Key summary points
Aim
To assess which method (or combination of methods) are relevant and feasible to diagnose dehydration in nursing home residents.
Findings
International experts agreed on the relevance and feasibility of 9 anamnestic items, 8 physical symptoms and 3 blood tests to diagnose dehydration. This resulted in a diagnostic strategy consisting of a suspicion phase (including anamnestic items and physical symptoms) and a confirmation phase (including blood tests).
Message
This is the first study reaching international consensus about a strategy to diagnose dehydration in the nursing home.
Electronic supplementary material
The online version of this article (10.1007/s41999-020-00304-3) contains supplementary material, which is available to authorized users.
Keywords: Expert opinion, Diagnostic strategy, Anamnesis, Physical symptoms, Blood tests
Abstract
Purpose
Even though dehydration is a big problem among nursing home residents, a universally agreed method to diagnose dehydration among nursing home residents is missing. Therefore, this study aimed to establish consensus on a method to diagnose dehydration in this population.
Methods
Using an international Delphi study, 53 experts (physicians and advanced nurse practitioners) were asked to judge various methods to diagnose dehydration on relevance and feasibility in the nursing home. Based on the methods that gained consensus in the first and second round (≥ 75% consensus), a step-by-step diagnostic strategy was developed which was presented to, and judged by, the experts in round three.
Results
After the first and second round, consensus was reached on nine anamnestic items, eight physical symptoms and three blood tests. In the third round, 24 experts agreed with the developed step-by-step diagnostic strategy as a standard to diagnose dehydration in nursing home residents.
Conclusion
This is the first study reaching international consensus on a strategy to diagnose dehydration in the nursing home. This strategy comprehends a presumption phase, where anamnestic items and physical symptoms are examined, followed by a confirmation phase with blood tests to confirm the diagnosis of dehydration. Using this strategy, it is important to take the individual characteristics (e.g. co-morbidity) of the resident and its care environment (e.g. ambient temperature) into account.
Electronic supplementary material
The online version of this article (10.1007/s41999-020-00304-3) contains supplementary material, which is available to authorized users.
Introduction
Dehydration is a condition that arises from excessive loss of body water with or without sodium and is a complex care problem, with adverse effects on health and wellbeing [1, 2]. Failure to identify and treat dehydration is associated with reduced quality of life and increased mortality [3–5]. Dehydration often leads to hospital admissions with associated high health care costs [4].
Dehydration often occurs in frail patient populations, such as nursing home residents. Research show widely varying prevalence of dehydration in nursing home residents, ranging from 0.8 to 38.8% depending on the methods used to diagnose dehydration [6]. Health care providers use multiple methods to diagnose dehydration, including physical symptoms (e.g. dry mucous membranes), blood tests (e.g. serum osmolality) and urine tests (e.g. urine specific gravity). However, it is not clear which method, or combination of methods, is the best and most feasible way to diagnose dehydration in nursing home residents [6].
One reason why it is particularly difficult to diagnose dehydration in this target group in a uniform way is that some clinical signs associated with dehydration can also be caused by other conditions common in older adults. For instance, symptoms like tongue furrows, dry mucous membranes, and measurements like urine specific gravity, can be indicative of dehydration but can also be influenced by medications [7, 8]. Another example is the Blood Urea Nitrogen Serum Creatinine Ratio (BUN/Scr) which may point to dehydration but also to conditions like renal or heart failure, both common in nursing home residents [9].
A further challenge to diagnose dehydration in nursing home residents is that it is not feasible to use some relevant diagnostic methods in nursing homes in every country [10, 11]. For example, some laboratory tests cannot be taken and/or analyzed in the nursing home itself and the involvement of hospital laboratories is required. This can be time-consuming and lead to delays in results. This in turn delays commencement of treatment resulting in deterioration in residents’ health and avoidable hospital admissions [8, 10, 12].
These factors challenge the adequate and timely detection of dehydration. A universally agreed approach to diagnose dehydration, which is feasible in nursing homes, is needed. The objective of this study is to reach a consensus on a relevant and feasible method (or combination of methods) to diagnose dehydration in nursing home residents by means of a Delphi study.
Methods
Research design
To gain consensus on the most relevant and feasible method, or combination of methods, to diagnose dehydration in nursing home residents, a Delphi study was conducted. Three structured rounds of questionnaires were completed. The study was approved by the Medical Ethics Committee of University Hospital Maastricht (2018–0728).
Data collection and data analysis
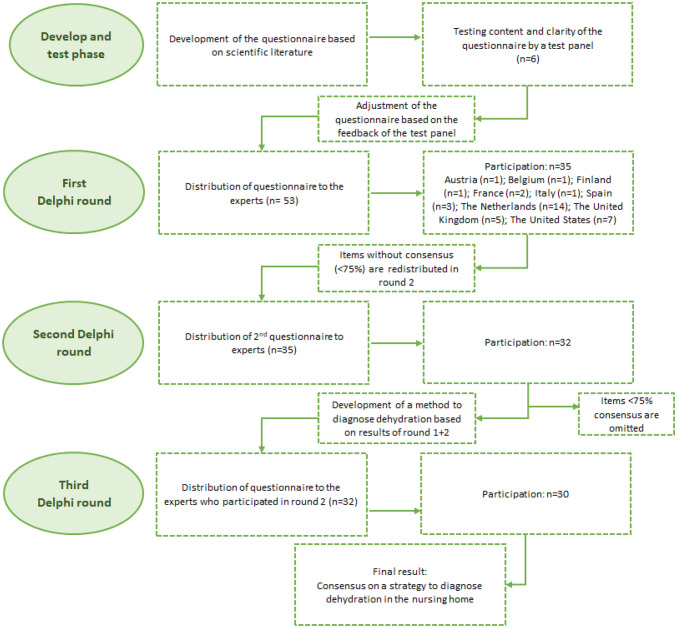
The study consisted of four phases, described in detail below (see Fig. 1).
Fig. 1.
Phases of data collection used in Delphi study
Phase 1: develop and test phase
The questionnaire was based on a systematic review which includes a comprehensive overview of recent literature about dehydration in nursing home residents [6]. The project team critically discussed diagnostic approaches retrieved from the literature and developed a first draft of the questionnaire. To pilot the questionnaire for content and clarity, an international test panel (n = 6) obtained from international contacts from the research team, was asked to critically reflect on the draft questionnaire. This international test panel (including panel members from the United Kingdom, The Netherlands and Austria) consisted of an elderly care physician, three geriatricians, a nurse practitioner and an internist. The panel received the questionnaire by e-mail, accompanied by questions about the completeness, structure and clarity of the questionnaire. After critical review by the test panel the questionnaire was adjusted by removing some methods as well as processing several textual changes (see Appendix 1 in Electronic Supplementary Material).
The final questionnaire comprised a general part, including five questions about background characteristics of the participating experts, and four sections representing different methods to diagnose dehydration comprising: anamnestic data (11 items); physical symptoms (10 items); blood tests (5 items); and urine tests (1 item). Experts were asked to indicate whether they thought a method was: (1) a relevant indicator to diagnose dehydration among nursing home residents (yes/no); and (2) feasible for the diagnosis of dehydration in nursing homes (yes/no). After each section, experts were given the opportunity to provide comments or explain their answers. In addition, experts were asked to suggest additional methods to diagnose dehydration, which were not yet included in the questionnaire. Additional methods which were mentioned by > 10% of the experts were included in the Delphi process [13].
Selection of experts
Both national and international experts were invited to participate in the Delphi study. Experts were eligible for participation if:
They were a physician (e.g. general practitioner, geriatrician or elderly care physician [14]), or advanced nurse practitioner, and
Were currently working with nursing home residents;
Experts were invited to participate from the research team’s professional networks from Austria, Belgium, France, Italy, Norway, Spain, the Netherlands, the United Kingdom and the United States. Not every country has advanced nurse practitioners in their care system. No stratification criteria were used regarding the distribution of experts (physicians vs. advanced nurse practitioners) by country.
The literature suggests a sample size between 30 to 40 experts for a Delphi study [15]. To account for dropout and non-response, 53 experts were invited to participate. Answers from experts who dropped-out during the Delphi rounds were included in the data analysis.
Phase 2: first Delphi round
All experts (n = 53) who initially agreed to participate in the Delphi study were sent an email on November 26, 2018 through the online Qualtrics software [16]. The email contained information on the aim and content of the Delphi method as well as instructions on questionnaire completion. After two weeks a reminder was sent to experts who had not yet responded.
Experts were asked to indicate whether or not they judged a method to be relevant to the diagnosis of dehydration in nursing home residents. A separate question asked if the method was feasible to conduct in nursing homes. Experts were also given the opportunity to describe methods they deemed important to diagnose dehydration, which were not yet included in the questionnaire. If > 10% of the experts mentioned the same additional method, it was included in the second round [13].
Level of consensus
Based on other studies with a similar Delphi methodology, consensus on a method was reached if ≥ 75% of the experts gave the same answer (yes/no) [17].
Phase 3: second Delphi round
A second Delphi round was conducted to seek further consensus (≥ 75% agreement) on methods for which no consensus (< 75% agreement) was reached in the first round. The second questionnaire was sent on February 28, 2019 (see Appendix 1 in Electronic Supplementary Material). A reminder was sent after two weeks to participants who had not yet responded. In this questionnaire, participants received feedback on the methods on which consensus had been reached. Also, all methods for which no consensus was reached in the first round in terms of relevance or feasibility were presented again. Experts were asked to reassess their answers given in the first round by answering ’yes’ or ’no’. To have experts reconsider their initial answers and to seek further consensus, every table contained the distribution of answers provided by experts in the first round together with the individual answers of the expert.
Experts were asked to also assess the relevance and feasibility of additional methods mentioned by > 10% of experts in the first round.
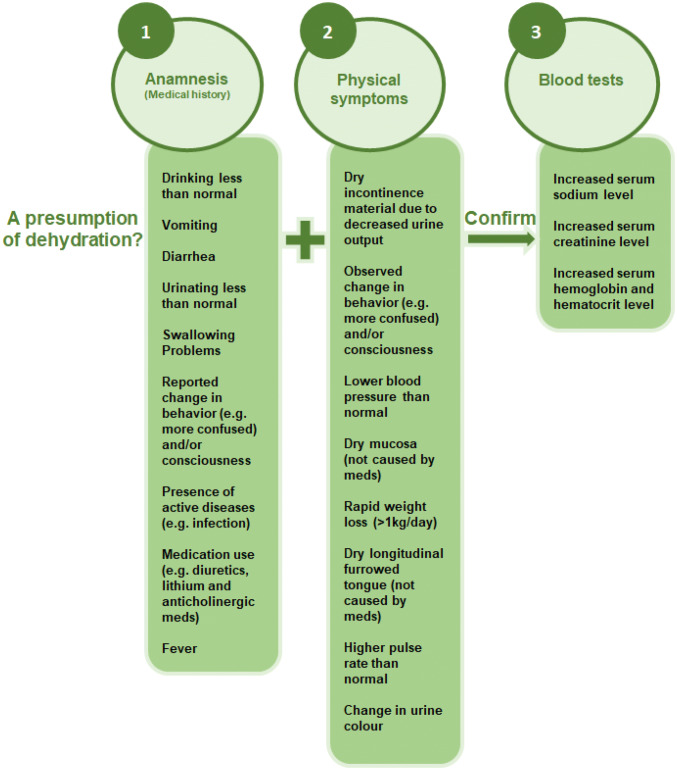
Methods which had failed to reach consensus after the second round were excluded. The reason for this was that after giving the same answer in two consecutive rounds, it was not likely that experts would change their answers in the third round [18, 19]. The methods for which consensus was reached on relevance and feasibility were used to develop a step-by-step diagnostic strategy based on regular clinical practice to diagnose dehydration in nursing home residents (see Fig. 2) [20].
Fig. 2.
Step-by-step strategy to diagnose dehydration in nursing home residents in the nursing home itself. This strategy can be regarded as a method in which health care professionals first have to pay attention to the items in the presumption phase, where after the diagnosis of dehydration is established in the confirmation phase taking into account the individual characteristics of a resident and the characteristics of the care environment
Phase 4: third Delphi round
In the third and final round, which started on May 28, 2019 experts were asked to indicate whether or not they agreed with this strategy as a standard to diagnose dehydration in nursing home residents (see Appendix 1 in Electronic Supplementary Material). Experts could answer this question with ’yes’ or ’no’. If the answer was ’no’, experts were asked to explain their answer. A reminder was sent after two weeks to the experts who did not respond yet in the third round.
All results were analyzed using descriptive statistics in the software program SPSS Statistics 25 (IBM) [21].
Results
First Delphi round
Demographic characteristics
In total, 35 out of 53 invited experts participated in the first Delphi round. Reasons for non-response were not provided. Twenty-two (62.9%) were physicians and 13 (37.1%) advanced nurse practitioners. Participants worked in Austria (n = 1), Belgium (n = 1), Finland (n = 1), France (n = 2), Italy (n = 1), Spain (n = 3), The Netherlands (n = 14), the United Kingdom (n = 5) and the United States (n = 7) (see Fig. 1). The mean years of working experience in providing care for nursing home residents were 18.4 years (SD 8.9). Most experts (68.6%) worked in a nursing home with no formal agreements with a hospital about 24-h laboratory diagnostic support.
Consensus after first round
Anamnesis: Of the 11 methods in the section ‘anamnesis’, consensus on relevance and feasibility of a method to diagnose dehydration was reached for 8 (72.7%) methods: ‘drinking less than normal/decreased fluid intake’, ‘presence of active disease(s)’, ‘fever’, ‘vomiting’, ‘diarrhea’, ‘swallowing problems’, ‘reported change in behavior’ and ‘use of medication’.
Physical symptoms: In the section ‘physical symptoms’, consensus was achieved for 3 out of 10 methods (30%). These were ‘lower blood pressure than normal’, ‘dry incontinence material due to decreased urine output’ and ‘observed change in behavior’.
Blood- and urine testing: In the section blood- and urine testing, no consensus could be reached in terms of relevance and feasibility (see Table 1). Experts mentioned some additional methods in the first round. ‘An increased Blood Urea Nitrogen (BUN)’ (n = 4) and ‘an increased serum urea’ (n = 6) were carried forward to the second round as they were mentioned by > 10% of the experts.
Table 1.
Methods consensus round 1 and 2
| Relevant to diagnose dehydration among nursing home residents (yes) Round 1 (n = 35) |
Feasible to diagnose dehydration in the nursing home (yes) Round 1 (n = 35) |
Relevant to diagnose dehydration among nursing home residents (yes) Round 2 (n = 32) |
Feasible to diagnose dehydration in the nursing home (yes) Round 2 (n = 32) |
|
|---|---|---|---|---|
| Anamnestic data | ||||
| Drinking less than normal/decreased fluid intake | n = 34 (97.1%)a | n = 32 (91.4%)a | ||
| Vomiting | n = 34 (97.1%)a | n = 33 (94.3%)a | ||
| Diarrhea | n = 34 (97.1%)a | n = 33 (94.3%)a | ||
| Urinating less than normal | n = 33 (94.3%)a | n = 23 (65.7%) | n = 28 (87.5%)a | |
| Swallowing problems | n = 32 (91.4%)a | n = 32 (91.4%)a | ||
| Change in behavior (e.g. more confused) and/ or consciousness | n = 32 (91.4%)a | n = 29 (82.9%)a | ||
| Presence of active disease(s) (e.g. renal failure, infection, active co-pathology such as DM | n = 31 (88.6%)a | n = 30 (85.7%)a | ||
|
Use of medication (e.g. diuretic medication, lithium, anticholinergic meds, ACE-inhibitors, beta-blockers) |
n = 31 (88.6%)a | n = 28 (80.0%)a | ||
| Fever | n = 30 (85.7%)a | n = 31 (88.6%)a | ||
| Sweating | n = 28 (80.0%)a | n = 24 (68.6%) | n = 20 (62.5%) | |
| Thirst | n = 22 (62.9%) | n = 15 (42.9%) | n = 17 (53.1%) | n = 10 (31.3%) |
| Physical symptoms | ||||
| Dry incontinence material due to decreased urine output | n = 33 (94.3%)a | n = 29 (82.9%)a | ||
| Change in behavior (e.g. more confused) and/or consciousness | n = 31 (88.6%)a | n = 27 (77.1%)a | ||
| Lower blood pressure than normal | n = 31 (88.6%)a | n = 29 (82.9%)a | ||
| Dry mucosa (not caused by medication) | n = 29 (82.9%)a | n = 26 (74.3%) | n = 28 (87.5%)a | |
| Rapid weight loss (> 1 kg per day) | n = 26 (74.3%) | n = 27 (77.1%)a | n = 28 (87.5%)a | |
| Dry longitudinal furrowed tongue (not caused by medication) | n = 25 (71.4%) | n = 22 (62.9%) | n = 30 (93.8%)a | n = 28 (87.5%)a |
| Higher pulse rate than normal | n = 25 (71.4%) | n = 26 (74.3%) | n = 29 (90.6%)a | n = 30 (93.8%)a |
| Change in urine colour | n = 25 (71.4%) | n = 27 (77.1%)a | n = 27 (84.4%)a | |
| Hyperthermia | n = 21 (60.0%) | n = 24 (68.6%) | n = 22 (68.8%) | n = 27 (84.4%)a |
| Poor skin turgor | n = 21 (60.0%) | n = 22 (62.9%) | n = 19 (59.4%) | n = 20 (62.5%) |
| Blood tests | ||||
| Increased serum sodium level | n = 30 (85.7%)a | n = 23 (65.7%) | n = 27 (84.4%)a | |
| Increased serum creatinine level | n = 28 (80.0%)a | n = 25 (71.4%) | n = 27 (84.4%)a | |
| Increased serum osmolality | n = 26 (74.3%) | n = 17 (48.6%) | n = 30 (93.8%)a | n = 18 (56.3%) |
| Higher blood glucose level (in case of diabetes mellitus) | n = 23 (65.7%) | n = 28 (80.0%)a | n = 22 (68.8%) | |
| Increased serum hemoglobin and hematocrit level | n = 23 (65.7%) | n = 25 (71.4%) | n = 25 (78.1%)a | n = 27 (84.4%)a |
| Urine tests | ||||
| Increased urine glucose level (in case of Diabetes Mellitus) | n = 13 (37.1%) | n = 19 (54.3%) | n = 5 (15.6%) | n = 12 (37.5%) |
aConsensus reached (≥ 75%)
Second Delphi round
In total, 32 experts participated in the second round (see Fig. 1). Reasons for drop-out were not provided.
Consensus after second round
Anamnesis: Of the anamnestic methods without consensus in the first round, no consensus could be reached in terms of relevance and feasibility on the method ‘thirst’ and ‘sweating’ to diagnose dehydration among nursing home residents. Consensus was reached for ‘urinating less than normal’.
Physical symptoms: Of the remaining seven physical symptoms without consensus after the first Delphi round, consensus was reached for relevance and feasibility on five methods: ‘dry mucosa’, ‘dry longitudinal furrowed tongue’, ‘a higher pulse rate than normal’, ‘rapid weight loss’ and ‘change in urine colour’.
Blood- and urine testing: Out of five methods in the section blood tests, ‘increased serum hemoglobin’ and ‘hematocrit level’, ‘increased serum creatinine level’ and ‘increased serum sodium level’ reached consensus on relevance and feasibility. No consensus could be reached on the methods in the section ‘urine tests’. Increased serum urea and BUN were mentioned as additional methods in the section blood tests in the first Delphi round. More than 75% of the experts assessed increased serum urea as relevant but felt that it was not feasible to test in the nursing home. BUN did not achieve consensus for relevance or feasibility.
Third Delphi round
Based on the items that reached consensus on relevance and feasibility after the second round, a diagnostic strategy to assess dehydration among nursing home residents was developed. This strategy comprised a presumption phase and a confirmation phase. The presumption phase included the anamnestic items and physical symptoms on which consensus was reached, whilst the confirmation phase included the blood tests on which consensus was reached. This strategy can be regarded as a step-by-step plan whereby health care professionals focus first on the presumption phase before moving on to confirm their diagnosis of dehydration in the confirmation phase (see Fig. 2).
In total, 30 experts participated in the third round (see Fig. 1) seeking agreement on the diagnostic strategy. Twenty-four experts agreed on the strategy as the standard to diagnose dehydration among nursing home residents. Experts who disagreed with the strategy were largely physicians (83%) and worked in the Netherlands (n = 3), the United Kingdom (n = 2) and Finland (n = 1). The main reason they disagreed with the strategy was that the strategy did not specify how many methods/items should be fulfilled to start the confirmation phase.
Discussion
This study established a structured approach to diagnose dehydration in nursing homes comprising anamnestic items, physical symptoms and blood tests. This approach is supported by professional consensus from healthcare experts across nine countries.
The E-SPEN guideline and a diagnostic accuracy study suggested that serum osmolality was the best test to diagnose dehydration among older adults and nursing home residents [22, 23]. Some elements from the E-SPEN guideline support the results of this Delphi study. The difference is that we focused this Delphi study entirely on nursing home residents and the feasibility of methods in nursing homes. Serum osmolality was not supported by our Delphi exercise when taking feasibility into account, allowing for the lack of laboratory access from nursing homes. In addition, our consensus strategy incorporated several physical signs (e.g. including the dry furrowed tongue, dry mucosa and changes in pulse rate and blood pressure), the importance of which has been previously contested due to their lack of accuracy [23]. Literature suggests that these physical symptoms are individually less useful, but a combination of physical symptoms may identify dehydration [22]. A patient-tailored approach in the nursing home setting seems desirable as indicated by experts in this Delphi study. Our approach here was pragmatic, with an emphasis on feasibility, and the resulting recommendation incorporates these signs in the presumption phase, only establishing the diagnosis with supporting blood tests in the confirmation phase.
In addition, although the strategy developed in our study indicates that if any of the above-mentioned anamnestic items and physical symptoms is present, this could be a reason for further diagnostics (blood testing), prioritization of the items was not done. Four out of six experts in the third Delphi round disagreed with the strategy due to the absence of prioritization. The rationale for excluding prioritization was that the importance of any of these items is highly dependent on the individual characteristics of a resident, as well as other risk factors such as characteristics of the care environment for developing dehydration that might be present [24–26]. This means that for one resident, only one anamnestic item and one physical symptom might trigger further blood testing, while for another resident 3 or 4 items be present before deciding to perform a blood test. Therefore, it is also recommended to take into account the individual characteristics (e.g. co-morbidity) and the resident’s care environment (e.g. ambient temperature) in the decision-making process if further diagnostics (blood tests) are needed [2, 5].
We did not rank items included in the diagnostic strategy in terms or order of importance. It is, though, evident from Table 1 that there was greater consensus around the importance of particular items. ‘Drinking less than normal/decreased fluid intake’, ‘vomiting’, ‘diarrhea’, ‘swallowing problems’, ‘urinating less than normal’, ‘reported change in behaviour’ and ‘a higher pulse rate than normal’ scored the highest level of agreement for both relevance and feasibility. There is a strong emphasis amongst these in detecting a change from normality. This requires detailed knowledge of the resident and emphasises the important of nursing home staff in triggering the presumption phase. The technical challenges of supporting the anamnestic items and physical symptoms in the strategy are recognised, with evidence that decreased oral intake, for example, may be difficult for nursing staff to recognise [27, 28]. More structured approaches to these anamnestic items and physical symptoms included in the strategy are required and should be the focus of future research.
Strengths and limitations
The strengths of this study rise from a highly structured and objective approach, comprising thorough literature review, followed by structured consensus informed by experts from a number of countries [15]. The international nature of the participants increases the generalisability of our findings. The study had good response rates (varying between 66 and 93.8%) compared with the broader Delphi literature [29, 30].
The results from this Delphi study may be generalizable to other settings, besides the nursing home, in which older adults receive care. The strategy is tailored to the needs of individual patients and takes into account the heterogeneity of older adults. Nevertheless, there may be differences in the feasibility of the methods when moving between different settings. We would recommend further consensus work before these findings are transferred to other settings.
A limitation is the failure to rank methods in terms of importance for the final diagnostic strategy. Whilst this made it easier to achieve consensus, it provides less specific guidance on which items should be prioritized. This was, though, a conscious decision, as inferring strength of association between anamnestic signs and physical symptoms and the likelihood of dehydration based upon consensus alone would be conceptually flawed. The resulting strategy allows healthcare professionals to structure their approach and draws their attention to areas of agreed importance when presuming and confirming a diagnosis of dehydration.
A potential bias in this study is that the majority of the participants came from one specific country (the Netherlands). This might have led to selection bias. However, subanalyses showed that experts from the Netherlands did not indicate more or different methods to be relevant or feasible to diagnose dehydration compared to experts from other countries. An additional strength was that the Delphi study consisted of multiple rounds, between which individual answers with summarized group responses were distributed to each expert. This allowed experts to make a well-considered (re)assessment on the relevance and feasibility of a method [31].
Finally, although this strategy was assessed on both relevance and feasibility, we are aware that formal agreements with a hospital about 24-h laboratory diagnostics is not standard practice in every country. Therefore, when implementing the strategy, clear agreements with hospitals on blood testing should be made.
Conclusion
This Delphi study produced a strategy to diagnose dehydration using a range of anamnestic items, physical symptoms and blood tests. Research to validate these recommendations is required especially because of the high heterogeneity and multimorbidity in this group. The strategy encompasses a broad range of items. As various items in the presumption phase of the strategy can only be identified by a health care professional who frequently interacts with the resident, effective interdisciplinary working between nursing staff and physicians in this phase is essential. Further research should focus on this collaboration and the barriers and facilitators to this from the perspective of both parties. Additionally, identifying changes in residents in busy routine care practice in the context of multimorbidity, functional dependency and cognitive impairment can be challenging. Research to develop reliable approaches to the anamnestic items and physical symptoms are, therefore, important.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the experts who were willing to put their knowledge, effort and time into participating in this Delphi study.
Author contributions
All authors meet the criteria for authorship stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals. Conception and design: all authors. Acquisition of data: SJCP. Analysis and interpretation of data: SJCP, IHJE, RJGH, JMGAS. Drafting the article: all authors. Revising the article critically for intellectual content: all authors
Funding
This research did not receive any funding from agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
Om behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Medical Ethics Committee of University Hospital Maastricht (2018-0728)) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organisation (2011) Initial treatment of dehydration for severe acute malnutrition. https://www.who.int/elena/titles/bbc/dehydration_sam/en/. Accessed 28 Aug 2019
- 2.Thomas DR, Cote TR, Lawhorne L, et al. Understanding clinical dehydration and its treatment. J Am Med Dir Assoc. 2008;9(5):292–301. doi: 10.1016/j.jamda.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Begum MN, Johnson CS. A review of the literature on dehydration in the institutionalized elderly. J Clin Nutr Metab. 2010;5:e47–e53. [Google Scholar]
- 4.Hooper L, Bunn D, Jimoh FO, et al. Water-loss dehydration and aging. Mech Ageing Dev. 2013;136:50–58. doi: 10.1016/j.mad.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Schols JMGA, De Groot CPGM, Van der Cammen TJM, et al. Preventing and treating dehydration in the elderly during periods of illness and warm weather. J Nutr Health Aging. 2009;13:150–157. doi: 10.1007/s12603-009-0023-z. [DOI] [PubMed] [Google Scholar]
- 6.Paulis SJC, Everink IHJ, Halfens RJG, et al. Prevalence and risk factors of dehydration among nursing home residents. J Am Med Dir Assoc. 2018;19(8):646–657. doi: 10.1016/j.jamda.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Bennett JA. Dehydration: hazards and benefits. Geriatr Nurs. 2000;21(2):84–88. doi: 10.1067/mgn.2000.107135. [DOI] [PubMed] [Google Scholar]
- 8.Fortes MB, Owen JA, Raymond Barker P, et al. Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. J Am Med Dir Assoc. 2015;16:221–228. doi: 10.1016/j.jamda.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Morley JE. Dehydration, hypernatremia and hyponatremia. Clin Geriatr Med. 2015;31:389–399. doi: 10.1016/j.cger.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Shirts BH, Perera S, Hanlon JT, et al. Provider management of and satisfaction with laboratory testing in the nursing home setting: results of a national internet-based survey. J Am Med Dir Assoc. 2009;10(3):161–166. doi: 10.1016/j.jamda.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen Mansfield J, Lipson S. To hospitalize or not to hospitalize? That is the question: an analysis of decision making in the nursing home. J Behav Med. 2006;32(2):64–70. doi: 10.3200/BMED.32.2.64-70. [DOI] [PubMed] [Google Scholar]
- 12.Ouslander JG, Lamb G, Faan RN, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs. J Am Geriatr Soc. 2010;58(4):627–635. doi: 10.1111/j.1532-5415.2010.02768.x. [DOI] [PubMed] [Google Scholar]
- 13.Thorn JC, Brookes ST, Ridyard C, et al. Core items for a standardized resource use measure: expert Delphi consensus survey. Value Health. 2018;21(6):640–649. doi: 10.1016/j.jval.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verenso, the Dutch Association of Elderly Care Physicians (2015) https://www.verenso.nl/_asset/_public/Vereniging/VER0026_Professionalprofile_broch_DEF.pdf. Accessed 10 Aug 2019
- 15.Von der Gracht HA. The future of logistics: scenarios for 2025. 1. Wiesbaden: Springer; 2008. [Google Scholar]
- 16.University of Michigan-Flint (2019) https://www.umflint.edu/sites/default/files/groups/Research_and_Sponsored_Programs/qualtricsusermanual.pdf. Accessed 28 Aug 2019
- 17.Strupp J, Romotzky V, Galushko M, et al. Palliative care for severely affected patients with multiple sclerosis: when and why? Results of a Delphi survey of health care professionals. J Palliat Med. 2014;17(10):1128–1136. doi: 10.1089/jpm.2013.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Zolingen SJ, Klaassen CA. Selection processes in a Delphi study about key qualifications in Senior Secondary Vocational Education. Technol Forecast Soc Chang. 2003;70(4):317–340. doi: 10.1016/S0040-1625(02)00202-0. [DOI] [Google Scholar]
- 19.Moutinho L, Sokele M. Innovative research methodologies in management. Futures, biometrics and neuroscience research. 1. Basel: Springer; 2018. [Google Scholar]
- 20.Rhoads J, Murphy JM. Differential diagnosis for the advanced practice nurse. 1. New York: Springer; 2014. [Google Scholar]
- 21.George D, Mallery P. IBM SPSS statistics 25 step by step: a simple guide and reference. 15. New York: Routledge; 2018. [Google Scholar]
- 22.Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38:10–47. doi: 10.1016/j.clnu.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Bunn DJ, Hooper L. Signs and symptoms of low-intake dehydration do not work in older care home residents—DRIE diagnostic accuracy study. J Am Med Dir Assoc. 2019;20(8):963–970. doi: 10.1016/j.jamda.2019.01.122. [DOI] [PubMed] [Google Scholar]
- 24.Johnson P, Hahn RG. Signs of dehydration in nursing home residents. J Am Med Dir Assoc. 2018;19(12):1124–1128. doi: 10.1016/j.jamda.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Siervo M, Bunn D, Prado CM, et al. Accuracy of prediction equations for serum osmolarity in frail older people with and without diabetes. Am J Clin Nutr. 2015;100(3):867–876. doi: 10.3945/ajcn.114.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liamis G, Liberopoulos E, Barkas F, et al. Diabetes mellitus and electrolyte disorders. World J Clin Cases. 2014;2(10):488–496. doi: 10.12998/wjcc.v2.i10.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett JA, Thomas V, Riegel B, et al. Unrecognized chronic dehydration in older adults: examining prevalence rate and risk factors. J Gerontol Nurs. 2004;30(11):22–28. doi: 10.3928/0098-9134-20041101-09. [DOI] [PubMed] [Google Scholar]
- 28.Reed PS, Zimmerman S, Sloane PD, et al. Characteristics associated with low food and fluid intake in long-term care residents with dementia. Gerontologist. 2005;1(1):74–80. doi: 10.1093/geront/45.suppl_1.74. [DOI] [PubMed] [Google Scholar]
- 29.Baruch Y, Holtom BC. Survey response rate levels and trends in organizational research. Hum Relat. 2008;61(8):1139–1160. doi: 10.1177/0018726708094863. [DOI] [Google Scholar]
- 30.Babbi E. The basics of social research. 4. Belmont: Cengage Learning; 2007. [Google Scholar]
- 31.Hallowell MR (2009) Techniques to minimize bias when using the delphi method to quantify construction safety and health risks. In: Construction research congress 2009: building a sustainable future, pp 1489–1498
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.