Abstract
The number of male gametes is critical for reproductive success and varies between and within species. The evolutionary reduction of the number of pollen grains encompassing the male gametes is widespread in selfing plants. Here, we employ genome-wide association study (GWAS) to identify underlying loci and to assess the molecular signatures of selection on pollen number-associated loci in the predominantly selfing plant Arabidopsis thaliana. Regions of strong association with pollen number are enriched for signatures of selection, indicating polygenic selection. We isolate the gene REDUCED POLLEN NUMBER1 (RDP1) at the locus with the strongest association. We validate its effect using a quantitative complementation test with CRISPR/Cas9-generated null mutants in nonstandard wild accessions. In contrast to pleiotropic null mutants, only pollen numbers are significantly affected by natural allelic variants. These data support theoretical predictions that reduced investment in male gametes is advantageous in predominantly selfing species.
Subject terms: Genetic variation, Genome-wide association studies, Pollen
Reduction of pollen grain number is widespread in selfing plants, but the determining gene is unknown. Here, the authors show that a ribosome-biogenesis factor encoding gene RDP1 is responsible for adaptive reduction of male gamete number in Arabidopsis thaliana.
Introduction
Male gamete numbers, reflected by pollen grain (each containing two sperm cells) numbers in seed plants and sperm numbers in animals, have been studied extensively from agricultural, medical, and evolutionary viewpoints1–9. Evolutionary theory predicts that the breeding system could act as a major selective force on male gamete numbers. In highly promiscuous outcrossing species, a large number of male gametes should be produced because of male–male gamete competition, so reduced male gamete numbers are considered to be deleterious1. In contrast, they may be advantageous at lower outcrossing rates because of the high cost of their production, decreasing fitness. In an agricultural context, low pollen number may have been selected during domestication10, but may serve as a barrier for hybrid breeding of wheat and other species11,12. In flowering plants, the transition from an outcrossing to a selfing breeding system through loss of self-incompatibility is one of the most prevalent evolutionary trends6,13. Selfing populations or species generally show lower pollen grain numbers per flower (hereafter, pollen number) as well as reduced flower size. There has been a sustained debate on whether the reduced pollen number is a result of adaptive evolution or the accumulation of deleterious mutations owing to reduced selection6,14,15, but little was known about the genetic basis of pollen number variation to assess molecular signatures of selection on it.
To unravel the genetic basis of quantitative natural variation in pollen number, we here focus on the predominantly selfing plant Arabidopsis thaliana6,16. Studies have shown that the evolution of predominant selfing in A. thaliana occurred much more recently than its evolutionary divergence from outcrossing relatives6,16,17. Thus, in addition to fixed, genetically based differences from these outcrossing relatives, we expect that variation with regard to pollen number may still be segregating among current accessions. By harnessing the genetic and genomic resources available in A. thaliana, we conduct a genome-wide association study on pollen number variation. We find that natural variants of the RDP1 gene confer variation in pollen number without detectable pleiotropy. Signatures of selection at the top genome-wide association study (GWAS) peaks, including the RDP1 locus, support the theoretical prediction that reduced investment in male gametes should provide an advantage in selfing species.
Results
Genome-wide association study and signatures of selection
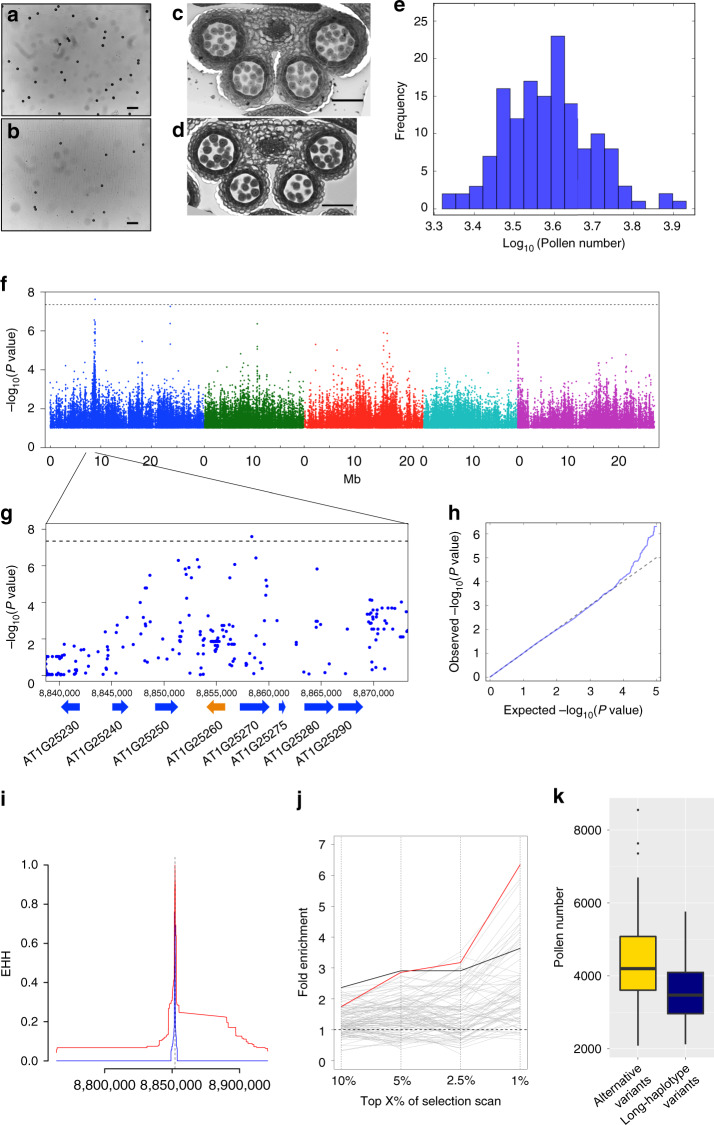
To examine variability in pollen number on a species-wide scale, we determined pollen number per flower in 144 natural A. thaliana accessions (Fig. 1a–d; Supplementary Table 1 and Supplementary Data 1) and found approximately fourfold variation (average ~4000) (Fig. 1e). Histological sections of stamens from representative accessions confirmed pollen number variation among accessions (Fig. 1c, d). We also measured the number of ovules per flower (Supplementary Table 2). We did not find significant correlations between numbers of pollen grains and ovules (P = 0.5164), although negative correlations have often been reported in between-species comparisons, as expected on theoretical grounds owing to trade-offs in resource allocation to male versus female function14,18. Furthermore, we found that pollen number per flower was not significantly correlated with any of the 107 published phenotypes of flowering, defense-related, ionomic, and developmental traits (Supplementary Table 3; Supplementary Note 1)19, nor with climate variables, geographic location, or S-haplogroups across the 144 accessions (Supplementary Tables 4 and 5, Supplementary Fig. 1)20,21. These data suggest that variation in pollen number is largely independent of other traits.
Fig. 1. Genome-wide association study of pollen number variation in Arabidopsis thaliana.
a, b Pollen grains of higher pollen number accession Bor-4 (a) and lower pollen number accession Mz-0 (b) mounted on glass slides for counting52. Scale bars = 100 μm. c, d Histological sections of Bor-4 (c) and Mz-0 (d) stamens. At least three independent observations showed similar results (a–d). Scale bars = 50 μm. e Distribution of pollen number variation across 144 natural accessions. f Manhattan plot of the genome-wide association study (GWAS). g Closer view of the region around the significant GWAS peak on chromosome 1 with gene models and coordinates. f, g SNPs with minor allele frequency > 0.15 are shown; horizontal dashed lines indicate the nominal P < 0.05 threshold after Bonferroni correction. h Quantile–quantile plot of the GWAS. i Extended haplotype homozygosity (EHH) detected in the RDP1 genomic region. Red and blue lines correspond to the long haplotype and alternative variants, respectively. j Signatures of selection at pollen number-associated loci. Each line indicates a phenotype (red: pollen number, black: ovule number, gray: 107 phenotypes19). The x axis quantifies the extreme tails of the integrated haplotype score (iHS) statistic. The pollen and ovule GWAS show significant enrichment (permutation test, P < 0.05 cutoff for all iHS statistical tails; Supplementary Fig. 3). k Accessions with the long-haplotype variants (defined by SNP 1-8852112) generally showed lower pollen number (P = 2.152 × 10–6, two-sided t test; population structure-corrected GWAS P = 2.95 × 10–6). Boxplots show center line: median; box limits: upper and lower quartiles; whiskers: not >1.5 times the interquartile range; dots: outliers. Source data underlying e, j and k are provided as a Source Data file.
To evaluate genome-wide signatures of natural selection on loci associated with gamete numbers, we first performed GWAS for pollen and ovule numbers using a genome-wide single-nucleotide polymorphism (SNP) data set for these lines, which was obtained by imputation based on genome-wide resequencing data and 250 k SNP data (Fig. 1f, h; Supplementary Fig. 2)22. In total, 68 peaks of association were identified (10-kb windows having SNPs with P < 10–4), although only one pollen number-associated peak remained significant after Bonferroni correction. Focusing on the identified GWAS peaks, we performed an enrichment analysis to ask whether pollen and ovule number-associated peaks are enriched in long-haplotype regions, which could be owing to partial or ongoing sweeps of segregating polymorphisms23–25. To identify long-haplotype regions, we first calculated the extended haplotype homozygosity (EHH), which measures decay of haplotypes that carry a specified core allele as a function of distance23. We then obtained the integrated haplotype score (iHS) statistic for each SNP, which compares EHH between two alleles of the SNP by controlling for the allele frequency of each SNP23. We found that 10-kb windows including pollen number-associated loci were significantly enriched in extreme iHS tails (P < 0.05, permutation test; Fig. 1i, j; Supplementary Fig. 3). These loci showed generally high iHS scores, and two of the top five GWAS peaks were outliers of the genome-wide iHS distribution (Supplementary Table 6). The enrichment was robust to changes in sample composition, allele frequency cutoffs, and the use of windows (Supplementary Figs. 4–6; see Supplementary Note 2 for details). Ovule number also showed enrichment, albeit less than pollen number (Fig. 1j). In principle, the iHS enrichment could be confounded by recombination rate and the accuracy of imputation. To deal with such potential confounding factors, we compared these results with the results of an iHS enrichment analysis for GWAS peaks (P < 0.0001) for 107 other phenotypes, as these confounding factors should also influence the enrichment for other traits. We found that the iHS enrichment for pollen number GWAS peaks (P = 0.002 for the top 1% iHS tail; Supplementary Fig. 3) was among the highest, compared with that for many known adaptive traits included in the 107 phenotypes, such as leaf number at flowering time and resistance to Pseudomonas pathogens19,25 (Supplementary Table 7). In addition, iHS enrichment of the ovule number GWAS peaks was also significant (P = 0.030 for the top 1% iHS tail; Supplementary Fig. 3). These enrichments support polygenic selection on a considerable number of loci associated with male and female gamete numbers throughout the genome.
Isolation of the REDUCED POLLEN NUMBER1 gene
To further understand the molecular basis of pollen number variation and to examine the nature of the putative targets of selection, we tried to identify the genes underlying pollen number variation; however, the top five peaks of association did not contain any genes with known functions in early stamen or pollen development. To obtain experimental evidence concerning genes underlying pollen number variation, we conducted functional analyses of the genes under the highest pollen number GWAS peak, which explains ~20% of the total phenotypic variance between accessions and satisfies the criterion for genome-wide significance (–log10 P = 7.60). This region is of particular interest because it also satisfies the criteria for genome-wide significance of the iHS statistic (P = 0.0149; Fig. 1i, Supplementary Fig. 7), suggesting a selective sweep. To test whether the signature of selection in this region might be owing to traits other than pollen number, we examined whether there is an association signal for any of the 107 published phenotypes, ovule number, or variants showing climatic correlations19,20. In the 10-kb window including the SNP of the highest GWAS score for pollen number, we found no genotype–phenotype associations below P < 10–5 or climate–SNP correlations below an empirical P < 0.01, i.e., there is no significant evidence for selection on traits other than pollen number in this region. We also found that accessions with the long-haplotype variants produced lower pollen numbers than those with alternative haplotype variants (P = 2.152 × 10–6, t test; population structure-corrected GWAS P = 2.95 × 10–6; Fig. 1k), as expected if this haplotype was under selection for reduced pollen number.
Of the three genes in this chromosomal region with the highest GWAS scores (AT1G25250, AT1G25260, and AT1G25270; Fig. 1g), the expression level of AT1G25260, a gene of unknown function, was much higher in flower buds than that of the other two genes (Supplementary Fig. 8). Therefore, we obtained two T-DNA insertion mutants of AT1G25260 in the standard Col-0 accession from the Nottingham Arabidopsis Stock Centre26. These mutants showed a 32% reduction in pollen number (Fig. 2a; Supplementary Table 8). We hereafter refer to AT1G25260 as REDUCED POLLEN NUMBER1 (RDP1). Because both rdp1-1 (insertion in the 5′ UTR) and rdp1-2 (insertion at the end of the coding sequence) (Fig. 2b) homozygotes showed low levels of expression, they are likely to be hypomorphic mutants (Supplementary Fig. 8). We generated two amorphic (null) frameshift mutants of RDP1 (rdp1-3 and rdp1-4) using the CRISPR/Cas9 system27,28 in the Col-0 background. These mutants indeed showed an even greater reduction in pollen number, but still produced about half the number of pollen grains of the corresponding wild type (53% for rdp1-3; Fig. 2a), suggesting a quantitative nature of the effect of RDP1. Pollen size was slightly increased, in agreement with the well-known negative relationship between pollen number and size, even within the same genotype (Supplementary Fig. 9; Supplementary Table 9)14. The mutant phenotype was complemented by transforming a 4.3-kb genomic fragment of the Col-0 accession encompassing RDP1 (Fig. 2a, Supplementary Fig. 9). In contrast to RDP1 mutants, CRISPR/Cas9 induced null mutants in AT1G25250 or AT1G25270 did not result in any significant change in pollen number (Supplementary Fig. 10). The phenotype of four independent mutants of RDP1 together with successful complementation using the wild-type allele thus demonstrated that RDP1 is involved in the control of pollen number.
Fig. 2. Functional characterization of the RDP1 gene.
a Pollen number differences between four homozygous mutants (rdp1-1/rdp1-1, rdp1-2/rdp1-2, rdp1-3/rdp1-3, and rdp1-4/rdp1-4), wild-type (RDP1/RDP1), and the complemented lines (rdp1-1/rdp1-1 + RDP1). Letters a, b, c indicate significant differences, determined by nested analysis of variance (ANOVA) and post hoc Tukey test; P < 0.05 (See Supplementary Table 8 for P values). Numbers of flowers pollen-counted (left to right, as plotted): N = 132, 45, 40, 60, 135, 411. b Schematic structure of the RDP1 gene. Untranslated regions (green boxes), coding regions (yellow boxes), introns (bars), two T-DNA insertion mutants (rdp1-1, rdp1-2; open triangles), and four CRISPR/Cas9-based frameshift mutants (rdp1-3 and rdp1-4 in Col-0, rdp1-5 in Uod-1, and rdp1-6 in Bor-4 backgrounds, respectively). The box in the wild-type sequences indicates the target site for CRISPR/Cas9, and red letters in mutants show the indel mutation. c, d Representative microsporocyte images of RDP1/RDP1 (c) and rdp1-3/rdp1-3 (d) stained with aniline blue. Dark spots indicate microsporocytes. Summary of observation is depicted in e. e The number of microsporocytes was significantly lower in rdp1-3/rdp1-3 than in the wild type (Wilcoxon rank sum test, P = 2.82e–06). Numbers of anthers counted (left to right, as plotted): N = 40 and 50. f, g In situ hybridization for RDP1. Similar expression patterns were observed in four (f) and two (g) individuals. f Stage 8 floral cross section; expression is detected in sporogenous cells (Sp). g Inflorescence longitudinal section; expression is observed in the inflorescence meristem (Ifm) and young flowers. h Pollen number of wild type and null mutants in the Uod-1 (blue) and Bor-4 (yellow) accessions. Dots and error bars indicate means and standard errors of means, respectively. The mutation in Bor-4 caused a stronger reduction in pollen number than that in Uod-1 (ANOVA interaction effect P = 1.07 × 10–5, one-sided test). Boxplots a, e show center line: median; box limits: upper and lower quartiles; whiskers: not >1.5 times the interquartile range; dots: outliers. Scale bars: 50 μm. Source data underlying a, e and h are provided as a Source Data file.
Based on phylogenetic analysis, RDP1 is a putative homolog of the yeast mRNA turnover 4 protein (Mrt4p) (Supplementary Figs. 11 and 12). The MRT4 gene is nonessential in yeast but null mutants show a phenotype of slightly slower growth. The Mrt4p shares similarity with the ribosome P0 protein and is necessary for the assembly of the P0 protein into the ribosome. The human ribosome P0 gene is reported to have an extra-ribosomal function in cancer by modulating cell proliferation29,30. During anther development, sporogenous cells first divide and differentiate into microsporocytes31. Following meiosis of the microsporocyte, four microspores are formed, each of which undergoes two mitotic divisions to form a mature pollen grain containing the male gametes. The null rdp1-3 mutant produced fewer microsporocytes than the wild type (Fig. 2c–e), indicating a reduction in cell numbers before meiosis. Consistent with this, in situ mRNA hybridization experiments detected strong expression of RDP1 in sporogenous cells and the microsporocytes derived from them, but not in microspores (Fig. 2f, g; Supplementary Fig. 13). RDP1 was also expressed in other proliferating cells, including those in inflorescences, floral meristematic regions, and ovules (Supplementary Figs. 8 and 13), supporting a more widespread role in proliferating cell types with putatively high demands for ribosome biogenesis32. Furthermore, fusing the RDP1 promoter to the uidA reporter gene encoding β-glucuronidase (GUS) to assess its activity confirmed the RDP1 expression pattern in stamens (Supplementary Fig. 14) and demonstrated marked expression in root tips and young leaf primordia during the vegetative phase; these data are supported by quantitative reverse transcription PCR experiments (Supplementary Fig. 8b). Consistent with RDP1 expression in proliferating tissues, the rdp1-3 null mutant showed pleiotropic phenotypes, including slower vegetative growth and reduced ovule numbers per flower (Supplementary Fig. 15). Because these pleiotropic phenotypes would be deleterious in natural environments, these data indicate that natural alleles of RDP1 are not null variants (see below). In summary, these data suggest that RDP1 is required in proliferating A. thaliana cells, yet the natural variants we have identified predominantly affect the proliferation of sporogenous cells in the anthers, consistent with the function of its yeast homolog in cell proliferation.
Natural variants of RDP1 confer pollen number variation
It has been difficult to experimentally determine whether a particular gene has natural alleles with subtle phenotypic effects on quantitative traits33. When allelic effects are subtle, transgenic analysis of natural alleles is not sufficiently powerful because the phenotypes of transgenic A. thaliana plants tend to be highly variable as a result of the variation between lines, e.g., owing to different transgene insertion sites. In contrast, a quantitative complementation test can identify responsible genes by testing the effect of natural alleles in a heterozygous state with a null allele if the effects of other loci are small, although this may be confounded by polygenic effects in the genetic background33,34. To conduct such quantitative complementation, we took advantage of the CRISPR/Cas9 technique to generate frameshift null alleles in nonstandard natural accessions, in which no prior mutant was available.
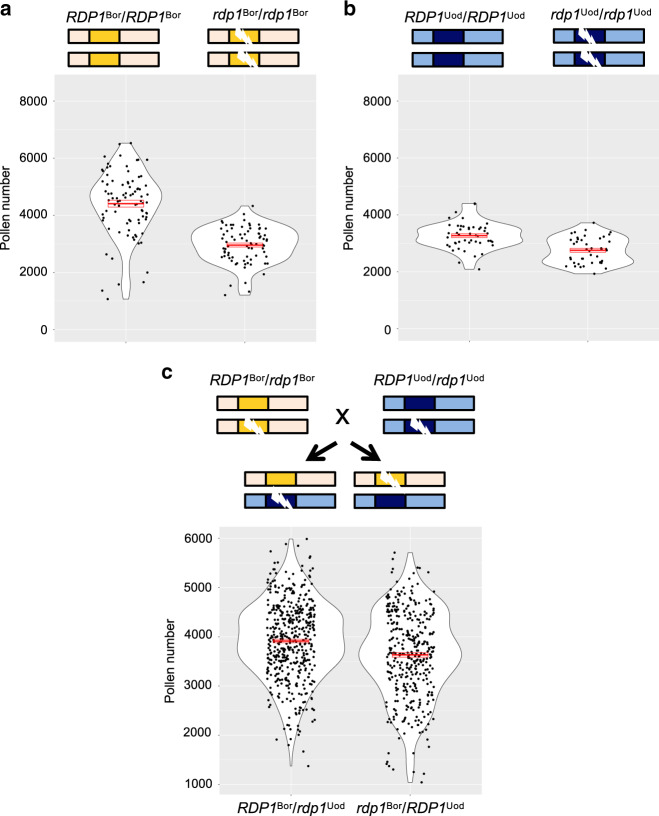
We used Bor-4 and Uod-1, which have high and low pollen number phenotypes, respectively (Fig. 2b; Fig. 3). There were a number of sequence differences between the two accessions in the region encompassing the RDP1 gene from 777 bp upstream of the start codon to 643 bp downstream of the stop codon. We found one non-synonymous and six synonymous substitutions in the coding region, and 62 substitutions and six indel mutations in the non-coding region (Supplementary Fig. 16). Yet, both accessions did not reveal obvious loss-of-function mutations (Supplementary Fig. 16), and rdp1 CRISPR null mutants of each accession showed reduced pollen number compared with the corresponding wild type (P < 2.2 × 10–16 for Bor-4, P = 9.84 × 10–7 for Uod-1; Fig. 3a, b; Fig. 2h). These results show that both of the naturally occurring variants of the RDP1 gene are not null mutants but rather encode a functional protein. Disruption of RDP1 had a stronger effect on pollen number in Bor-4 than in Uod-1 (analysis of variance (ANOVA) interaction effect P = 1.07 × 10–5; Fig. 2h). This finding supports the notion that the Bor-4 allele has a stronger promotive effect on pollen number than the Uod-1 allele, although other loci in the genetic backgrounds of Bor-4 and Uod-1 may contribute to this difference through epistasis.
Fig. 3. Quantitative complementation test of the RDP1 gene.
Violin plots with means and standard errors of means indicated by red bold bars and boxes, respectively. a, b Pollen number differences between wild-type and homozygous plants of a frameshift allele generated by the CRISPR/Cas9 technique in the Bor-4 background (a Numbers of flowers pollen-counted: RDP1Bor/RDP1Bor, N = 89; rdp1Bor/rdp1Bor, N = 77) and in the Uod-1 background (b Numbers of flowers pollen-counted: RDP1Uod/RDP1Uod, N = 47; rdp1Uod/rdp1Uod, N = 43) (same data sets with Fig. 2h). c The difference in the effect on pollen number by two natural alleles, RDP1Bor and RDP1Uod. Pollen number of plants with RDP1Uod was significantly lower than that of plants with RDP1Bor (nested analysis of variance; P = 4.85 × 10–8; Numbers of flowers pollen-counted: RDP1Bor/rdp1Uod, N = 468 from 26 individuals; rdp1Bor/RDP1Uod, N = 368 from 20 individuals). The two alleles were compared in the heterozygous state with a frameshift CRISPR/Cas9 allele, with otherwise identical genetic backgrounds. F1 plants were obtained from the cross of two heterozygotes, RDP1Bor/rdp1Bor and RDP1Uod/rdp1Uod. Source data are provided as a Source Data file.
To test the allelic effect of RDP1, we utilized a quantitative complementation test that controls for genetic background (Fig. 3). Among F1 plants obtained by crossing heterozygotes for the frameshift mutation in each genetic background, we compared two genotypes: RDP1Bor/rdp1Uod vs. rdp1Bor/RDP1Uod. These F1 genotypes are identical except for the differences at RDP1, where they both carry a frameshift allele but differ with respect to the functional allele; because of the crossing design, any independently segregating off-target effects, resulting from CRISPR/Cas9 mutagenesis would be equally distributed between the two genotype cohorts of interest. We found that pollen number in plants with RDP1Uod was significantly lower than in plants with RDP1Bor (nested ANOVA, 468 flowers from 26 individuals of RDP1Bor/rdp1Uod and 368 flowers from 20 individuals of rdp1Bor/RDP1Uod, P = 4.85 × 10–8; Fig. 3c). The significant difference cannot be attributed to stochastic individual differences, because the significant difference between plants bearing functional RDP1Bor and RDP1Uod alleles was also observed in an individual-based test using averaged data of each individual separately (P = 0.0331). Thus, in an otherwise identical genetic background, the respective functional RDP1 haplotypes cause differences in pollen number. We also measured rosette leaf size, flowering date, ovule number, dry weight, and seed weight of plants in the two cohorts, but none of these traits showed significant differences between RDP1Bor/rdp1Uod and rdp1Bor/RDP1Uod (Supplementary Fig. 17). Thus, in contrast to the experimentally generated null mutants that showed pleiotropic growth defects, these results indicate that natural allelic differences at RDP1 affect pollen number in the absence of any detectable deleterious pleiotropy. This finding is also supported by no genotype–phenotype associations for other traits, as described above.
Discussion
We here isolated the RDP1 gene underlying natural variation in male gamete numbers. Our study provides evidence for polygenic selection on pollen number-associated loci, including RDP1. Even though RDP1 encodes a ribosome-biogenesis factor that would be required globally for proliferative growth, the naturally selected alleles predominantly confer reduced pollen number. This is analogous to a hypomorphic allele of the human G6PD gene, which encodes an enzyme in the pentose phosphate pathway and features a long haplotype because of selection for malaria resistance24.
The mean number of pollen grains of an outcrossing population of Arabidopsis lyrata is ~18,000 (ref. 35) and thus several times higher than our counts in A. thaliana (~2000–8000; Fig. 1e). Although the evolutionary split between the lineages leading to A. thaliana and A. lyrata is estimated to have occurred ~5 million years ago or before36–38, several studies suggested that predominant selfing in A. thaliana evolved much more recently (0–0.413 million years ago based on the timing of the loss of a self-incompatibility gene, ~0.5 million years ago based on the abundance of transposable elements, and ~0.3–1 million years ago based on the pattern of genome-wide linkage disequilibrium)6,39,40. Thus, in addition to presumably fixed differences to distantly related outcrossing congeners, it is quite conceivable that some underlying loci, not limited to but including RDP1, are still segregating within A. thaliana. These might reflect an ongoing selection process for the further reduction in pollen number, which has been considered a hallmark of the so-called selfing syndrome6,8,14. Although we note the possibility that partial sweeps of RDP1 and other segregating loci are not directly related to the transition to predominant selfing, our analysis did not find evidence of other selective forces including local adaptation, climate association, or pleiotropic selection on other traits. Therefore, our study supports the theoretical predictions that reduced investment in male gametes is advantageous in predominantly selfing species.
Our work also illustrates that a combination of GWAS and functional analysis using a quantitative complementation test based on the CRISPR/Cas9-based alleles provides a powerful approach to dissect allelic differences underlying quantitative natural variation.
Methods
Pollen and ovule counting for genome-wide association studies
To perform GWAS, numbers of pollen grains and ovules per flower were counted for 144 and 151 world-wide natural accessions, respectively (Supplementary Tables 1, 2 and Supplementary Data 1). Plants were grown at 21 °C under a 16 h light/8 h dark cycle without vernalization. We grew four plants per accession. Three flower buds per plant were harvested from the main inflorescence, and each flower bud was collected into a 1.5 mL tube and dried at 65 °C overnight. We sampled individual flower buds of young main inflorescences but avoided the first and second flowers of the inflorescence because these flowers tend to show developmentally immature morphologies. We collected flower buds with mature pollen but before the anthers were opened (flower stage 13), and added 30 μL of 5% Tween 20 (Sigma-Aldrich, St. Louis, MO, USA) to each tube. The tubes were sonicated using a Bioruptor (Diagenode, Seraing, Belgium) in high power mode with 10 cycles of sonication-ON for 30 s and sonication-OFF for 30 s so that the pollen grains were released from the anther sacs. After a short centrifugation and vortexing, 10 μL of the solution was mounted on a Neubauer slide. We took three images per sample using a light microscope. The number of pollen grains per image was counted using the particle counting implemented in ImageJ (http://imagej.nih.gov/ij/) and in Fiji (http://fiji.sc/Fiji). We then estimated the total pollen number per flower based on the image size and the total volume. Ovule numbers were counted by dissecting young siliques (5.3 siliques per accession on average) under a dissecting microscope.
Because of limited chamber space, we split the plants into two batches. The two batches were treated under the same conditions in the same chambers, but at different times. We controlled for this potential batch effect for pollen number by setting equal medians and standard deviations for the two batches and used as the GWAS input of pollen number phenotype (Source Data file, Supplementary Table 1).
Sometimes, there were no or very few pollen grains per image. This was mainly in situations where anthers did not open. To eliminate these artefacts, we discarded flowers with pollen counts of <10 per image. We confirmed that such extremely low pollen numbers did not occur in specific accessions, indicating that this is not heritable.
Plant materials and growth conditions for functional analyses
For functional analyses, we mainly used Arabidopsis thaliana wild-type and mutant plants of the Col-0, Bor-4, and Uod-1 accessions. The T-DNA lines SALK_064854/N666274 (rdp1-1) from the Salk collection41 and GK-879G09/N484369 (rdp1-2) from GABI-Kat collection42 were obtained from the European Arabidopsis Stock Centre26.
The T-DNA insertion in each line was confirmed using PCR with the primers listed in Supplementary Table 10 as described at http://signal.salk.edu/tdnaprimers.2.html. DNA was extracted from young leaves using the cetrimonium bromide (CTAB) method.
We generated single-nucleotide insertion/deletion lines in the Col-0, Uod-1, and Bor-4 accessions using the FAST-CRISPR-Cas9 construct (see the section CRISPR mutant) and designated them rdp1-3 and rdp1-4 (in Col-0), rdp1-5 (in Uod-1), and rdp1-6 (in Bor-4).
Arabidopsis seeds were sown on soil mixed with the insecticide ActaraG (Syngenta Agro, Switzerland) and stratified for 3–4 days at 4 °C in the dark. The plants were grown under 16 h of light at 22 °C, and 8 h of dark at 20 °C, with weekly treatments of insecticide (Kendo Gold, Syngenta Agro) unless noted otherwise (for GWAS, see above).
Statistical analysis
Unless stated otherwise, statistical and population genetic analyses were performed using the statistical software R43. In boxplots, bars indicate the median, boxes indicate the interquartile range, and whiskers extend to the most extreme data point that is no >1.5 times the interquartile range from the box, with outliers shown by dots.
Histological analysis of anthers
For histological analysis, inflorescences were fixed with formaldehyde:acetic acid:70% ethanol = 1:1:18 (FAA), dehydrated and embedded in Technovit 7100 according to the manufacturer’s instructions (Heraeus Kulzer GmbH, Wehrheim, Germany). Five-micrometer sections were cut with a microtome (RM2145, Leica, Germany) and stained with toluidine blue before observation under a Leica microscope (DM5000, Leica) equipped with a black-and-white camera (DFC345, Leica).
Correlations with published GWAS results and climatic data
To examine whether pollen and ovule numbers were correlated with any of the other 107 published phenotypes19, or with climate and geographic variables20, Pearson’s correlation coefficients were calculated (Supplementary Tables 3 and 5). We also surveyed whether there were any SNPs significantly associated with climate variables in the 10-kb window including the SNP of the highest GWAS score for pollen number (Chr1:8,850,000–8,860,000). The significance was based on the genome-wide empirical P values20,25. Focusing on the same 10-kb window, we also surveyed whether there were significant SNPs (P < 10–5; minor allele frequency (MAF)>0.1) for the 107 published phenotypes in the GWAS. The correlation of pollen number and the S-haplogroups21 was also tested.
SNP imputation
To perform GWAS, we generated a set of dense SNP markers that overlapped with the phenotyped accessions using imputation22. First, we constructed a reference set of 186 haplotypes from resequencing data44,45. MaCH version 1.0.16.c46 was then used to impute non-genotyped SNPs for 1311 accessions in the 250 k SNP array data set25. The command line used for each overlapping bin was:
mach1 --dosage --greedy -r -d [sample_bin].dat -p [sample_bin].ped -s [ref_bin].snps -h [ref_bin].haplos --prefix [output_prefix]
The output was then merged and converted into the homozygous SNP data set.
Genome-wide association study
GWAS was performed to identify loci associated with pollen number variation in 144 natural accessions. We also performed GWAS for ovule number and for 107 published phenotypes19 using the same SNP set as for pollen number. The median values of pollen number were used to represent each accession. We used the log-transformed pollen number values for GWAS, as they did not deviate significantly from a normal distribution (Shapiro–Wilk normality test: P = 0.438). To deal with confounding effects as a result of population structure, we employed the mixed model implemented in the software Mixmogam, in which a genome-wide kinship matrix was incorporated as a random effect (population structure-corrected GWAS)47. By using Mixmogam, we also generated a quantile–quantile (Q–Q) plot, which shows the relationship between the observed and expected negative logarithm of P values (Fig. 1h). For the Manhattan plot (Fig. 1f, g), we removed SNPs with minor allele frequencies <0.15, leaving 1,115,178 SNPs overall.
Quantitative reverse transcription PCR
For gene expression analysis, total RNA was isolated using the ZR Plant RNA MiniPrep (Zymo Research, Irvine, CA) according to the manufacturer’s instructions. Total RNA was treated with a DNA-free DNA removal kit (Thermo Fisher Scientific, Waltham, MA), and cDNA was synthesized using a High-Capacity RNA-to-cDNA kit (Thermo Fisher Scientific, Waltham, MA). For the quantitative PCR step, cDNA was used with SYBR Select Master Mix (Thermo Fisher Scientific, Waltham, MA) and gene-specific primers (Supplementary Table 10). Data were collected using the StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA) in accordance with the instruction manual. Expression levels were normalized using EF1-α (AT5G60390), which was used as an internal control.
CRISPR/Cas9-based mutant alleles
To generate RDP1 frameshift mutants, the CRISPR/Cas9 system was used28. A 20-nucleotide target sequence (5′-GCAGAAGATGAACTCCGTTC-3′) was selected using the CRISPR-P tool48. The target sequence was subcloned into a pTTK194 vector (pUC19 U6.26pro::sgRNA). Then, the HindIII sgRNA fragment was subcloned into a pTTK182 (pFAST-R02 35Spro::Cas9) vector or pTTK227 (pFAST-R01 RPS5Apro::Cas9). For plant transformation, the binary vector was first introduced into Agrobacterium tumefaciens (GV3101) and then into A. thaliana by the floral-dip method.
Transgenic experiment
The RDP1 genomic sequence was amplified by PCR using Phusion High Fidelity PCR polymerase (New England Biolabs, Beverly, MA). We used the primers 3550_At1g25260F1 (5′-TTTCTCCCCACATTTCTC-3′) and 3551_At1g25260R1 (5′-GTTTAAAATGAGAGAACCCG-3′) to amplify the full length of RDP1 and the surrounding genomic sequence that may contain promoter and terminator regions (ca. 4.3 kbp, positions 8,857,725 to 8,853,420 on chromosome 1). The RDP1 promoter sequence was amplified by PCR using the following primers: 3550_At1g25260F1 (5′-TTTCTCCCCACATTTCTC-3′) and 4155_At1g25260R (5′-AGCTGAGGTTTCAAATGTTGTATG-3′). These sequences were cloned into a pCR8 vector using the pCR8/GW/TOPO TA Cloning kit (Thermo Fisher Scientific, Waltham, MA). pCR8-RDP1 complete sequences were cloned into a pFAST-G01 vector49, and the pCR8-RDP1 promoter vector was cloned into a pGWB3 vector50 using Gateway LR clonase II (Thermo Fisher Scientific, Waltham, MA). We designated these constructs pFAST-RDP1 and pGWB3-RDP1pro, respectively. Plants were grown at 22 °C under a 16 h light/8 h dark cycle until transformation via A. tumefaciens strain GV3101 using the floral-dip method51. pFAST-RDP1 was transformed into rdp1-1 homozygous mutants. Transformed seeds were selected using a fluorescence stereomicroscope with a GFP filter (Olympus SZX12, Japan). pGWB3-RDP1pro was transformed to Col-0. Seedlings carrying pGWB3-RDP1pro were selected on 1/2 Murashige and Skoog medium supplemented with 50 μg mL−1 kanamycin and 50 μg mL−1 hygromycin.
Measuring pollen number and size with a cell counter
To expedite pollen number counting, we established a rapid method using a cell counter (CASY TT, OMNI Life Science GmbH, Germany)52. We found that the pollen numbers of flowers on side inflorescences and side branches of the main inflorescence were similar, but those of flowers on the main inflorescence were higher (Supplementary Fig. 18). To obtain a large number of replicates, we sampled flowers from the former two positions. We sampled during the first 3 weeks of flowering and excluded the first and second flowers on each branch; this yielded up to 40 flowers per individual. Collecting, suspending, and sonicating of flowers for GWAS were conducted as described above. All pollen solutions were suspended in 10 mL of CASYton (OMNI Life Science GmbH), and pollen numbers were counted with a CASY TT cell counter53. Three 400 μL aliquots of each pollen solution were counted. We counted particles within a size range of 12.5–25 μm (estimated diameter) as pollen. Samples with a clear peak at 7.5–12.5 μm were discarded as broken or unhealthy samples.
Counting microsporocyte number
Flower buds were collected and fixed in 3:1 (vol:vol) ethanol:acetic acid for 16 h. The fixed flower buds were rehydrated in 99.5%, 70%, and 50% ethanol for 30 min each. Flower buds were transferred to 1 n NaOH for 3 h and then stained with aniline blue solution (0.1% aniline blue, 100 mm K3PO4) for 3 h. Anthers at the microsporocyte stage were dissected under a microscope with UV illumination (DM5000, Leica, Germany). Z-stack images were obtained with a confocal microscope (SP5, Leica, Germany).
In situ hybridization
Flower buds were fixed with 3.7% formaldehyde, 5% acetic acid, 50% ethanol (FAA) and dehydrated through an ethanol series. Fixed samples were embedded in paraplast using an embedding machine (ASP200, Leica, Germany). RDP1 cDNA was PCR-amplified using the primer pair (At1g25260g2F; 5′-tgcctaatcaaagcgagtagacc-3′ and At1g25260gR; 5′-cagagcaagttcagcttgaaagtagc-3′) and cloned into pCR4-TOPO (Thermo Fisher Scientific) vector. Cloned cDNA was used as a template for in vitro transcription using a MAXIscript T7 labeling kit (Thermo Fisher Scientific) for hybridization54.
GUS assays
Plant samples were incubated in 90% acetone for 20 min at room temperature, washed with 50 mm phosphate buffer containing 0.1% Triton X-100, 2 mm potassium ferrocyanide, and 2 mm potassium ferricyanide, and incubated in the same buffer supplemented with 1 mg mL−1 X-Gluc for 5 h at 37 °C55.
Phylogenetic analysis
Multiple sequence alignment was performed using ClustalW implemented in the CLC Workbench (version 7.7). A phylogenetic tree was generated by the neighbor-joining distance algorithm, using the aligned region (amino acid positions 44–153) and a bootstrap value of 1000. Yvh1 of Saccharomyces cerevisiae was used as an outgroup. Accession numbers of used sequences are listed in Supplementary Table 11.
Selection scan
For the selection scan, we used the imputed SNP data set that was also used for GWAS. We used 298 accessions (Supplementary Data 1), covering all the accessions used for our GWAS of pollen and ovule numbers and the GWAS of 107 phenotypes reported by Atwell et al.19,25,56. We used the iHS statistic for the selection scan; this statistic compares the EHH between two alleles by controlling for the allele frequency of each SNP23. The iHS statistic uses the contrast of EHH values on each SNP; iHS values strongly deviate from zero when one allele has a long haplotype (high EHH) and the other has a short one (low EHH); the R library rehh57 was used to calculate the iHS statistic. The Arabidopsis lyrata reference genome58 was used to infer the ancestral state for each SNP. We first calculated the iHS for each SNP. Then, we split the genome into 10-kb windows and used the maximum score from the iHS scan for each window as the test statistic, as LD is known to decay within ~10-kb on average in geographically world-wide samples of A. thaliana59. Empirical P values were calculated for all windows and for all SNPs, based on their ranks in genomic distributions. To deal with the geographically biased sampling, which could be a possible confounding factor, we also performed the selection scan with 144 accessions that were used for GWAS (see Supplementary Note 2 for details; Supplementary Fig. 5).
We then asked whether the GWAS-associated windows were enriched in the extreme tails of the iHS statistic. Enrichment analysis was performed across the 10-kb windows. To examine whether enrichment of the iHS was commonly observed in GWAS peaks, we also performed this analysis for the GWAS results of the other 107 publicly available phenotypes19 and compared them with the GWAS of pollen and ovule numbers. Four thresholds of empirical P value tails were considered: 10%, 5%, 2.5%, and 1%. GWAS SNPs were considered if P values were smaller than 10–4 and the MAF was >0.1. We also performed the same enrichment analysis with MAF > 0.15 (Supplementary Fig. 4). To assess the effect of using a 10-kb window size, we also performed the iHS enrichment analysis on a per-SNP basis, in addition to the 10-kb window size (Supplementary Fig. 6).
The statistical significance of the fold-enrichment was addressed based on permutation tests that preserve the linkage disequilibrium structure in the data. A set of windows was resampled for each permutation, preserving the relative positions of the windows, but shifting them by a randomly chosen uniformly drawn number of windows for each permutation. A similar method of permutation has been used in several population genomic studies19,25,56. Permutation was performed 1000 times.
Measuring plant phenotypes
For measuring rosette leaf size, we took images of plants that included a ruler at 3 weeks after germination. A minimum circumscribed circle was drawn manually on the picture using Fiji; then, the area was measured and transformed depending on the scale. The flowering date was counted as days from sowing to flowering. The dry weight of plants was measured using aerial parts, and seed weight was determined by collecting seeds from dried plants. P values for quantitative complementation test (Supplementary Fig. 17) are shown in Supplementary Table 12.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Matt Horton, Robert Martin, Atsushi Tajima, and Hirokazu Tsukaya for discussions, Karen Thomsen for sample preparation and pollen counting, and the Functional Genomics Center Zurich for technical support. This study was supported by the Swiss National Science Foundation 31003A_182318, 31003A_159767, JST CREST Grant Number JPMJCR16O3, Japan, to K.K.S., a Syngenta Fellowship through the Zurich-Basel Plant Science Center to T.T., T.S., and K.K.S., the University Research Priority Program of Evolution in Action of the University of Zurich to K.K.S. and U.G., MEXT KAKENHI Grant Numbers 16H06469, 16K21727, 26113709 to K.K.S., 16H06467, 17H05833, 18H04813, 19H04851 to T.T., and 16H06465 to M.M.K., JSPS KAKENHI Grant Number 19K05976 to H.K., the Forschungskredit of the University of Zurich and an EMBO long-term fellowship to T.T., a JSPS postdoctoral fellowship for research abroad to H.K. and T.T., and, in part, an Advanced Grant of the European Research Council to U.G.
Source data
Author contributions
T.T., H.K., M.Y., T.S., M.L., M.N., and K.K.S. conceived and designed the study; T.T., H.K., M.Y., D.M., and K.K.S. analyzed the data with help from A.H., U.G., T.S., M.L., and M.N.; T.T., H.K., M.Y., C.M., H.T., A.H., Y.S., and M.M.K. performed experiments and generated the data; T.T., H.K., M.Y., and K.K.S. wrote the paper with inputs from U.G., T.S., M.L., and M.N.; All authors discussed the results and commented on the manuscript.
Data availability
Data supporting the findings of this work are available within the paper and its Supplementary Information files. A Reporting Summary for this Article is available as a Supplementary Information file. The data sets generated and analyzed during the current study are available from the corresponding author upon request. RDP1 gene sequence data generated in this article were registered in GenBank (National Center for Biotechnology Information) databases under the following accession numbers: LC164158 (Mz-0), LC164159 (Bor-4), LC504218 (Uod-1), LC164160 (A. lyrata), and LC164161 (Arabidopsis halleri). P0 gene sequence data of A. halleri generated in this Article were registered in GenBank databases under the following accession numbers: LC164162 and LC164163. Raw and processed sequencing data for GWAS and population genetic analyses are publicly available at 10.5061/dryad.jh9w0vt7z (ref. 60). The source data underlying Figs. 1e, 1j, 1k, 2a, 2e, 2h, and 3, as well as Supplementary Figs. 1, 3–6, 7a, 7d, 8, 9, 10c, 10d, 15, 17, and 18 are provided as a Source Data file.
Code availability
Code used for selection enrichment analysis is publicly available at https://github.com/tsuchimatsu/arabidopsis_pollen_number.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks James Prendergast, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Takashi Tsuchimatsu, Hiroyuki Kakui.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-16679-7.
References
- 1.Harvey PH, May RM. Out for the sperm count. Nature. 1989;337:508–509. doi: 10.1038/337508a0. [DOI] [PubMed] [Google Scholar]
- 2.Birkhead, T. R. & Møller, A. P. Sperm Competition and Sexual Selection (Academic Press, 1998).
- 3.Merzenich H, Zeeb H, Blettner M. Decreasing sperm quality: a global problem? BMC Public Health. 2010;10:24. doi: 10.1186/1471-2458-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czeizel AE, Rothman KJ. Does relaxed reproductive selection explain the decline in male reproductive health? A new hypothesis. Epidemiology. 2002;13:113–114. doi: 10.1097/00001648-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Kosova G, Scott NM, Niederberger C, Prins GS, Ober C. Genome-wide association study identifies candidate genes for male fertility traits in humans. Am. J. Hum. Genet. 2012;90:950–961. doi: 10.1016/j.ajhg.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu KK, Tsuchimatsu T. Evolution of selfing: recurrent patterns in molecular adaptation. Annu. Rev. Ecol. Evol. Syst. 2015;46:593–622. [Google Scholar]
- 7.Darwin, C. The Effects of Cross and Self Fertilisation in the Vegetable Kingdom (John Murray, 1876).
- 8.Sicard A, Lenhard M. The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants. Ann. Bot. 2011;107:1433–1443. doi: 10.1093/aob/mcr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett SCH. The evolution of plant sexual diversity. Nat. Rev. Genet. 2002;3:274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- 10.Oka H-I, Morishima H. Variations in the breeding systems of a wild rice, Oryza perennis. Evolution. 1967;21:249–258. doi: 10.1111/j.1558-5646.1967.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 11.Boeven PHG, et al. Genetic architecture of male floral traits required for hybrid wheat breeding. Theor. Appl. Genet. 2016;129:2343–2357. doi: 10.1007/s00122-016-2771-6. [DOI] [PubMed] [Google Scholar]
- 12.Langer SM, Longin CFH, Würschum T. Phenotypic evaluation of floral and flowering traits with relevance for hybrid breeding in wheat (Triticum aestivum L.) Plant Breed. 2014;133:433–441. [Google Scholar]
- 13.Goldberg EE, et al. Species selection maintains self-incompatibility. Science. 2010;330:493–495. doi: 10.1126/science.1194513. [DOI] [PubMed] [Google Scholar]
- 14.Cruden RW. Pollen grains: why so many? Plant Syst. Evol. 2000;222:143–165. [Google Scholar]
- 15.Willis JH. The contribution of male-sterility mutations to inbreeding depression in Mimulus guttatus. Heredity. 1999;83:337–346. doi: 10.1038/sj.hdy.6885790. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchimatsu T, et al. Evolution of self-compatibility in Arabidopsis by a mutation in the male specificity gene. Nature. 2010;464:1342–1346. doi: 10.1038/nature08927. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchimatsu T, Kaiser P, Yew C-L, Bachelier JB, Shimizu KK. Recent loss of self-incompatibility by degradation of the male component in allotetraploid Arabidopsis kamchatica. PLoS Genet. 2012;8:e1002838. doi: 10.1371/journal.pgen.1002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charnov, E. L. The Theory of Sex Allocation (Princeton University Press, 1982).
- 19.Atwell S, et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature. 2010;465:627–631. doi: 10.1038/nature08800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock AM, et al. Adaptation to climate across the Arabidopsis thaliana genome. Science. 2011;334:83–86. doi: 10.1126/science.1209244. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu KK, Shimizu-Inatsugi R, Tsuchimatsu T, Purugganan MD. Independent origins of self-compatibility in Arabidopsis thaliana. Mol. Ecol. 2008;17:704–714. doi: 10.1111/j.1365-294X.2007.03605.x. [DOI] [PubMed] [Google Scholar]
- 22.The 1001 Genomes Consortium. 1,135 genomes reveal the global pattern of polymorphism in Arabidopsis thaliana. Cell. 2016;166:481–491. doi: 10.1016/j.cell.2016.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voight BF, Kudaravalli S, Wen X, Pritchard JK. A map of recent positive selection in the human genome. PLoS Biol. 2006;4:e72. doi: 10.1371/journal.pbio.0040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tishkoff SA, et al. Haplotype diversity and linkage disequilibrium at human G6PD: recent origin of alleles that confer malarial resistance. Science. 2001;293:455–462. doi: 10.1126/science.1061573. [DOI] [PubMed] [Google Scholar]
- 25.Horton MW, et al. Genome-wide patterns of genetic variation in worldwide Arabidopsis thaliana accessions from the RegMap panel. Nat. Genet. 2012;44:212–216. doi: 10.1038/ng.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scholl RL, May ST, Ware DH. Seed and molecular resources for Arabidopsis. Plant Physiol. 2000;124:1477–1480. doi: 10.1104/pp.124.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsutsui H, Higashiyama T. pKAMA-ITACHI vectors for highly efficient CRISPR/Cas9-mediated gene knockout in Arabidopsis thaliana. Plant Cell Physiol. 2017;58:46–56. doi: 10.1093/pcp/pcw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez-Mateos M, et al. Role and dynamics of the ribosomal protein P0 and its related trans-acting factor Mrt4 during ribosome assembly in Saccharomyces cerevisiae. Nucleic Acids Res. 2009;37:7519–7532. doi: 10.1093/nar/gkp806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhavsar RB, Makley LN, Tsonis PA. The other lives of ribosomal proteins. Hum. Genomics. 2010;4:327–344. doi: 10.1186/1479-7364-4-5-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders PM, et al. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod. 1999;11:297–322. [Google Scholar]
- 32.Breuninger H, Lenhard M. Control of tissue and organ growth in plants. Curr. Top. Dev. Biol. 2010;91:185–220. doi: 10.1016/S0070-2153(10)91007-7. [DOI] [PubMed] [Google Scholar]
- 33.Turner TL. Fine-mapping natural alleles: quantitative complementation to the rescue. Mol. Ecol. 2014;23:2377–2382. doi: 10.1111/mec.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stern DL. A role of Ultrabithorax in morphological differences between Drosophila species. Nature. 1998;396:463–466. doi: 10.1038/24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willi Y. Mutational meltdown in selfing Arabidopsis lyrata. Evolution. 2013;67:806–815. doi: 10.1111/j.1558-5646.2012.01818.x. [DOI] [PubMed] [Google Scholar]
- 36.Koch MA, Haubold B, Mitchell-Olds T. Comparative evolutionary analysis of chalcone synthase and alcohol dehydrogenase loci in Arabidopsis, Arabis, and related genera (Brassicaceae) Mol. Biol. Evol. 2000;17:1483–1498. doi: 10.1093/oxfordjournals.molbev.a026248. [DOI] [PubMed] [Google Scholar]
- 37.Ossowski S, et al. The rate and molecular spectrum of spontaneous mutations in Arabidopsis thaliana. Science. 2010;327:92–94. doi: 10.1126/science.1180677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu KK, Kudoh H, Kobayashi MJ. Plant sexual reproduction during climate change: gene function in natura studied by ecological and evolutionary systems biology. Ann. Bot. 2011;108:777–787. doi: 10.1093/aob/mcr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang C, et al. The evolution of selfing in Arabidopsis thaliana. Science. 2007;317:1070–1072. doi: 10.1126/science.1143153. [DOI] [PubMed] [Google Scholar]
- 40.de la Chaux N, Tsuchimatsu T, Shimizu KK, Wagner A. The predominantly selfing plant Arabidopsis thaliana experienced a recent reduction in transposable element abundance compared to its outcrossing relative Arabidopsis lyrata. Mob. DNA. 2012;3:2. doi: 10.1186/1759-8753-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alonso JM, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 42.Kleinboelting N, Huep G, Kloetgen A, Viehoever P, Weisshaar B. GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Res. 2012;40:D1211–D1215. doi: 10.1093/nar/gkr1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R Core Team. R Foundation for Statistical Computing (The R Foundation, 2013).
- 44.Cao J, et al. Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat. Genet. 2011;43:956–965. doi: 10.1038/ng.911. [DOI] [PubMed] [Google Scholar]
- 45.Long Q, et al. Massive genomic variation and strong selection in Arabidopsis thaliana lines from Sweden. Nat. Genet. 2013;45:884–890. doi: 10.1038/ng.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet. Epidemiol. 2010;34:816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segura V, et al. An efficient multi-locus mixed-model approach for genome-wide association studies in structured populations. Nat. Genet. 2012;44:825–830. doi: 10.1038/ng.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lei Y, et al. CRISPR-P: a web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol. Plant. 2014;7:1494–1496. doi: 10.1093/mp/ssu044. [DOI] [PubMed] [Google Scholar]
- 49.Shimada TL, Shimada T, Hara-Nishimura I. A rapid and non-destructive screenable marker, FAST, for identifying transformed seeds of Arabidopsis thaliana. Plant J. 2010;61:519–528. doi: 10.1111/j.1365-313X.2009.04060.x. [DOI] [PubMed] [Google Scholar]
- 50.Nakagawa T, et al. Improved gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 2007;71:2095–2100. doi: 10.1271/bbb.70216. [DOI] [PubMed] [Google Scholar]
- 51.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 52.Kakui, H., Yamazaki, M., Hamaya, N.-B. & Shimizu, K. K. in Pollen and Pollen Tube Biology: Methods and Protocols (ed. Geitmann, A.) (MIMB Humana Press, 2020).
- 53.Tedder A, et al. Evolution of the selfing syndrome in Arabis alpina (Brassicaceae) PLoS ONE. 2015;10:e0126618. doi: 10.1371/journal.pone.0126618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peterson KM, et al. Arabidopsis homeodomain-leucine zipper IV proteins promote stomatal development and ectopically induce stomata beyond the epidermis. Development. 2013;140:1924–1935. doi: 10.1242/dev.090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vielle-Calzada JP, Baskar R, Grossniklaus U. Delayed activation of the paternal genome during seed development. Nature. 2000;404:91–94. doi: 10.1038/35003595. [DOI] [PubMed] [Google Scholar]
- 56.Nordborg M, et al. The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol. 2005;3:1289–1299. doi: 10.1371/journal.pbio.0030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gautier M, Vitalis R. rehh: an R package to detect footprints of selection in genome-wide SNP data from haplotype structure. Bioinformatics. 2012;28:1176–1177. doi: 10.1093/bioinformatics/bts115. [DOI] [PubMed] [Google Scholar]
- 58.Hu TT, et al. The Arabidopsis lyrata genome sequence and the basis of rapid genome size change. Nat. Genet. 2011;43:476–481. doi: 10.1038/ng.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim S, et al. Recombination and linkage disequilibrium in Arabidopsis thaliana. Nat. Genet. 2007;39:1151–1155. doi: 10.1038/ng2115. [DOI] [PubMed] [Google Scholar]
- 60.Tsuchimatsu, T. et al. Adaptive reduction of male gamete number in the selfing plant Arabidopsis thaliana. Dryad 10.5061/dryad.jh9w0vt7z (2020). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Data supporting the findings of this work are available within the paper and its Supplementary Information files. A Reporting Summary for this Article is available as a Supplementary Information file. The data sets generated and analyzed during the current study are available from the corresponding author upon request. RDP1 gene sequence data generated in this article were registered in GenBank (National Center for Biotechnology Information) databases under the following accession numbers: LC164158 (Mz-0), LC164159 (Bor-4), LC504218 (Uod-1), LC164160 (A. lyrata), and LC164161 (Arabidopsis halleri). P0 gene sequence data of A. halleri generated in this Article were registered in GenBank databases under the following accession numbers: LC164162 and LC164163. Raw and processed sequencing data for GWAS and population genetic analyses are publicly available at 10.5061/dryad.jh9w0vt7z (ref. 60). The source data underlying Figs. 1e, 1j, 1k, 2a, 2e, 2h, and 3, as well as Supplementary Figs. 1, 3–6, 7a, 7d, 8, 9, 10c, 10d, 15, 17, and 18 are provided as a Source Data file.
Code used for selection enrichment analysis is publicly available at https://github.com/tsuchimatsu/arabidopsis_pollen_number.