Abstract
Background and Purpose: Olfactory dysfunction is one of the most common non-motor symptoms in Parkinson's disease (PD) preceding the motor symptoms for years. This study aimed to evaluate different olfactory domains in PD patients in comparison with healthy controls and to explore the relationships among olfactory deficit and other clinical manifestations in patients with PD.
Methods: Sniffin' Sticks test, which detects olfactory threshold, discrimination, and identification (TDI), were conducted in 500 PD patients and 115 controls. Furthermore, demographic and clinical data including motor and other non-motor symptoms were collected.
Results: In the single olfactory model, the identification test showed the area under the receiver operating characteristic (ROC) curve (AUC = 0.818), followed by threshold test (AUC = 0.731) and discrimination test (AUC = 0.723). Specifically, the identification test has a similar discriminative power as the TDI score (0.818 and 0.828, respectively, p = 0.481). In the integrated olfactory model involved with other non-motor manifestations, identification test scores performed as good as the TDI score in differentiating PD patients from controls (0.916 and 0.918, respectively, p = 0.797). In PD patients, age and cognition together explained 7.5% of the variance of the threshold score, while age, cognition, and gender accounted for the 15.2% explained variance of the discrimination score, while cognition, age, the ability of daily living, and gender together interpreted 11.1% of the variance of the identification score.
Conclusion: Our results indicated that the identification domain was the most practical olfactory factor in differentiating PD patients, and the combination of several different manifestations was better than a single symptom. Furthermore, the olfactory identification score may be associated with the ability of daily living.
Keywords: Parkinson's disease, Sniffin' sticks, olfactory dysfunction, Chinese population, olfactory domains
Introduction
The olfactory deficit is one of the most important non-motor symptoms that could appear to precede motor symptoms in Parkinson's disease (PD) (1–4). Olfactory dysfunction has been incorporated both in Movement Disorder Society clinical diagnostic criteria for PD and research criteria for prodromal PD, demonstrating its role in the diagnosis and prediction of PD (5–7). PD-associated smell dysfunction involves several domains of odor perception, i.e., detection threshold, identification, discrimination, and memory (8–10). The structural changes in the olfactory bulb, neurotransmitter system dysfunction, and inflammatory activity in the brain are all possible mechanisms of olfactory impairment in PD (11).
In terms of differentiating PD from control subjects, some studies have shown that the sensitivity and specificity of olfactory testing are better than other biomarkers, including single-photon emission computed tomography (SPECT) and positron-emission tomography (PET) imaging of the dopamine (DA) transporter (12). In PD patients, different odor domains have relatively uniform impairment (13–15); however, data on the magnitude of different odor domains impairment and its ability in distinguishing PD from healthy control remains insufficient. Mahlknecht and colleagues investigated the power of olfactory function in distinguishing PD with a proper sample size, but the olfactory test was limited to the identification domain (16). Krismer and colleagues researched different olfactory domains, but the sample size is relatively small (17). Studies have indicated that combining olfactory tests and other prodromal non-motor features could recognize the risk of PD more efficiently (18); however, similar studies have never been conducted in Chinese PD populations.
In some studies, considered as an independent feature of PD, the olfactory deficit was not found to have significant associations with other symptoms of the disease (19). However, Mahlknecht and colleagues suggested that olfactory dysfunction may facilitate the development of PD from associated with rapid eye movement sleep behavior disorder (RBD) (20). There were still inconsistent conclusions about the relationship between olfactory function and other clinical manifestations in PD (21, 22).
In this study, we comprehensively evaluated the discriminative power of different olfactory domains, as well as in condition of combining other non-motor symptoms for early diagnosis of Chinese PD patients, and explored the potential relationship between olfactory deficit and other motor or non-motor features in Chinese PD patients. The aim was to identify the specific olfactory domain that has the best discriminative power and to ascertain if the olfactory deficits were independent features of PD.
Methods
Participants
All the PD patients were recruited from the inpatients and outpatients of the Department of Neurology of Xiangya Hospital, Central South University, Hunan, China, between September 2014 and July 2017 at Parkinson's Disease & Movement Disorders Multicenter Database and Collaborative Network in China (PD-MDCNC, http://pd-mdcnc.com:3111/). Patients with idiopathic PD were diagnosed by no less than two experienced neurologists according to the United Kingdom Parkinson's Disease Brain Bank criteria (23). Healthy controls without neurological diseases were recruited from Health Management Centers of Xiangya Hospital. Participants with a history of respiratory system diseases, nasal or sinonasal diseases, and neurological or sinonasal surgery were excluded. The Medical Ethics Committee of Xiangya Hospital approved the study, and the participants gave informed consent for the investigation.
Assessments
Demographic data of all subjects were collected including gender, age, years of education, smoking status, and family history of PD. Seven domains of non-motor symptoms were evaluated by the Non-Motor Symptom Scale (NMSS) (24, 25), including cardiovascular, sleep/fatigue (26), mood, perceptual problems, gastrointestinal, urinary, and sexual issues. Cognitive functions were evaluated by the Mini-Mental State Examination (MMSE) (27, 28). Olfactory function was evaluated by the Sniffin' Sticks test.
In addition, age at onset, course of disease, and anti-PD medication were recorded for patients. Motor functions were evaluated by the Unified Parkinson's Disease Rating Scale (UPDRS) and Hoehn Yahr Scale (H-Y). In addition, tremor score was measured by adding up scores of tremors at rest and action and postural tremor of hands from the UPDRS score, while bradykinesia score was calculated by score on finger taps, hand movements, rapid alternating movements of hands, and leg agility. Rigidity score was added up by the scores on rigidity of the neck, hands, and feet (29). Disease motor subtype (30) was classified as tremor-dominant (TD) phenotype when the ratio of tremor score and postural instability and gait difficulty (PIGD) score was no <1.5, whereas patients with a ratio of no more than 1.0 were defined to PIGD phenotype, and rest of patients belonged to the indeterminate phenotype. UPDRS is made up of four sections. Of them, UPDRS part II is characterized by questionnaires about self-evaluation of the activities of daily life, including speech, swallowing, handwriting, dressing, hygiene, falling, salivating, turning in bed, walking, and cutting food. UPDRS part III was used to assess motor ability. A higher UPDRS score means more severe symptoms. A higher MMSE score means better cognitive condition. A higher NMSS score means more severe non-motor symptoms. Dyskinesia was affirmed by experienced neurologists (31).
Sniffin' Sticks
Sniffin' Sticks test consist of three parts, and they were tests for olfactory threshold, discrimination, and identification domain. Threshold and discrimination tests were conducted in the condition of subjects' eyes closed or blindfolded to prevent them from recognizing through the color of pen caps.
Both threshold and discrimination tests comprised 16 triplets' pens (total of 48 pens) numbered from 1 to 16. The color of three pen caps differed from each other, which are red, blue, and green. Identification tests were comprised of 16 common odors, each of which presented 4 alternative odors to choose from. Odor threshold test could evaluate the ability to perceive the lowest concentration of an odorant by the subject, odor discrimination test measured the ability to differentiate two different odors, and odor identification test measured the ability to perceive and name the presented odor out of four alternative answers (32). The threshold score (T-score) equals the mean of the last four of seven scores, while the discrimination score (D-score) and identification score (I-score) equals the numbers of correct responses, respectively (33). The threshold, discrimination, and identification (TDI) score equals to the total score of three tests. The cutoff of the TDI score was 30.3 for ages from 16 to 35 years, 27.3 for ages from 36 to 55 years, and 19.6 for subjects older than 55 years, according to the standard of Hummel et al. (34). A higher score means better olfactory perception.
Statistics
All data were not normally distributed by the Kolmogorov–Smirnov test. All continuous variables were described as median and interquartile range (IQR), such as age, years of education, disease duration, UPDRS II, UPDRS III, MMSE, NMSS scores, and so on, while the categorical variables were described as a percentage, such as a gender, smoking status, dyskinesia status, and so on.
To establish which of the olfactory test is of service for differentiating PD patients from healthy controls, we calculated the receiver operating characteristic (ROC) curves for each of the olfactory tests separately and for any two or three tests combined. The single binary logistic regression models were developed with diagnosis as the dependent variable: using age, years of education with threshold score; then age, years of education with discrimination score; next age, years of education with identification score; and then age, years of education with any two or three of olfactory domain score added together. Afterward, integrated binary logistic regression models were developed with diagnosis as the dependent variable: using the above variable with each model combining other non-motor features, including MMSE and NMSS (cardiovascular, sleep/fatigue, mood, perceptual problems, gastrointestinal, urinary, and sexual issues). We graphed ROC curves with sensitivity and specificity estimates and corresponding area under the ROC curve (AUC), as well as positive likelihood ratios (LR+), negative likelihood ratios (LR–), positive predictive values (PPV), and negative predictive values (NPV). The ROC cutoffs were chosen when Youden's Index to get the maximum value. We compared AUC between TDI score single model and other single models by MedCalc software, as well as in the integrated models.
To compare demographic information and clinical features between PD with hyposmia and PD with normosmia, we used the analysis of chi-square tests for measurement data and non-parametric tests for continuous data.
To explore the contribution of different variables to the olfactory score, we used four stepwise multiple linear regression analyses (methods = stepwise, F-to-enter = 0.05, F-to-remove = 0.1). In the multiple linear regression analysis, independent variables include demographic factors (age, sex, educational years, smoking status), motor clinical symptoms (disease duration, UPDRS II points, UPDRS III points, dyskinesia), and other clinical symptoms (MMSE, NMSS). We did stepwise multiple linear regression analyses with threshold score, differentiation score, identification score, and TDI score as dependent variables, respectively.
Data were analyzed using SPSS version 18. p < 0.05 were considered significant.
Results
Demographic and Clinical Characteristics
In total, we recruited 500 patients (male, 269, 53.8%) with a median age at assessments of 60 years and a median age at onset of 55 years. The 115 healthy controls (male, 50, 43.5%) have a median age of 55 years. Median disease duration of PD was 3 years, whereas the median UPDRS II and UPDRS III scores were 12 and 26, respectively (Table 1).
Table 1.
Basic information and motor features in patients with Parkinson's disease (PD) and controls.
| Items | Patients with PD (n = 500) | Normal controls (n = 115) |
|---|---|---|
| Sex (male %) | 269 (53.8%) | 50 (43.5%) |
| Age (year) | 60 (52–67) | 55 (49–64) |
| Educational years (year) | 9 (6–12) | 9 (9–12) |
| Smoking or not | 144 (28.8%) | 32 (27.8%) |
| Age of onset (year) | 55 (47–62) | – |
| Duration(year) | 3 (2–6) | – |
| UPDRS II | 12 (9–17) | – |
| UPDRS III | 26 (19–38) | – |
| H-Y stage | 2 (1.5–3) | – |
Data for continuous variables are presented as medial levels (IQRs).
Olfactory Test Alone
Of the 500 included patients, 343 patients had hyposmia, whereas 157 patients had a normal sense of smell, according to the standard of Hummel's (34). The median TDI score of PD patients was 19.50 and that of the control subjects was 28.25. Median threshold, discrimination, and identification scores of PD patients were 4.75, 7, and 7, respectively, while control subjects were 7.50, 10, and 10, respectively. After age and years of education correction, every single olfactory score of PD patients were significantly lower than controls subjects (all p ≤ 0.001), as well as the total TDI score (Table 2).
Table 2.
Olfaction function in patients with Parkinson's disease (PD) and controls.
| Items | Patients with PD (n = 500) | Normal controls (n = 115) | p-value* |
|---|---|---|---|
| Threshold score (T) | 4.75 (2.25–7.00) | 7.50 (5.50–9.25) | 0.001 |
| Discrimination score (D) | 7 (5–9) | 10 (8–11) | <0.001 |
| Identification score (I) | 7 (5–9) | 10 (9–12) | <0.001 |
| TD score | 12.50 (8.50–15.75) | 16.75 (14.50–19.50) | <0.001 |
| TI score | 11.87 (7.75–15.50) | 17.75 (15.50–20.75) | <0.001 |
| DI score | 14 (11–18) | 20 (17–23) | <0.001 |
| TDI score | 19.50 (14.25–24.25) | 28.25 (24.50–31.00) | <0.00 |
p-value was calculated after adjustment of age and educational years.
ROC curves of the TDI and I scores were drawn by SPSS (Figure 1). Every model had diagnostic value between these two groups (all p < 0.05). AUC of different olfactory domains and their sensitivity, specificity, LR+, LR–, PPV, and NPV in single olfactory models and integrated models were reported (Table 3).
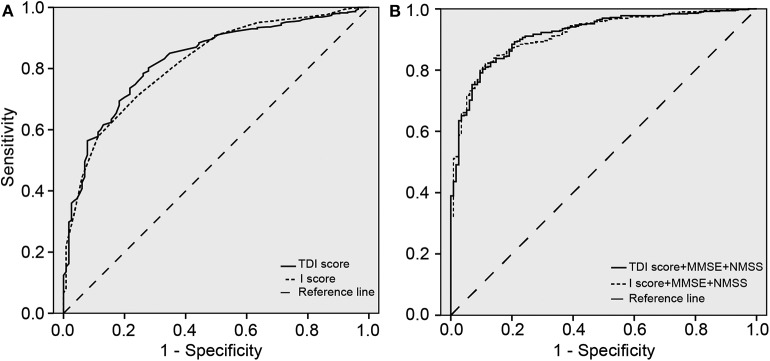
Figure 1.
Receiver operating characteristic (ROC) curves. (A) Relating sensitivity and specificity for olfactory threshold, discrimination, and identification (TDI) and I scores in differentiating Parkinson's disease (PD) patients from healthy controls. (B) Relating sensitivity and specificity for olfactory TDI and I scores combining Mini-Mental State Examination (MMSE) and Non-Motor Symptom Scale (NMSS) in differentiating PD patients from healthy controls.
Table 3.
Area under the receiver operating characteristic curve (AUC) of different olfactory domains and their sensitivity, specificity, LR+, LR–, PPV, and NPV in single olfactory models and integrated models.
| Models | Olfactory tests | Sensitivity | Specificity | PPV | NPV | LR+ | LR– | AUC | p1-values | p2-values |
|---|---|---|---|---|---|---|---|---|---|---|
| (A) SINGLE MODELS* | ||||||||||
| TDI score | 0.802 | 0.722 | 0.891 | 0.259 | 2.885 | 0.274 | 0.828 | <0.05 | ||
| TD score | 0.692 | 0.748 | 0.884 | 0.235 | 2.746 | 0.412 | 0.772 | <0.05 | <0.05 | |
| TI score | 0.796 | 0.730 | 0.894 | 0.262 | 2.948 | 0.279 | 0.819 | <0.05 | 0.306 | |
| DI score | 0.738 | 0.748 | 0.893 | 0.251 | 2.929 | 0.350 | 0.815 | <0.05 | 0.239 | |
| T score | 0.612 | 0.783 | 0.888 | 0.229 | 2.820 | 0.496 | 0.731 | <0.05 | <0.05 | |
| D score | 0.544 | 0.791 | 0.875 | 0.215 | 2.603 | 0.576 | 0.723 | <0.05 | <0.05 | |
| I score | 0.712 | 0.757 | 0.894 | 0.247 | 2.930 | 0.380 | 0.818 | <0.05 | 0.481 | |
| (B) INTEGRATED MODELS** | ||||||||||
| TDI score | 0.803 | 0.896 | 0.971 | 0.515 | 7.721 | 0.220 | 0.918 | <0.05 | ||
| TD score | 0.846 | 0.835 | 0.956 | 0.559 | 5.127 | 0.184 | 0.902 | <0.05 | <0.05 | |
| TI score | 0.838 | 0.870 | 0.965 | 0.556 | 6.446 | 0.186 | 0.913 | <0.05 | 0.298 | |
| DI score | 0.783 | 0.913 | 0.975 | 0.495 | 9.000 | 0.238 | 0.913 | <0.05 | 0.374 | |
| T score | 0.756 | 0.887 | 0.966 | 0.459 | 6.690 | 0.275 | 0.890 | <0.05 | <0.001 | |
| D score | 0.707 | 0.922 | 0.975 | 0.424 | 9.064 | 0.318 | 0.886 | <0.05 | <0.001 | |
| I score | 0.813 | 0.896 | 0.971 | 0.528 | 7.817 | 0.209 | 0.916 | <0.05 | 0.797 | |
p1-values show the significance of differentiating PD patients from controls calculated by SPSS. p2-values were calculated by MedCalc software. p2-values of (A) were calculated by comparing AUC between each score with TDI score in single models, p2-values of (B) were calculated by comparing AUC between each score with TDI score in integrated models. p < 0.05 were considered significant.
Single models were built upon each corresponding olfactory test, after adjustment of age and educational years.
Integrated models were built upon each corresponding olfactory test and MMSE, NMSS, after adjustment of age and educational years.
PPV, positive predictive values; NPV, negative predictive values; LR+, positive likelihood ratios; LR–, negative likelihood ratios; T, threshold; D, Discrimination; I, Identification.
In single models, the TDI score (AUC = 0.828) and identification score (AUC = 0.818) were better than threshold score (AUC = 0.731) and discrimination score (AUC = 0.723) at differentiating PD patients from controls. By comparing AUC between identification score with TDI score in single models, there was no significant difference of discriminative power between TDI and identification scores (difference between areas = 0.01, z statistic = 0.706, p = 0.481).
Olfactory Test Combining Other Non-motor Features
Compared to control subjects, PD patients had poorer performance on other non-motor features, including cognitive, cardiovascular, sleep/fatigue, emotional, perceptual problems, gastrointestinal, and urinary and sexual dysfunction. These integrated models were much better than the corresponding single olfactory models (Figure 1; Table 3). Similarly, with a combination of other non-motor features mentioned above, the TDI score (AUC = 0.918) and the identification test score (AUC = 0.916) were slightly better than threshold score (AUC = 0.890) and discrimination score (AUC = 0.886) at differentiating PD patients from controls. Identification and TDI scores have no significant difference of discriminative power (difference between areas = 0.002, z statistic = 0.257, p = 0.797).
Olfaction in PD Patients
Of the 500 included PD patients, 343 (68.6%) patients had hyposmia. The median TDI scores for the hyposmia and normosmia groups were 16.50 and 24.75 points, respectively. Median threshold, median discrimination, and median identification scores of the normosmia group were 7.25, 9, and 9. By contrast, those of the hyposmia group were 3.50, 6.5, and 6 (Table S1).
Compared with the normosmia group, we observed that patients in the hyposmia group were significantly more often men, with fewer educational years, more severe rigidity symptom, and more severe cognitive problems (p < 0.05).
Finally, stepwise multiple linear regression analysis removed confounding factors whose p ≥ 0.05. By multiple regression analysis, the final models interpreted 16.0% of variance in TDI score (p < 0.001, R2 = 0.160, Table S2a), 7.5% of the variance in threshold score (p < 0.001, R2 = 0.075, Table S2b), 15.2% of the variance in discrimination score (p < 0.001, R2 = 0.152, Table S2c), 11.1% of the variance in identification score (p < 0.001, R2 = 0.111, Table S2d). The variance inflation factor (VIF) showed no evidence of a multicollinearity problem among the independent variables. Older age, lower MMSE scores, and male sex were significantly associated with lower TDI scores. Older age and lower MMSE scores were associated with lower threshold scores. Older age, lower MMSE scores, and male sex were significantly associated with lower discrimination scores. Lower MMSE, older age, higher UPDRS II score, and male sex were associated with lower identification scores.
Discussion
In our study, first of all, we confirmed olfactory deficit in PD, including the impairment of olfactory threshold, discrimination, and identification ability. Meanwhile, the olfactory identification test distinguished best between PD patients and control subjects among three olfactory tests in the single or integrated models.
Previous studies have compared different olfactory domains in discriminating patients with PD and control subjects, as well as other neurodegenerative diseases. For instance, Berendse et al. (35) supported that odor identification was better in differentiating patients with PD from control subjects than the odor discrimination task. Then, the same group (14) supported that a combination of an olfactory threshold test and a 16-item olfactory identification test scored the best in sensitivity and specificity in discriminating between PD patients and controls. A meta-analysis (36) once concluded that the olfactory threshold test should be included in the test for subclinical patients with PD. Hummel et al. (37) reported that PD patients performed relatively well in the olfactory threshold task, while they perform poorly in olfactory discrimination and identification compared to other diseases, such as sinonasal disease, postinfectious and posttraumatic status, and so on.
In the background of these published studies, our current study had resembled but more detailed implications. In our study, a combination of olfactory identification and discrimination tests could not improve the diagnostic accuracy of a single olfactory identification test, which was partly in accordance with other studies (14, 35). However, combining three olfactory tests did slightly improve the diagnostic value, which still supported that olfactory deficit was based on the dysfunction of multiple olfactory domains (38).
However, besides PD, the olfactory deficit was also the feature of other causes (39). Therefore, we usually combine other non-motor manifestations to distinguish between PD patients and controls. In our integrated model of differentiation, no matter which single or combined odor tests were chosen to represent for olfactory function, olfactory dysfunction was always included in the model. In summary, we may believe that the olfactory test was an essential part of the PD clinical studies, especially in a large scale of screening of PD or in PD modeling establishment. It was consistent with the study of Antje et al. (40) that the combination of olfactory tests and other tests may constitute a screening tool for PD.
When the entire three olfactory tasks represented olfactory function, its corresponding integrated model had the highest AUC based on the corresponding ROC curve, whereas the AUC of an integrated model constructed by odor identification was not significantly lower. Therefore, in large scales of studies containing an olfactory evaluation of patients with PD, we may believe that the olfactory identification test was sufficient enough to represent olfactory function in an integrated model to differentiated PD patients from controls subjects. After all, the entire olfactory test was more time and energy consuming. In large-scale studies, it can save researchers' and patients' time and energy to accomplish other necessary non-motor manifestations in the integrated model.
According to the standard of olfactory dysfunction (34), patients with olfactory deficit were more often men, had fewer educational years, and presented more severe cognitive problems. Liu et al. (41) also supported that male patients had significantly more deficits in olfaction than female patients. It may also suggest that not only the age of subjects but also gender and educational years should be considered into the future standard of olfactory deficits.
Moreover, in the olfactory threshold and discrimination domains, other clinical manifestations did not remain in their corresponding regression models. It indicated that olfactory threshold and discrimination domains were independent features of PD, just like tremors (42), which were not clearly related to other PD manifestations. We found a lack of relationship between dyskinesia and olfactory function in PD, which was consistent with the conclusion of Stephenson et al. (43) that there was no significant effect of olfactory performance on the risk of motor complications, such as falls and dyskinesia. This result indicated that olfactory threshold and discrimination deficit developed and progressed before the development of motor symptoms and maintained throughout the process of the disease (44). However, in the olfactory identification domain, the UPDRS II score was included in its regression model except for age, gender, and MMSE score, which partly resembled the observations in other studies that disease stage explained part of the variance in olfactory discrimination score of PD patients (13, 35). The lesser UPDRS II scores were associated with higher identification scores, which indicated that the odor identification task is associated with the activities of daily living. As previous studies pointed out, the performance of daily activities can be limited and conditioned by non-motor symptoms (45). An alternative explanation for the association between the odor identification and daily activities, but not UPDRS III, is methodological, since the former section is mainly based on a patient/caregiver self-completed questionnaire, whereas UPDRS III is based on professional rating (46). Therefore, it deserves further replication in larger cohorts in the future.
In conclusion, our study showed that the odor identification domain can basically represent olfactory functions in discriminating PD patients from controls, suggesting a specific aspect of one symptom may be an adequate representation of this certain symptom, which was energy and time saving especially in data collection of a large cohort study. Our data also indicated that the combination of different kinds of symptoms would be better in discriminating PD than a single symptom. Furthermore, the olfactory threshold and discrimination domains were independent features of PD, while worse daily living ability was associated with lower olfactory identification scores.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Xiangya Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
YZha, YH, JG, BT, and QS designed the experiments. YZha, YH, RH, YZho, XiZ, XuZ, LZ, ZL, QX, JT, and XY collected clinical data and performed phenotype analyses. YZha, YH, and HP analyzed the data and reference management. YZha, YH, and JG wrote and revised the manuscript. All authors contributed to read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all the PD patients and healthy controls for participating in the study.
Footnotes
Funding. This study was supported by the National Key Research and Development Program of China (Grant No. 2017YFC0909100; No. 2016YFC1306000) to JG and BT, the Central Public-Interest Scientific Institution Basal Research Fund of Chinese Academy of Medical Sciences (Grant No. 2018-12M-HL-025) to JG, the National Natural Science Foundation of China (Grant No. 81873785) to JG, Science and Technology Major Project of Hunan Provincial Science and Technology Department (2018SK1030) to JG, the innovative team program from Department of Science & Technology of Hunan Province (No. 2019RS1010) to JG, and the Innovation-Driven Team Project (No. 2020CX016) from Central South University.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00420/full#supplementary-material
References
- 1.Masala C, Solla P, Liscia A, Defazio G, Saba L, Cannas A, et al. Correlation among olfactory function, motors' symptoms, cognitive impairment, apathy, and fatigue in patients with Parkinson's disease. J Neurol. (2018) 265:1764–71. 10.1007/s00415-018-8913-9 [DOI] [PubMed] [Google Scholar]
- 2.Cecchini MP, Federico A, Zanini A, Mantovani E, Masala C, Tinazzi M, et al. Olfaction and taste in Parkinson's disease: the association with mild cognitive impairment and the single cognitive domain dysfunction. J Neural Transm. (2019) 126:585–95. 10.1007/s00702-019-01996-z [DOI] [PubMed] [Google Scholar]
- 3.Haehner A, Masala C, Walter S, Reichmann H, Hummel T. Incidence of Parkinson's disease in a large patient cohort with idiopathic smell and taste loss. J Neurol. (2019) 266:339–45. 10.1007/s00415-018-9135-x [DOI] [PubMed] [Google Scholar]
- 4.Solla P, Masala C, Liscia A, Piras R, Ercoli T, Fadda L, et al. Sex-related differences in olfactory function and evaluation of possible confounding factors among patients with Parkinson's disease. J Neurol. (2020) 267:57–63. 10.1007/s00415-019-09551-2 [DOI] [PubMed] [Google Scholar]
- 5.Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson's disease. Mov Disord. (2015) 30:1600–11. 10.1002/mds.26431 [DOI] [PubMed] [Google Scholar]
- 6.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. (2015) 30:1591–601. 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- 7.He R, Yan X, Guo J, Xu Q, Tang B, Sun Q. Recent advances in biomarkers for Parkinson's disease. Front Aging Neurosci. (2018) 10:305. 10.3389/fnagi.2018.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Shrestha S, Huang X, Jain S, Guo X, Tranah GJ, et al. Olfaction and incident Parkinson disease in US white and black older adults. Neurology. (2017) 89:1441–7. 10.1212/WNL.0000000000004382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dall'Antonia I, Sonka K, Dusek Olfaction P, Colour Vision. What can they tell us about Parkinson's disease? Prague Med Rep. (2018) 119:85–96. 10.14712/23362936.2018.8 [DOI] [PubMed] [Google Scholar]
- 10.Nielsen T, Jensen MB, Stenager E, Andersen AD. The use of olfactory testing when diagnosing Parkinson's disease - a systematic review. Dan Med J. (2018) 65:A5481. [PubMed] [Google Scholar]
- 11.Doty RL. Olfaction in Parkinson's disease and related disorders. Neurobiol Dis. (2012) 46:527–52. 10.1016/j.nbd.2011.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeb J, Shah M, Muhammed N, Gunasekera R, Gannon K, Findley LJ, et al. A basic smell test is as sensitive as a dopamine transporter scan: comparison of olfaction, taste DaTSCAN in the diagnosis of Parkinson's disease. QJM. (2010) 103:941–52. 10.1093/qjmed/hcq142 [DOI] [PubMed] [Google Scholar]
- 13.Tissingh G, Berendse HW, Bergmans P, DeWaard R, Drukarch B, Stoof JC, et al. Loss of olfaction in de novo and treated Parkinson's disease: possible implications for early diagnosis. Mov Disord. (2001) 16:41–6. [DOI] [PubMed] [Google Scholar]
- 14.Boesveldt S, de Muinck Keizer RJ, Knol DL, Wolters E, Berendse HW. Extended testing across, not within, tasks raises diagnostic accuracy of smell testing in Parkinson's disease. Mov Disord. (2009) 24:85–90. 10.1002/mds.22325 [DOI] [PubMed] [Google Scholar]
- 15.Fullard ME, Morley JF, Duda JE. Olfactory dysfunction as an early biomarker in Parkinson's disease. Neurosci Bull. (2017) 33:515–25. 10.1007/s12264-017-0170-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahlknecht P, Pechlaner R, Boesveldt S, Volc D, Pinter B, Reiter E, et al. Optimizing odor identification testing as quick and accurate diagnostic tool for Parkinson's disease. Mov Disord. (2016) 31:1408–13. 10.1002/mds.26637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krismer F, Pinter B, Mueller C, Mahlknecht P, Nocker M, Reiter E, et al. Sniffing the diagnosis: olfactory testing in neurodegenerative parkinsonism. Parkinsonism Relat Disord. (2017) 35:36–41. 10.1016/j.parkreldis.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 18.Siderowf A, Jennings D, Eberly S, Oakes D, Hawkins KA, Ascherio A, et al. Impaired olfaction and other prodromal features in the Parkinson at-risk syndrome study. Mov Disord. (2012) 27:406–12. 10.1002/mds.24892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verbaan D, Boesveldt S, van Rooden SM, Visser M, Marinus J, Macedo MG, et al. Is olfactory impairment in Parkinson disease related to phenotypic or genotypic characteristics? Neurology. (2008) 71:1877–82. 10.1212/01.wnl.0000336651.48596.c7 [DOI] [PubMed] [Google Scholar]
- 20.Mahlknecht P, Iranzo A, Hogl B, Frauscher B, Muller C, Santamaria J, et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology. (2015) 84:654–8. 10.1212/WNL.0000000000001265 [DOI] [PubMed] [Google Scholar]
- 21.Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. (1988) 38:1237–44. 10.1212/WNL.38.8.1237 [DOI] [PubMed] [Google Scholar]
- 22.Haehner A, Boesveldt S, Berendse HW, Mackay-Sim A, Fleischmann J, Silburn PA, et al. Prevalence of smell loss in Parkinson's disease–a multicenter study. Parkinsonism Relat Disord. (2009) 15:490–4. 10.1016/j.parkreldis.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 23.Gibb WR, Lees AJ. The relevance of the lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. (1988) 51:745–52. 10.1136/jnnp.51.6.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, et al. The metric properties of a novel non-motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov Disord. (2007) 22:1901–11. 10.1002/mds.21596 [DOI] [PubMed] [Google Scholar]
- 25.Wang G, Hong Z, Cheng Q, Xiao Q, Wang Y, Zhang J, et al. Validation of the Chinese non-motor symptoms scale for Parkinson's disease: results from a Chinese pilot study. Clin Neurol Neurosurg. (2009) 111:523–6. 10.1016/j.clineuro.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 26.Xiang YQ, Xu Q, Sun QY, Wang ZQ, Tian Y, Fang LJ, et al. Clinical features and correlates of excessive daytime sleepiness in Parkinson's disease. Front Neurol. (2019) 10:121. 10.3389/fneur.2019.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang YQ, Tang BS, Yan XX, Chen ZH, Xu Q, Liu ZH, et al. A neurophysiological profile in Parkinson's disease with mild cognitive impairment and dementia in China. J Clin Neurosci. (2015) 22:981–5. 10.1016/j.jocn.2014.11.030 [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Tang BS, Guo JF. Parkinson's disease and cognitive impairment. Parkinsons Dis. (2016) 2016:6734678 10.31193/ssap.01.9787509791011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janvin C, Aarsland D, Larsen JP, Hugdahl K. Neuropsychological profile of patients with Parkinson's disease without dementia. Dement Geriatr Cogn Disord. (2003) 15:126–31. 10.1159/000068483 [DOI] [PubMed] [Google Scholar]
- 30.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord. (2006) 21:1123–30. 10.1002/mds.20897 [DOI] [PubMed] [Google Scholar]
- 31.Zhou X, Guo J, Sun Q, Xu Q, Pan H, Yu R, et al. Factors associated with dyskinesia in Parkinson's disease in mainland China. Front Neurol. (2019) 10:477. 10.3389/fneur.2019.00477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 'Sniffin' sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. (1997) 22:39–52. 10.1093/chemse/22.1.39 [DOI] [PubMed] [Google Scholar]
- 33.Rumeau C, Nguyen DT, Jankowski R. How to assess olfactory performance with the Sniffin' Sticks test[(R)]. Eur Ann Otorhinolaryngol Head Neck Dis. (2016) 133:203–6. 10.1016/j.anorl.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 34.Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the “Sniffin' Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. (2007) 264:237–43. 10.1007/s00405-006-0173-0 [DOI] [PubMed] [Google Scholar]
- 35.Boesveldt S, Verbaan D, Knol DL, Visser M, van Rooden SM, van Hilten JJ, et al. A comparative study of odor identification and odor discrimination deficits in Parkinson's disease. Mov Disord. (2008) 23:1984–90. 10.1002/mds.22155 [DOI] [PubMed] [Google Scholar]
- 36.Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol. (1998) 55:84–90. 10.1001/archneur.55.1.84 [DOI] [PubMed] [Google Scholar]
- 37.Whitcroft KL, Cuevas M, Haehner A, Hummel T. Patterns of olfactory impairment reflect underlying disease etiology. Laryngoscope. (2016) 127:291–5. 10.1002/lary.26229 [DOI] [PubMed] [Google Scholar]
- 38.Martzke JS, Kopala LC, Good KP. Olfactory dysfunction in neuropsychiatric disorders: review and methodological considerations. Biol Psychiatry. (1997) 42:721–32. 10.1016/S0006-3223(96)00442-8 [DOI] [PubMed] [Google Scholar]
- 39.London B, Nabet B, Fisher AR, White B, Sammel MD, Doty RL. Predictors of prognosis in patients with olfactory disturbance. Ann Neurol. (2008) 63:159–66. 10.1002/ana.21293 [DOI] [PubMed] [Google Scholar]
- 40.Haehner A, Hummel T, Hummel C, Sommer U, Junghanns S, Reichmann H. Olfactory loss may be a first sign of idiopathic Parkinson's disease. Mov Disord. (2007) 22:839–42. 10.1002/mds.21413 [DOI] [PubMed] [Google Scholar]
- 41.Liu R, Umbach DM, Peddada SD, Xu Z, Troster AI, Huang X, et al. Potential sex differences in nonmotor symptoms in early drug-naive Parkinson disease. Neurology. (2015) 84:2107–15. 10.1212/WNL.0000000000001609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louis ED, Tang MX, Cote L, Alfaro B, Mejia H, Marder K. Progression of parkinsonian signs in Parkinson disease. Arch Neurol. (1999) 56:334–7. 10.1001/archneur.56.3.334 [DOI] [PubMed] [Google Scholar]
- 43.Stephenson R, Houghton D, Sundarararjan S, Doty RL, Stern M, Xie SX, et al. Odor identification deficits are associated with increased risk of neuropsychiatric complications in patients with Parkinson's disease. Mov Disord. (2010) 25:2099–104. 10.1002/mds.23234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JY, Lee WY, Chung EJ, Dhong HJ. Analysis of olfactory function and the depth of olfactory sulcus in patients with Parkinson's disease. Mov Disord. (2007) 22:1563–6. 10.1002/mds.21490 [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR, Group NV. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson's disease. Mov Disord. (2011) 26:399–406. 10.1002/mds.23462 [DOI] [PubMed] [Google Scholar]
- 46.Song J, Fisher BE, Petzinger G, Wu A, Gordon J, Salem GJ. The relationships between the unified Parkinson's disease rating scale and lower extremity functional performance in persons with early-stage Parkinson's disease. Neurorehabil Neural Repair. (2009) 23:657–61. 10.1177/1545968309332878 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.