological alterations and neuroimaging findings with discriminant value in eating behavior disorders. Acta Esp. Psiquiatr. 2011;39:203–210. [PubMed] [Google Scholar]
- 18.Karhunen LJ, et al. Regional cerebral blood flow during exposure to food in obese binge eating women. Psychiatry Res. 2000;99:29–42. doi: 10.1016/s0925-4927(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 19.Bateman TM. Advantages and disadvantages of PET and SPECT in a busy clinical practice. J. Nucl. Cardiol. 2012;19:S3–S11. doi: 10.1007/s12350-011-9490-9. [DOI] [PubMed] [Google Scholar]
- 20.Hodkinson DJ, et al. Quantifying the test-retest reliability of cerebral blood flow measurements in a clinical model of on-going post-surgical pain: a study using pseudo-continuous arterial spin labelling. Neuroimage Clin. 2013;3:301–310. doi: 10.1016/j.nicl.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf RL, Detre JA. Clinical neuroimaging using arterial spin-labeled perfusion magnetic resonance Imaging. Neurotherapeutics. 2007;4:346–359. doi: 10.1016/j.nurt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson, B. S. et al. Hyperperfusion of frontal white and subcortical gray matter in autism spectrum disorder. Biol. Psychiatry.10.1016/j.biopsych.2018.11.026 (2018). [DOI] [PMC free article] [PubMed]
- 23.Ota M, et al. Pseudo-continuous arterial spin labeling MRI study of schizophrenic patients. Schizophr. Res. 2014;154:113–118. doi: 10.1016/j.schres.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 24.Colloby SJ, et al. Regional cerebral blood flow in late-life depression: arterial spin labelling magnetic resonance study. Br. J. Psychiatry. 2012;200:150–155. doi: 10.1192/bjp.bp.111.092387. [DOI] [PubMed] [Google Scholar]
- 25.Wang DJJ, Chen YF, Fernandez-Seara MA, Detre JA. Potentials and challenges for arterial spin labeling in pharmacological magnetic resonance imaging. J. Pharmacol. Exp. Therapeutics. 2011;337:359–366. doi: 10.1124/jpet.110.172577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart SB, Koller JM, Campbell MC, Black KJ. Arterial spin labeling versus BOLD in direct challenge and drug-task interaction pharmacological fMRI. PeerJ. 2014;2:e687. doi: 10.7717/peerj.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plessow F, Eddy KT, Lawson EA. The neuropeptide hormone oxytocin in eating disorders. Curr. Psychiatry Rep. 2018;20:91. doi: 10.1007/s11920-018-0957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giel K, Zipfel S, Hallschmid M. Oxytocin and eating disorders: a narrative review on emerging findings and perspectives. Curr. Neuropharmacol. 2018;16:1111–1121. doi: 10.2174/1570159X15666171128143158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romano A, Tempesta B, Micioni Di Bonaventura MV, Gaetani S. From autism to eating disorders and more: the role of oxytocin in neuropsychiatric disorders. Front. Neurosci. 2015;9:497. doi: 10.3389/fnins.2015.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Micali N, Crous-Bou M, Treasure J, Lawson EA. Association between oxytocin receptor genotype, maternal care, and eating disorder behaviours in a community sample of women. Eur. Eat. Disord. Rev.: J. Eat. Disord. Assoc. 2017;25:19–25. doi: 10.1002/erv.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YR, Kim JH, Kim CH, Shin JG, Treasure J. Association between the oxytocin receptor gene polymorphism (rs53576) and bulimia nervosa. Eur. Eat. Disord. Rev. 2015;23:171–178. doi: 10.1002/erv.2354. [DOI] [PubMed] [Google Scholar]
- 32.Davis, C., Patte, K., Zai, C. & Kennedy, J. L. Polymorphisms of the oxytocin receptor gene and overeating: the intermediary role of endophenotypic risk factors. Nutr. Diabetes7, 10.1038/nutd.2017.24 (2017). [DOI] [PMC free article] [PubMed]
- 33.Swaab DF, Purba JS, Hofman MA. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J. Clin. Endocrinol. Metab. 1995;80:573–579. doi: 10.1210/jcem.80.2.7852523. [DOI] [PubMed] [Google Scholar]
- 34.Young J, et al. A measure of food seeking in individuals with Prader-Willi syndrome. J. Intellect. Disabil. Res. 2006;50:18–24. doi: 10.1111/j.1365-2788.2005.00724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank GK, Kaye WH, Altemus M, Greeno CG. CSF oxytocin and vasopressin levels after recovery from bulimia nervosa and anorexia nervosa, bulimic subtype. Biol. Psychiatry. 2000;48:315–318. doi: 10.1016/s0006-3223(00)00243-2. [DOI] [PubMed] [Google Scholar]
- 36.Demitrack MA, et al. CSF oxytocin in anorexia nervosa and bulimia nervosa: clinical and pathophysiologic considerations. Am. J. Psychiatry. 1990;147:882–886. doi: 10.1176/ajp.147.7.882. [DOI] [PubMed] [Google Scholar]
- 37.Leslie, M., Silva, P., Paloyelis, Y., Blevins, J. & Treasure, J. A systematic review and quantitative meta‐analysis of oxytocin’s effects on feeding. J. Neuroendocrinol. e12584, 10.1111/jne.12584 (2018). [DOI] [PMC free article] [PubMed]
- 38.Thienel M, et al. Oxytocin’s inhibitory effect on food intake is stronger in obese than normal-weight men. Int. J. Obes. 2016;40:1707–1714. doi: 10.1038/ijo.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ott V, et al. Oxytocin reduces reward-driven food intake in humans. Diabetes. 2013;62:3418–3425. doi: 10.2337/db13-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawson EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nat. Rev. Endocrinol. 2017;13:700–709. doi: 10.1038/nrendo.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerem, L., Hadjikhani, N., Holsen, L., Lawson, E. A. & Plessow, F. Oxytocin reduces the functional connectivity between brain regions involved in eating behavior in men with overweight and obesity. Int. J. Obes.10.1038/s41366-019-0489-7 (2019). [DOI] [PMC free article] [PubMed]
- 42.Spetter MS, et al. Oxytocin curbs calorie intake via food-specific increases in the activity of brain areas that process reward and establish cognitive control. Sci. Rep. 2018;8:2736. doi: 10.1038/s41598-018-20963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YR, Eom JS, Yang JW, Kang J, Treasure J. The impact of oxytocin on food intake and emotion recognition in patients with eating disorders: a double blind single dose within-subject cross-over design. PLoS ONE. 2015;10:e0137514. doi: 10.1371/journal.pone.0137514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YR, Eom JS, Leppanen J, Leslie M, Treasure J. Effects of intranasal oxytocin on the attentional bias to emotional stimuli in patients with bulimia nervosa. Psychoneuroendocrinology. 2018;91:75–78. doi: 10.1016/j.psyneuen.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 45.Leslie M, Leppanen J, Paloyelis Y, Nazar BP, Treasure J. The influence of oxytocin on risk-taking in the balloon analogue risk task among women with bulimia nervosa and binge eating disorder. J. Neuroendocrinol. 2019;31:e12771. doi: 10.1111/jne.12771. [DOI] [PubMed] [Google Scholar]
- 46.Leslie ML, Paloyelis J, Treasure Y. A pilot study investigating the influence of oxytocin on attentional bias to food images in women with bulimia nervosa or binge eating disorder. J. Neuroendocrinol. 2020;32:e12843 . doi: 10.1111/jne.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leslie M, Leppanen J, Paloyelis Y, Treasure J. The influence of oxytocin on eating behaviours and stress in women with bulimia nervosa and binge eating disorder. Mol. Cell. Endocrinol. 2019;497:110354. doi: 10.1016/j.mce.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 48.Martins DA, et al. Effects of route of administration on oxytocin-induced changes in regional cerebral blood flow in humans. Nat. Commun. 2020;11:1160. doi: 10.1038/s41467-020-14845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paloyelis Y, et al. A spatiotemporal profile of in vivo cerebral blood flow changes following intranasal oxytocin in humans. Biol. Psychiatry. 2016;79:693–705. doi: 10.1016/j.biopsych.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Davies C, et al. Oxytocin modulates hippocampal perfusion in people at clinical high risk for psychosis. Neuropsychopharmacology. 2019 doi: 10.1038/s41386-018-0311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fairburn CG, Beglin SJ. Assessment of eating disorders–interview or self-report questionnaire. Int. J. Eat. Disord. 1994;16:363–370. [PubMed] [Google Scholar]
- 52.Lovibond PF, Lovibond SH. The structure of negative emotional states - comparison of the depression anxiety stress scales (Dass) with the beck depression and anxiety inventories. Behav. Res. Ther. 1995;33:335–343. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- 53.Chan CW, Leung SF. Validation of the eating disorder examination questionnaire: an online version. J. Hum. Nutr. Diet. 2015;28:659–665. doi: 10.1111/jhn.12275. [DOI] [PubMed] [Google Scholar]
- 54.Shea TL, Tennant A, Pallant JF. Rasch model analysis of the depression, anxiety and stress scales (DASS) BMC Psychiatry. 2009;9:21. doi: 10.1186/1471-244X-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fafrowicz M, et al. Beyond the low frequency fluctuations: morning and evening differences in human brain. Front. Hum. Neurosci. 2019;13:288. doi: 10.3389/fnhum.2019.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kagerbauer SM, et al. Absence of a diurnal rhythm of oxytocin and arginine-vasopressin in human cerebrospinal fluid, blood and saliva. Neuropeptides. 2019;78:101977. doi: 10.1016/j.npep.2019.101977. [DOI] [PubMed] [Google Scholar]
- 57.Snyder AZ, Raichle ME. A brief history of the resting state: the Washington University perspective. Neuroimage. 2012;62:902–910. doi: 10.1016/j.neuroimage.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thedens DR, Irarrazaval P, Sachs TS, Meyer CH, Nishimura DG. Fast magnetic resonance coronary angiography with a three-dimensional stack of spirals trajectory. Magn. Reson. Med. 1999;41:1170–1179. doi: 10.1002/(sici)1522-2594(199906)41:6<1170::aid-mrm13>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 59.Alsop DC, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015;73:102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mato Abad V, Garcia-Polo P, O’Daly O, Hernandez-Tamames JA, Zelaya F. ASAP (Automatic Software for ASL Processing): a toolbox for processing arterial spin labeling images. Magn. Reson. Imaging. 2016;34:334–344. doi: 10.1016/j.mri.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 61.Pauli WM, Nili AN, Tyszka JM. Data descriptor: a high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci. Data. 2018;5:180063. doi: 10.1038/sdata.2018.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neubert FX, Mars RB, Sallet J, Rushworth MFS. Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc. Natl Acad. Sci. USA. 2015;112:E2695–E2704. doi: 10.1073/pnas.1410767112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martins D., et al. Do direct nose-to-brain pathways underlie intranasal oxytocin-induced changes in regional cerebral blood flow in humans? bioRxiv, 10.1101/563056 (2018).
- 64.Feser WJ, Fingerlin TE, Strand MJ, Glueck DH. Calculating average power for the Benjamini-Hochberg procedure. J. Stat. Theory Appl. 2009;8:325–352. [PMC free article] [PubMed] [Google Scholar]
- 65.Mutsaerts H, et al. Cerebral perfusion changes in presymptomatic genetic frontotemporal dementia: a GENFI study. Brain. 2019;142:1108–1120. doi: 10.1093/brain/awz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takeuchi H, et al. Cerebral blood flow during rest associates with general intelligence and creativity. PLoS ONE. 2011;6:e25532. doi: 10.1371/journal.pone.0025532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joe AY, et al. Response-dependent differences in regional cerebral blood flow changes with citalopram in treatment of major depression. J. Nucl. Med. 2006;47:1319–1325. [PubMed] [Google Scholar]
- 68.Thomas BP, et al. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J. Magn. Reson. Imaging. 2013;38:1177–1183. doi: 10.1002/jmri.24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loggia ML, et al. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nwokolo M, et al. Hypoglycemic thalamic activation in type 1 diabetes is associated with preserved symptoms despite reduced epinephrine. J. Cereb. Blood Flow Metab. 2020;40:787–798. doi: 10.1177/0271678X19842680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paloyelis Y, Stahl DR, Mehta M. Are steeper discounting rates in attention-deficit/hyperactivity disorder specifically associated with hyperactivity-impulsivity symptoms or is this a statistical artifact? Biol. Psychiatry. 2010;68:e15–e16. doi: 10.1016/j.biopsych.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 72.Clement P, et al. Variability of physiological brain perfusion in healthy subjects - a systematic review of modifiers. Considerations for multi-center ASL studies. J. Cereb. Blood Flow Metab. 2018;38:1418–1437. doi: 10.1177/0271678X17702156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheele D, Plota J, Stoffel-Wagner B, Maier W, Hurlemann R. Hormonal contraceptives suppress oxytocin-induced brain reward responses to the partner’s face. Soc. Cogn. Affect. Neurosci. 2016;11:767–774. doi: 10.1093/scan/nsv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vasic N, et al. Baseline brain perfusion and brain structure in patients with major depression: a multimodal magnetic resonance imaging study. J. Psychiatry Neurosci. 2015;40:412–421. doi: 10.1503/jpn.140246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steffener J, Brickman AM, Habeck CG, Salthouse TA, Stern Y. Cerebral blood flow and gray matter volume covariance patterns of cognition in aging. Hum. Brain Mapp. 2013;34:3267–3279. doi: 10.1002/hbm.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Delvenne V, Goldman S, De Maertelaer V, Lotstra F. Brain glucose metabolism in eating disorders assessed by positron emission tomography. Int. J. Eat. Disord. 1999;25:29–37. doi: 10.1002/(sici)1098-108x(199901)25:1<29::aid-eat4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 77.Delvenne V, Goldman S, Simon Y, DeMaertelaer V, Lotstra F. Brain hypometabolism of glucose in bulimia nervosa. Int. J. Eat. Disord. 1997;21:313–320. doi: 10.1002/(sici)1098-108x(1997)21:4<313::aid-eat2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 78.Andreason PJ. Regional cerebral glucose-metabolism in bulimia-nervosa (the American Journal of Psychiat, Vol 149, Pg 1509, 1992) Am. J. Psychiatry. 1993;150:174–174. doi: 10.1176/ajp.149.11.1506. [DOI] [PubMed] [Google Scholar]
- 79.Krieg JC, Holthoff V, Schreiber W, Pirke KM, Herholz K. Glucose-metabolism in the caudate nuclei of patients with eating disorders, measured by PET. Eur. Arch. Psychiatry Clin. Neurosci. 1991;240:331–333. doi: 10.1007/BF02279762. [DOI] [PubMed] [Google Scholar]
- 80.Hagman JO, et al. Comparison of regional brain metabolism in bulimia-nervosa and affective-disorder assessed with positron emission Tomography. J. Affect. Disord. 1990;19:153–162. doi: 10.1016/0165-0327(90)90085-m. [DOI] [PubMed] [Google Scholar]
- 81.Wong TP, et al. Loss of presynaptic and postsynaptic structures is accompanied by compensatory increase in action potential-dependent synaptic input to layer V neocortical pyramidal neurons in aged rats. J. Neurosci. 2000;20:8596–8606. doi: 10.1523/JNEUROSCI.20-22-08596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu F, et al. Use of 3D-ASL and VBM to analyze abnormal changes in brain perfusion and gray areas in nasopharyngeal carcinoma patients undergoing radiotherapy. Biomed. Res. India. 2017;28:7879–7885. [Google Scholar]
- 83.Olloquequi J, et al. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: Therapeutic implications. J. Psychopharmacol. 2018;32:265–275. doi: 10.1177/0269881118754680. [DOI] [PubMed] [Google Scholar]
- 84.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 85.Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol. Behav. 2009;97:537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heatherton TF. Neuroscience of self and self-regulation. Annu. Rev. Psychol. 2011;62:363–390. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uher R, Treasure J. Brain lesions and eating disorders. J. Neurol. Neurosurg. Psychiatry. 2005;76:852–857. doi: 10.1136/jnnp.2004.048819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen J, Papies EK, Barsalou LW. A core eating network and its modulations underlie diverse eating phenomena. Brain Cognition. 2016;110:20–42. doi: 10.1016/j.bandc.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 89.Tuulari JJ, et al. Neural circuits for cognitive appetite control in healthy and obese individuals: an fMRI study. PLoS ONE. 2015;10:e0116640. doi: 10.1371/journal.pone.0116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marsh R, et al. An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am. J. Psychiatry. 2011;168:1210–1220. doi: 10.1176/appi.ajp.2011.11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marsh R, et al. Deficient activity in the neural systems that mediate self-regulatory control in bulimia nervosa. Arch. Gen. Psychiatry. 2009;66:51–63. doi: 10.1001/archgenpsychiatry.2008.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Balodis IM, et al. Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity (Silver Spring) 2013;21:367–377. doi: 10.1002/oby.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eryilmaz H, et al. Neural determinants of human goal-directed vs. habitual action control and their relation to trait motivation. Sci. Rep. 2017;7:6002. doi: 10.1038/s41598-017-06284-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tschernegg M, et al. Impulsivity relates to striatal gray matter volumes in humans: evidence from a delay discounting paradigm. Front. Hum. Neurosci. 2015;9:384. doi: 10.3389/fnhum.2015.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang YY, Posner MI, Rothbart MK, Volkow ND. Circuitry of self-control and its role in reducing addiction. Trends Cogn. Sci. 2015;19:439–444. doi: 10.1016/j.tics.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 96.Lieberz, J. et al. Kinetics of oxytocin effects on amygdala and striatal reactivity vary between women and men. Neuropsychopharmacology, 10.1038/s41386-019-0582-6 (2019). [DOI] [PMC free article] [PubMed]
- 97.Xu X, et al. Oxytocin biases men but not women to restore social connections with individuals who socially exclude them. Sci. Rep. 2017;7:40589. doi: 10.1038/srep40589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoge EA, et al. Gender moderates the effect of oxytocin on social judgments. Hum. Psychopharmacol. Clin. Exp. 2014;29:299–304. doi: 10.1002/hup.2402. [DOI] [PubMed] [Google Scholar]
- 99.Gao S, et al. Oxytocin, the peptide that bonds the sexes also divides them. Proc. Natl Acad. Sci. USA. 2016;113:7650–7654. doi: 10.1073/pnas.1602620113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng C, et al. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav. 2015;9:754–764. doi: 10.1007/s11682-014-9333-9. [DOI] [PubMed] [Google Scholar]
- 101.Spengler FB, et al. Kinetics and dose dependency of intranasal oxytocin effects on amygdala reactivity. Biol. Psychiatry. 2017;82:885–894. doi: 10.1016/j.biopsych.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 102.Meule A, et al. Food cue-induced craving in individuals with bulimia nervosa and binge-eating disorder. PLoS ONE. 2018;13:e0204151. doi: 10.1371/journal.pone.0204151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collins B, et al. The impact of acute stress on the neural processing of food cues in bulimia nervosa: replication in two samples. J. Abnorm. Psychol. 2017;126:540–551. doi: 10.1037/abn0000242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be provided upon request.