Abstract
Interoception refers to the process by which the nervous system senses and integrates signals originating from within the body, providing a momentary mapping of the body’s internal landscape and its relationship to the outside world. Active inference is based on the premise that afferent sensory input to the brain is constantly shaped and modified by prior expectations. In this review we propose that interoceptive psychopathology results from two primary interoceptive dysfunctions: First, individuals have abnormally strong expectations of the situations that elicit bodily change (i.e., hyperprecise priors), and second, they have great difficulty adjusting these expectations when the environment changes (i.e., context rigidity). Here we discuss how these dysfunctions potentially manifest in mental illness and how interventions aimed at altering interoceptive processing can help the brain create a more realistic model of its internal state.
Keywords: anxiety, computational psychiatry, depression, interoception, mental health, predictive coding
It’s not the strongest species that survive, nor the most intelligent, but the most responsive to change.
—Leon C. Megginson on Darwin’s Origin of Species
INTRODUCTION
Interoception, the process by which the nervous system senses, interprets, and integrates signals originating from within the body, provides organisms with a momentary mapping of the body’s internal landscape and its relationship to the outside world. This is fundamentally important to facilitate the sort of adaptive change that Darwin believed was critical for survival, and dysregulated interoceptive states are increasingly recognized as a defining feature of mental illness (Khalsa et al. 2018). Traditionally, interoception has been considered to be a one-way street in which bodily signals traveling to the brain cause sensation and elicit regulatory responses when bodily homeostasis is disrupted. More recently, interoception has been adopted into the conceptual framework of active inference (Barrett & Simmons 2015, Paulus & Stein 2010, Pezzulo et al. 2015, Stephan et al. 2016), which is based on the premise that afferent sensory input to the brain is constantly shaped and modified by the individual’s expectations. Thus, interoception can be conceptualized as a bidirectional process between the brain and the body, with feedback and feedforward loops, and as a rich internal model aimed at predicting future states of the body.
Despite these theoretical advances and the evidence showing that the brain and the body are inseparable, most explanatory approaches in psychology and neuroscience attempting to understand cognitive, emotional, and behavioral functioning have not integrated these two dimensions. In this review, we aim to (a) provide a brief overview of different conceptualizations of interoception; (b) offer an intuitive understanding of the active inference framework; (c) develop two main hypotheses about how interoceptive dysfunctions contribute to psychopathology in disorders characterized by anxiety and depression; (d) relay updates of recent empirical findings focused on interoceptive processing; and (e) apply the active inference framework of interoception to psychologically based interventions. The central idea proposed in this review is that interoceptive psychopathology arises from altered active inference due to disorder-specific expectations, which are the result of hierarchically based feedback and feedforward loops that are reinforced by mental rehearsal. These expectations alter interoceptive awareness, which in turn changes how the internal and external environment affect the selection of adaptive behaviors. We hypothesize that individuals with depression and anxiety exhibit two interoceptive dysfunctions. First, these individuals have overly strong expectations, or hyperprecise priors, which shape the perception of the interoceptive afferences. Second, these individuals have difficulty adjusting these expectations when the internal or external environment changes; that is, they show context rigidity. Moreover, faulty prediction error signaling contributes to this context rigidity. Within an active inference framework, these hypotheses lead to experimentally testable predictions using computational approaches, such as the hierarchical Gaussian filter (Mathys et al. 2011), which can be used to help explain how certain psychological interventions aim to correct these dysfunctional interoceptive processes.
CLASSIFICATION MODELS AND INTEROCEPTION
Classification models based on syndromes include interoceptive symptoms prominently for anxiety, mood, eating, addictive, and somatic symptom disorders (Am. Psychiatr. Assoc. 2013). However, despite this reliance on interoceptive phenomenology, the concept of interoception is completely missing in diagnostic nosology, and this has hindered progress in understanding the clinical impact of interoception on mental health. For example, little progress has been made to identify interoceptive biomarkers to improve the diagnosis, prognosis, or treatment of mental health disorders (Khalsa et al. 2018), which may be due to a limited conceptual understanding of interoception and a lack of good methodological tools that can be broadly disseminated. The reliance of classification systems upon valid and reliable terminology, and the relative paucity of such information with respect to interoception, may be another limiting factor (see the sidebar titled Terminology and Nomenclature). Interoception is also not clearly represented in dimensional frameworks for biologically phenotyping mental illness, such as the Research Domain Criteria (RDoC) initiative (Kozak & Cuthbert 2016), even though interoception provides a direct bridge between biological and psychological processes in numerous mental disorders. For example, interoception intersects with the negative valence systems, arousal/regulatory systems, and cognitive systems, and it might have been initially overlooked as a domain because of this overlap. Thus, interoception has not been given the appropriate attention for its role in various diagnostic categories or in dimensional psychopathology.
TERMINOLOGY AND NOMENCLATURE.
There is an emerging consensus about the nomenclature of different components of interoception (Khalsa et al. 2018). According to this view, interoception is a multidimensional process with several features spanning conscious and unconscious states. Interoceptive sensing occurs across spectrums of high/low arousal and negative/positive valence. It may be painful or nonpainful. It usually occurs outside of conscious awareness (e.g., blood glycemic index), and it is often but not necessarily consciously experienced during instances of homeostatic perturbation. Interoception can be contrasted with interoceptive awareness, which is the act of consciously sensing, interpreting, and integrating information about the state of inner body systems. Interoceptive awareness can be parsed into different elements and examined across organ systems (Garfinkel et al. 2016). Interoceptive attention refers to the process of observing internal bodily sensation; it can be goal directed in a top-down manner (Schulz 2016) or can be driven by bodily signals in a bottom-up manner (Hassanpour et al. 2018). Interoceptive detection refers to the presence or absence of conscious reportable sensation. Interoceptive discrimination is the process of localizing a sensation to a particular organ or physiological system and differentiating it from other sensations. Interoceptive magnitude is the perceived intensity of the sensation, akin to the loudness of a speaker volume. Interoceptive accuracy is the process of correctly and precisely monitoring the sensation as assessed by comparisons between subjective and objective indices. Interoceptive insight is a metacognitive evaluation of experience usually measured with respect to a specific task (e.g., confidence-accuracy correspondence). Interoceptive sensibility is a self-perceived tendency to focus on interoceptive signals, representing a trait-like feature. Finally, interoceptive self-report scales refer to the process of conducting psychometric assessments with a questionnaire. There are numerous measures that cover state and trait features (see Khalsa et al. 2018), reflecting a multitude of psychological constructs that await further classification. Homeostasis and allostasis are important constructs in the context of interoception. Homeostasis is a dynamic process by which a state of balance is maintained according to a predetermined set point. The body is constantly engaged in maintaining a multitude of homeostatic interoceptive set points, the majority of which never reach conscious awareness. The active processes responsible for maintaining this balance are not readily apparent, giving rise to a misleading sense of quiescence under resting physiological conditions. Allostasis is a proactive process aimed at minimizing energy costs by adaptively anticipating future needs of the body. An allostatic load occurs when there is a persistent deviation that continuously engages this adaptive process. It can sometimes lead to allostatic stress, whereby the body’s recovery mechanisms fail to adequately compensate for the increased burden, leading to a new and potentially harmful set point (McEwen & Wingfield 2003).
A COMPUTATIONAL FRAMEWORK FOR INTEROCEPTION
One way that mental disorders can be broadly characterized is by a dysfunction in the way the brain computes and integrates representations of the inner and outer worlds of the body across time. According to this view, changes in mood and anxiety are a by-product of the brain’s biased translation of what it expects will happen versus what is actually happening in these worlds, producing a persistent discrepancy or error signal when outcomes are observed. Whereas adaptive functioning relies on error signals to adjust expectations, perception, and subsequently actions, dysfunctional processing is characterized by a persistent presence of error signals due to inadequate adjustment. In the case of interoceptive dysfunction, persistent prediction errors reflect dynamic breakdowns in the brain’s tightrope act of simultaneously mapping objects in the world external to the body, mapping objects in the world internal to the body, integrating these maps with neural representations of the predicted state of the world, and subsequently modifying these maps over time to maintain an updated representation of the self.
Recent developments in computational neuroscience have provided a conceptual framework and quantitative approaches to better understand and measure interoception. These approaches have helped to reexamine the traditional view that visceral sensations arise solely from perception of the body’s internal milieu via ascending afferent pathways. Based on the notion of active inference (Friston et al. 2009), these conceptual models suggest that interoceptive perceptions are dynamically constructed by the brain, meaning that interoception is the result of an iterative process of comparing the brain’s expectation of sensation with the incoming sensation (Barrett & Simmons 2015, Paulus & Stein 2010, Pezzulo et al. 2015, Stephan et al. 2016). Before delving further into these conceptual models, it is important to emphasize that they represent current speculations on how the brain and the body interactively produce adaptive responses to environmental change, and they await validation from empirical testing. It is our view that certain mental disorders are characterized by an inflexible internal model in the context of a dynamically changing external world.
Active inference starts with the premise that the perceptual process is an interaction between the brain’s model of what is to be expected and its comparison to the actual sensory evidence. The goal of this process is to generate the most accurate model of the world to help guide the most adaptive behavior, despite the inherent uncertainties of nature. Perception is not what we sense but a computational compromise between our expectation of what we believe we should be sensing and the actual sensation experienced. Thus, perception emerges from processing the external or internal world within the context of a prior model. At any point in time an individual has a number of models in mind, and for each of these models the individual holds a belief as to how likely this model is to be correct, that is, to be a true representation of the world. Interoceptive afferences provide the brain with sensory evidence for what is happening inside the body and are evaluated relative to a given expectation (model). Perception changes future expectations, meaning that as a consequence of processing these afferences, the individual’s beliefs about the probabilities of the different models will change. The manner in which these changes occur is best described in probabilistic terms according to Bayes’ rule:
An important aspect of the Bayes’ rule is that model and evidence contribute to the perception as a function of their precision, which can be defined as the certainty with which a model is believed to be true and the certainty of a particular afferent given an expectation. For example, a person at home experiencing a tap on their shoulder by their partner might have a strongly positive perception, whereas the same experience in a dark alley might be perceived as frightening. However, a tap on their shoulder in a crowded space when waiting for their partner might elicit a much more complex perception based on the certainty with which the person believes that their partner should be present. Thus, the perceptual process updates the expectations for future perceptions. In that sense, perception is the iterative process of updating models with evidence from the inside or outside world. If the sum of the evidence provides information that is different from the model, the brain generates a prediction error. For example, a tap on the shoulder by a burglar at home would generate a strong prediction error. In the context of interoceptive afferences, we have previously called this a somatic error (Khalsa & Feinstein 2018) or a body prediction error (Paulus & Stein 2006) that serves as a signal to the brain to adjust the underlying model. As stated above, it is important to recognize that the degree of adjustment is related to the precision of the model and the precision of the evidence. Specifically, if the model is highly precise (i.e., the individual holds a strong expectation about the state of the inside or outside world and does not believe that there are alternative models of the world that can properly account for the afferent signal), then even in the presence of somatic error the model will be adjusted only slightly. In comparison, if the evidence is not very precise (i.e., it is not clear what the afferences are signaling), the model will not be strongly adjusted even if it differs significantly from the evidence. Two proposed interoceptive processing dysfunctions are depicted in Figures 1 and 2, which provide a step-by-step walkthrough of the active inference process.
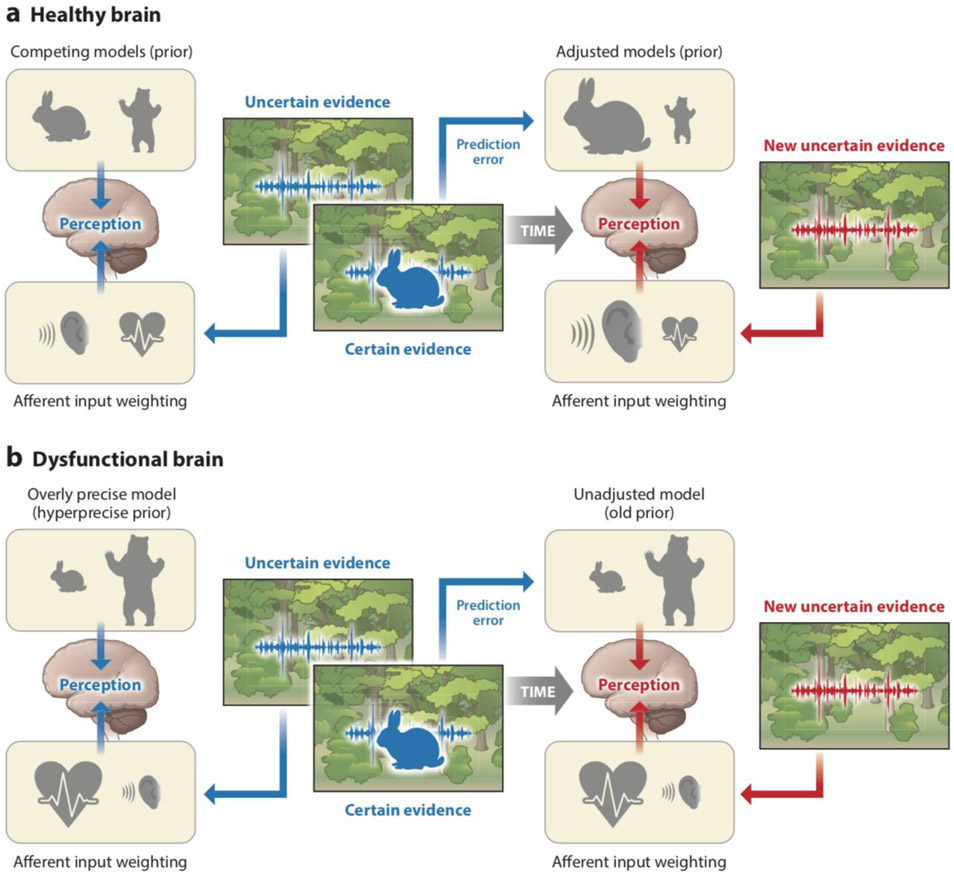
Figure 1.
Active inference interoceptive dysfunction 1: hyperprecise priors. This image depicts priors symbolically; the size of the individual symbol is proportional to the degree to which the individual believes this expectation. For example, say a person is walking in the Rocky Mountain forest and is listening for animals known to inhabit the area. Suddenly, they hear a sound (afferent evidence) that is paired with an interoceptive prediction (heartbeat) that is proportional to the expected percept. Soon after, they see the source of the sound, in this case a rabbit. In a typical case (right), if the person expects to encounter a rabbit and a bear with equal frequency (i.e., these competing models have modest precision), then hearing a sound will trigger competing models (bear or rabbit) and elicit preparatory interoceptive responses (e.g., heightened sympathetic tone). If a rabbit subsequently appears, a prediction error ensues that should adjust (increase) the prior expectation of seeing a rabbit when the same sound is heard again (new afferent evidence). The perception of the animal thus creates a prediction error in the presence of the prior expectation and modifies these expectations for the next encounter. In this case, the same sound presented after seeing a rabbit no longer elicits a prediction error. On the other hand (left), if the person is highly convinced to begin with that a bear will come charging out of the woods (i.e., the model is very precise), then even if they hear a rabbit, the expectation of a bear charging out of the woods will continue with little adjustments. Importantly, a prediction error persists over time because the model is not brought into congruence with the evidence.
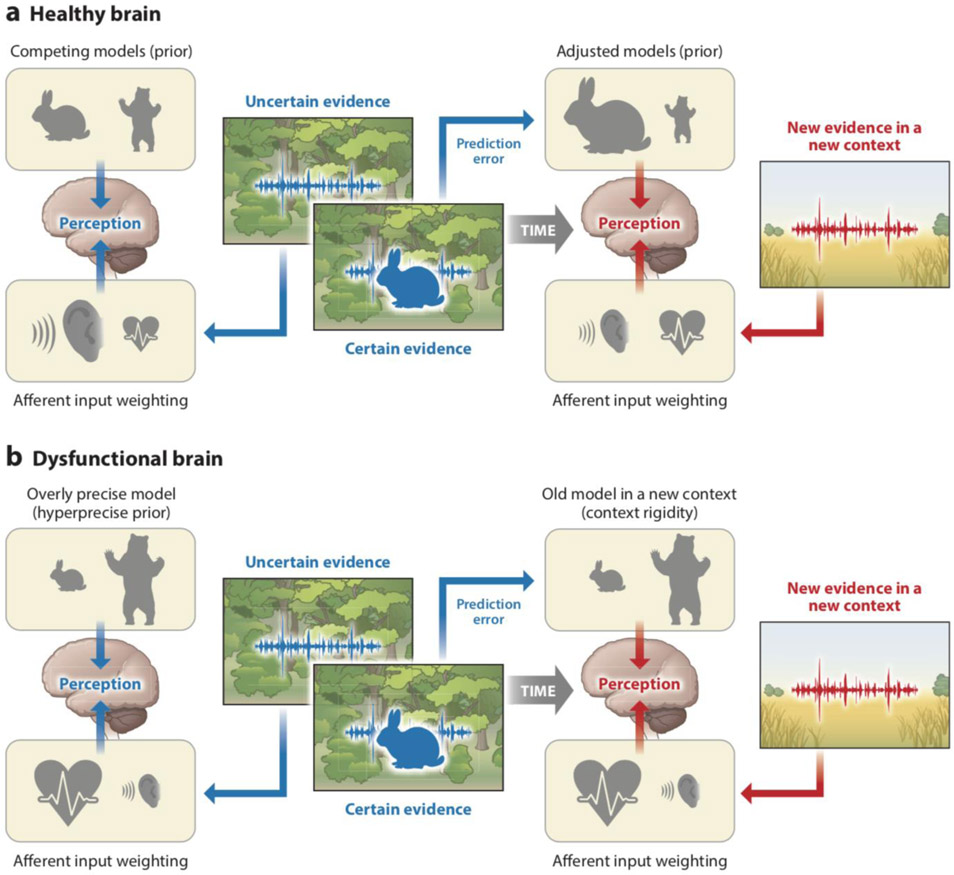
Figure 2.
Active inference interoceptive dysfunction 2: context rigidity. A second form of dysfunction occurs when the individual maintains the same model in a new context. For example, upon emerging from the Rocky Mountain forest into the prairie grasslands of Oklahoma (devoid of bears but with an abundance of rabbits), an individual may still associate the sound of an approaching rabbit with the perception of a charging bear. Whereas an adaptive person initially establishes a new and context-specific model (there may be bears or rabbits in this new environment; right), the nonadaptive person continues to maintain the previously held belief of a high prevalence of bears despite being in a different environment (left).
It has been hypothesized that the central nervous system implements active inference perceptual processing hierarchically and bidirectionally (Friston 2008). This means that models exist at different levels, and models at a lower level of the hierarchy serve as evidence for models at a higher level. In return, higher-level models modify the expectations of the lower-level ones. For example, an accelerated heartbeat may serve to increase the expectation of arousal, which in turn could provide evidence for fear or excitement, depending on the context. Each of these models is thought to consume energy, a finite resource, leading to competition within the organism for an optimal trade-off between model complexity, accuracy, and fitness—that is, a weighing of the model’s accuracy in representing the world against the energy spent to entertain it, the free energy principle proposed by Friston, aims to provide the computational explanation for how the brain optimizes (selects) perceptions in the presence of multiple expectations and models (Friston et al. 2013). Via the active inference process, the brain ultimately settles on a perception that results in the least divergence between the evidence coming from the inside or outside world and the expected model.
A consequence of active inference models is that certain psychiatric disorders, especially those characterized by chronic and unrelenting anxiety, are preferentially susceptible to top-down constructed dysfunctions; that is, they are the consequences of a persistent mismatch between predicted body states and afferent signals from the body. It has been proposed that sustained and exaggerated mismatches dysregulate the ability to sense accurately what is happening in the body, resulting in a turbulent reference state (i.e., a noisy baseline) (Paulus & Stein 2006), attentional bias toward threat (Hakamata et al. 2010), increased worry and self-related cognitions, dysfunctional learning about bodily states over time (Van den Bergh et al. 2017), and increased allostatic load leading to increased stress and mental illness (Peters et al. 2017, Sterling 2014).
In an adaptive individual, corrective action in the presence of somatic error can be achieved by adjusting expectations (priors) to match the current physiological state or by engaging in regulatory actions that change the afferent signal, leading the current physiological state to conform more closely with expectations. In either case, successful corrective action reduces somatic error, resulting in homeostatic balance within the nervous system. However, this adaptive adjustment can break down in a number of ways. First, hyperprecise priors (i.e., very strong beliefs that a certain model is correct) may be reinforced by prolonged periods of worry and rumination (i.e., mental rehearsals). Consequently, the priors may not get appropriately updated by the incoming sensory evidence. This can create persistent somatic errors or a noisy baseline state such that bodily perceptions fail to conform with the evidence (Paulus & Stein 2006).
In a nonadaptive person, persistent somatic errors are hypothesized to be important for generating feeling states aimed at motivating actions towards homeostasis. For example, a persistently hyperprecise prior of expecting a bear instead of a rabbit (Figure 1) may lead the individual to experience anxiety that motivates withdrawal or avoidance—that is, the person might withdraw from the environment associated with these overly strong beliefs. Second, context rigidity (Figure 2), or the lack of the ability to adjust expectation as a function of context, may contribute to the persistent experience of somatic error because the individual does not adjust prior beliefs about different models in a new environment, a hypothesis that can be tested with a hierarchical Gaussian filter approach using separate parameters for precision, prediction errors, and mean expectations on different temporal hierarchies (Mathys et al. 2011).
Most of the processing and regulation of somatic errors occurs beneath the surface of conscious awareness. However, if the somatic error magnitude becomes large enough, it can directly lead to subjective feeling states marked by prominent (often aversive) interoceptive symptoms, as well as an obligatory motivation to act to reduce the associated discomfort and distress. Prolongation of somatic error for an unsustainable period of time will eventually force the system into attempting long-term allostatic adjustments (see sidebar titled Terminology and Nomenclature). In the adaptive individual, successful corrective actions resolve somatic error, resulting in homeostasis. In the nonadaptive individual, allostatic adjustments prevail, yet they often fail to quell somatic error and instead promote states of chronic and uncontrollable anxiety, leading to helplessness and depression. In severe instances, the somatic error becomes so pervasive that the only corrective action that seems to quell the error is complete avoidance of all perceived triggers (e.g., agoraphobia).
This interoceptively focused model of psychopathology is predicated on the assumption that a primary function of the nervous system is to develop accurate models of the world in an effort to establish conditions ideal for optimizing bodily functioning. Corrective action failure results in homeostatic dysregulation, which can manifest as sudden changes in symptom intensity, mislabeling of symptoms, a predominant focus on bodily signals in daily life, extreme hypervigilance toward disturbed body states, aberrant self-related thinking patterns, and attempts to reduce these aversive experiences via avoidance or escape behaviors. We propose that in the case of certain disorders, a systematic failure to correct somatic errors leads individuals to make choices that fail to establish ideal conditions for optimizing internal bodily functioning and survival; that is, repeated attempts to reduce somatic error conflict lead to suboptimal functioning that is energetically costly to regulate, resulting in maladaptive behaviors and cognitions and often leading to further discrepancies between predicted and observed body states. These discrepancies propagate and/or amplify the presence of somatic errors, leading to a positive feedback loop that often manifests as anxious psychopathology (Khalsa & Feinstein 2018).
MEASURING INTEROCEPTION
To date, most studies assessing interoceptive awareness (see sidebar titled Terminology and Nomenclature) have focused on interoceptive accuracy within the cardiac system using measurements of heartbeat attention, heartbeat tapping, heartbeat counting, heartbeat detection, and heartbeat perturbation (Khalsa & Lapidus 2016). Heartbeat counting tasks have been the most popular likely due to measurement convenience, given that they require only a cardiac monitor, and ease of analysis, given that they use a simple formula that yields a single accuracy score. However, a number of problems with this task cast doubt on its construct validity; for example, prior knowledge of one’s resting heart rate, IQ scores, and time estimation ability substantially influence accuracy (Murphy et al. 2018). Counting accuracy correlates poorly with measured heart rates (Zamariola et al. 2018), is actually insensitive to heart rate increases (Windmann et al. 1999), and is substantially influenced by cognitive strategies (Desmedt et al. 2018). Moreover, counting accuracy shows limited test-retest reliability [intraclass correlation coefficient (ICC) = 0.41] (Wittkamp et al. 2018) and does not correlate with more rigorous measures of cardiac interoception (Ring & Brener 2018). One way that the active inference framework can reconcile these limitations with studies showing that heartbeat counting accuracy is related to clinical symptoms is that accuracy scores predominantly reflect altered prior beliefs about the heartbeat (i.e., hyperprecise priors) rather than being an objective index of sensation.
An important caveat for the use of heartbeat perception tasks as an objective measure of interoception is that only a third of individuals demonstrate accurate heartbeat detection at rest (Khalsa & Lapidus 2016), suggesting that under baseline conditions, most individuals cannot actually sense their heartbeat regardless of their self-report. Because clinically and/or emotionally significant events are arousing and frequently associated with homeostatic deviations, restricting assessment to resting conditions fails to capture an important generator of symptom variability. In comparison, the method of dynamically perturbing interoceptive systems has several advantages: (a) Perturbations may unmask clinically relevant phenomena that are not present during baseline states; (b) based on the amount of perturbation, these approaches avoid task-related floor effects, which are especially problematic for cardiovascular interoception; (c) perturbation approaches can be used in combination with manipulation of environmental and contextual expectancies, such as perceived controllability; and (d) perturbations provide a naturalistic assessment of interoceptive systems by triggering homeostatic responses sculpted by evolution. Homeostatic perturbations are feasible via noninvasive methods (e.g., Trier social stress test), although to adequately disentangle the effects of expectation from sensation it is preferable to utilize tasks in which the predictability and controllability of sensory stimulation vary (see Khalsa et al. 2018).
One experimental approach to explicitly measure interoception within an active inference framework is to set up experiments that provide an inherent ambiguity between knowable and fundamentally uncertain information. For example, in a recent exteroceptive experiment using a conditioning procedure, Powers and colleagues (2017) differentiated individuals who were hearing voices but were not psychotic from individuals who showed both hallucinatory and psychotic traits. In general, those individuals who were hallucinators were also more likely to hear voices when no voices were present and were more confident about hearing a voice. A hierarchical active inference model revealed that individuals with hallucinations had a stronger prior belief in experiencing hallucinations. Moreover, individuals who were psychotic at baseline were less likely to change their belief about how conditions in the experiment were changing. Taken together, these data show that whereas hallucinating individuals express greater prior belief in having a particular percept, psychotic individuals have a higher-order hierarchical belief that the context in which one might expect to hear a voice does not change. This landmark study provides an experimental and heuristic basis for future investigations of interoceptive dysfunctions among individuals with psychiatric disorders. For example, one could examine the precision of interoceptive afferences by pairing them with specific exteroceptive stimuli. This approach would enable one to determine whether there are fundamental group differences at a lower level of perceptual hierarchy. However, one could also examine higher-order changes—for example, by changing the experimental condition and the association of external stimuli with an interoceptive afferent—to test how stable these belief systems are. Applying this experimental approach to interoceptive psychopathology could directly test the hyperprecise prior hypothesis (according to which individuals have strong beliefs about what sensation to expect) in individuals with mood and anxiety disorders. In addition, one could also use this approach to test the context rigidity hypothesis, according to which, even if the context changes, individuals with mood and anxiety disorders fail to appropriately adjust their expectations.
NEURAL BASIS OF INTEROCEPTION
The brain sits at the interface between two worlds: the external world, which it samples through the exteroceptive senses, and the inner world of the body, which it accesses through interoceptive sensory channels. Body-sensing brain regions include those that play primary roles in directly mapping the autonomic, chemosensory, endocrine, and immune systems and which relay information from the lamina I spinothalamic system and the vagus and glossopharyngeal cranial nerves through the brainstem [e.g., nucleus tractus solitarius (NTS)], hypothalamus, and thalamus (e.g., ventromedial posterior thalamic nucleus) and into cortical sectors, including principally the insula and somatosensory cortices (Craig 2002, Critchley et al. 2004, Damasio 1999, Feinstein et al. 2013, Khalsa et al. 2009). The processing of information across these channels occurs in a hierarchical fashion, with multiple feedback loops starting in early levels of the brainstem.
Each waking moment the brain is in a state of flux, attempting to match the demands of the outside world with the world within. Prediction signals reflect one way in which the brain anticipates the influence of these sensory signals on the body. Body prediction generators include visceromotor brain regions such as the anterior insula, anterior cingulate, posterior ventromedial prefrontal cortices (vmPFC), and amygdala, the majority of which are evolutionarily older than neocortex and have a simple cellular structure lacking a granule cell layer; those generators also include newer neocortical regions with granule cell layers including supplementary motor areas and premotor cortex implicated in other forms of autonomic control (Barrett & Simmons 2015). Collectively, these gatekeepers help to merge the internal and external worlds so that the next time they collide, the body will be better prepared to cope with the challenges posed by the environment.
A somatic error is produced within the brain whenever the predicted body state differs from the actual body signals being continuously delivered and mapped in viscerosensory regions of the brainstem, diencephalon, insular, and somatosensory cortices. Such error is fundamentally a summative reflection of the discrepancies in predicted and observed body states as information flows from lower to higher levels within the nervous system and back (see Smith et al. 2017 for a detailed example of this pertaining to the autonomic nervous system and vagal control). Ultimately, the calculation of somatic errors occurs in brainstem nuclei such as the NTS and in cortical structures such as the dorsal mid-insula, both of which are uniquely situated, anatomically, to (a) compare incoming interoceptive information with the sensory predictions submitted by nearby structures such as the parabrachial nucleus and anterior insula, and (b) transmit error signals to other closely connected visceromotor structures, such as the periaqueductal gray and dorsal anterior cingulate, so that corrective action can be initiated and the error signal can be regulated. We conjecture that discrete hubs within these cortical and subcortical networks underlie the prediction, detection, and regulation of somatic errors. Important hubs for the generation of prediction signals include the vmPFC (Barrett & Simmons 2015), anterior insula (Simmons et al. 2006), amygdala/extended amygdala (Davis et al. 2010), and parabrachial nucleus (Herbert et al. 1990), particularly in the context of anxious anticipation and subjective uncertainty and ambiguity. In contrast, important hubs for the detection of somatic errors include NTS, one of the primary recipients of interoceptive signals from the cranial nerves and spinal cord, and the dorsal mid/posterior insula, which is the first cortical recipient of these afferent interoceptive signals (Craig 2002) and a region highly responsive to bottom-up physiological changes in the viscera (Gianaros et al. 2017). Finally, the periaqueductal gray (Roy et al. 2014), amygdala (Feinstein et al. 2013), and dorsal anterior cingulate cortex (ACC) (Shackman et al. 2011) are important hubs for the regulation of somatic errors and the inhibition of pain and panic. Differences in the laminar organization may explain how signal transmission flows between hubs, and more specifically why granular regions of the insula detect somatic errors (Barrett & Simmons 2015); moreover, agranular regions such as the anterior insula and ACC have outsized roles in the prediction and regulation of somatic errors. Based on this reasoning, it is plausible that sustained somatic error dysregulation may result in long-term brain alterations localized to the regions involved in the prediction, detection, and regulation of somatic errors. Supportive evidence comes from functional abnormalities seen in the amygdala, insula, and ACC during emotion processing across multiple types of anxiety disorders (Etkin & Wager 2007) as well as structural abnormalities localized to the insula and ACC across all mental disorders (Goodkind et al. 2015).
Considerable evidence suggests that the insula plays a vital role in anxiety (Paulus & Stein 2006), and the anterior insula appears to be particularly involved in making body predictions and gauging uncertainty (Paulus et al. 2003). However, we do not subscribe to the more extreme view that holds that the insula is necessary for interoceptive awareness (Craig 2002), self-awareness (Craig 2009), emotional awareness (Gu et al. 2013), and pain (Segerdahl et al. 2015). Although the insula is important for all of these functions, it is necessary for none, both because the hierarchical organization of the nervous system provides the basis for bodily consciousness within lower-order viscerosensory mappings in the brainstem, midbrain, and thalamus, and because we have evidence of rare human lesion patients who lack the insular cortex bilaterally but do not lack these basic features of consciousness (Feinstein et al. 2016b, Khalsa et al. 2009). Likewise, we do not view the role of the amygdala as being exclusive to fear, because we have also worked with patients with bilateral amygdala lesions who exhibit fascinating fear dissociations. On the one hand, they exhibit blunted fear in response to exteroceptive threats in the environment (Feinstein et al. 2016a); on the other hand, they show heightened fear, and even panic, in response to interoceptive threats such as inhaling high doses of carbon dioxide (CO2) (Feinstein et al. 2013) or receiving bolus infusions of isoproterenol (a synthetic form of adrenaline) (Khalsa et al. 2016). Clearly the neural circuitry for generating interoceptive states is more complex and nuanced than any single brain region, and more research needs to focus on deciphering the initial interoceptive maps contained within the brainstem (Chang et al. 2015, Davenport & Vovk 2009).
INTEROCEPTION AND MENTAL HEALTH
Recent Developments
Given the strong emphasis on the relationship between self awareness and interoception proposed by Craig (2009), it is not surprising that a number of investigators have aimed to experimentally elucidate the role of these constructs. Some have argued that interoceptive awareness acts to stabilize the mental representation of one’s self as distinct from others (Palmer & Tsakiris 2018). Moreover, an exteroceptively driven sense of body ownership interacts with interoceptive body signals to create a unique sense of embodiment and bodily self (Tsakiris 2017). Consistent with the integrative role of interoception and self is the finding that those with greater interoceptive accuracy also report a narrower peripersonal space boundary (Ardizzi & Ferri 2018). Others have argued that interoception and exteroception for body signals are two different ways of perceiving the self—the first from within, the second from outside—that interact significantly (Zamariola et al. 2017), and this interaction serves as a central process to understand disorders of brain, body, and behavior (Owens et al. 2018). However, much work needs to be done to better delineate self-relevant processing and interoceptive processing and to better understand how these processes influence each other.
A number of constructs contribute substantially to interoceptive processing; among these are motivation (McMorris et al. 2018), hippocampal (Stevenson et al. 2018) and nonhippocampal learning (Pfeifer et al. 2017), and conditioning (Zaman et al. 2016). Cardiac interoception has been linked to better learning (Stevenson et al. 2018), greater retrieval of emotional faces (Pfeifer et al. 2017), and stronger expectancies for unconditioned stimuli (Zaman et al. 2016). Individuals with high interoceptive accuracy have shown higher sensitivity to negative affect but lower accuracy in the recognition of fear and sadness (Georgiou et al. 2018). Lastly, interoceptive accuracy was associated with more specific topographical changes after emotional stimuli (Jung et al. 2017). However, other researchers have reported interference patterns between exteroceptive processing and interoception. For example, visual stimuli presented synchronously to the heartbeat take longer to enter visual awareness than the same stimuli presented asynchronously to the heartbeat, and this is reflected in anterior insular activation (Salomon et al. 2018). Visually evoked potentials to affective stimuli can also be interfered with by heartbeat-evoked potentials (Marshall et al. 2018), which in turn are modulated by attention (Villena-Gonzalez et al. 2017). Finally, interoceptive signals may also confer outcomes of actions; for example, some have found an accuracy-dependent pattern of cardiac activity such that the heart accelerates less after an incorrect stimuli discrimination than after a correct one (Lukowska et al. 2018). Taken together, interoceptive processing can both facilitate and interfere with exteroceptive processing. One interpretation is that body-relevant signals aid adaptive functioning by modulating the prior expectation of certain stimuli or augmenting the evidence of a certain stimulus coming from the external world to allow the individual to better select actions.
The broad relevance of interoceptive processing for various behaviors has led investigators to more closely examine the relationship between important constructs in psychology and interoception. For example, individuals who reported greater mindfulness during psychological challenges were more likely to meet established interoceptive accuracy criteria (Kiken et al. 2018). In various time-perception experiments, accurate time perception has been linked to interoceptive accuracy (Wittmann et al. 2011). Interoceptive sensitivity has also been associated with greater altruism (Piech et al. 2017), greater feeling of knowing (Fiacconi et al. 2017), better prospective memory performance (Umeda et al. 2016), better sleep (Ewing et al. 2017), and less ruminative processing (Lackner & Fresco 2016). These findings, which should be considered with caution given the different assessment procedures used, seem to suggest that interoceptive processing ability confers some behavioral advantages. This is generally consistent with the notion proposed above that interoceptive processing aids adaptive behavior.
Clinical Examples of Interoceptive Dysfunction
A number of both mental health and physical health conditions have been associated with dysfunctional interoceptive processing. Specifically, individuals who have attempted suicide or who engaged in nonsuicidal self-injury reported greater interoceptive deficits than those with no self-injury history (Smith et al. 2018). Other researchers found that these individuals had a greater tendency to distract themselves from bodily sensations and used less self-regulation based on bodily sensations (Rogers et al. 2018). Several investigators have examined both children and adults with autism spectrum disorder and found reduced interoceptive accuracy (Palser et al. 2018) and interoceptive sensitivity (Mul et al. 2018). Similarly, several studies have been conducted with eating disorder subjects (Jenkinson et al. 2018) and reviewed sensory processing deficits in bulimia (Klabunde et al. 2017), general interoceptive appraisal dysfunctions (Brown et al. 2017), and reduced interoceptive accuracy (Kunstman et al. 2016). Interoceptive dysfunctions of various kinds have also been found in individuals with obsessive compulsive disorder (Yoris et al. 2017), borderline personality disorder (Loffler et al. 2018), and female sexual arousal disorder (Handy & Meston 2018). Several medical conditions have also been linked to interoceptive processing deficits. Specifically, individuals with hypertensive disease show poorer interoceptive performance and reduced heart-evoked potential modulations (Yoris et al. 2018). Patients with Parkinson’s disease (Santangelo et al. 2018), functional motor disorders (Ricciardi et al. 2016), and fibromyalgia (Borg et al. 2018) also exhibit lower interoceptive accuracy. Similarly, individuals with frontotemporal dementia show deficits in both interoceptive accuracy and performance (Marshall et al. 2017). These studies indicate that interoceptive dysfunction is not limited to psychiatric disorders but may be an important aspect of dysfunctional processes across medical conditions. More robust, sensitive, and quantitative assessments of these deficits may provide a truly transdiagnostic approach to assess risk, progression, and intervention response across a broad swath of medical conditions. Below we review in detail certain psychiatric conditions which feature prominently within the domain of interoceptive dysfunction.
Panic disorder.
Individuals with panic disorder demonstrate a prototypical sensitivity to interoceptive cues signaling increased bodily arousal. Surprisingly, however, most resting measures of cardiac interoceptive accuracy have failed to clearly demonstrate whether panic disorder patients perceive heartbeat sensations differently or whether they have a systematic bias toward reporting such feelings (Domschke et al. 2010). On the other hand, many studies have shown that panic disorder patients perceive interoceptive sensations more intensely when the body is stimulated by caffeine ingestion (Charney et al. 1985), CO2 inhalation (Rassovsky & Kushner 2003), or intravenous infusion with sodium lactate (Dillon et al. 1987), yohimbine (Gurguis et al. 1997), cholecystokinin (Schunck et al. 2006), or isoproterenol (Pohl et al. 1988). This discrepancy may be explained by the view that under resting homeostatic conditions, interoceptive signals do not have as much biological salience as during nonhomeostatic perturbations that can signal threats to survival—be they physical, emotional or social. Individuals with panic disorder show increased autonomic arousal, heightened anxiety, and escape behaviors during exposure to experimental paradigms involving nonpharmacological approaches (e.g., being trapped in a small dark chamber) (Richter et al. 2012) as well as pharmacological ones (e.g., oral caffeine ingestion) (Benke et al. 2015). Interoceptive dysfunction in panic disorder can be conceptualized as a problem of hyperprecise priors and a lack of corrective adjustment following the sensory evidence. Therefore, sensing heartbeats in the presence of a high expectation of having a heart attack drives such patients to emergency departments and mental health clinics under the false premise that they are dying.
Depression.
The available data seem to suggest that depression is associated with blunted cardiac interoceptive awareness. Individuals with major depressive disorder (MDD) show lower accuracy on heartbeat counting tasks (Furman et al. 2013), and depression symptoms negatively correlate with heartbeat counting accuracy (Pollatos et al. 2009). Depressed individuals also exhibit lower counting accuracy compared to patients with panic or anxiety disorders (Ehlers & Breuer 1992). However, poorer resting heartbeat interoception is not always seen in depression. In a study comparing healthy participants to moderately depressed and severely depressed patient samples, the severely depressed sample showed higher heartbeat counting scores than the moderately depressed sample (Dunn et al. 2007). Although the authors controlled for many individual differences between the moderately depressed community and the clinical population as a group, the more severely depressed patients were also higher in anxiety, which might explain differences in accuracy. Indeed, in a follow-up study by the same group, poor counting accuracy was observed in a depressed sample but was found to increase as a function of reported anxiety (Dunn et al. 2010). Homeostatic perturbations in depressed patients yield mixed observations, with some studies suggesting hyperactive sympathetic responding and others reporting attenuated responsiveness (Nemeroff & Goldschmidt-Clermont 2012). Patients with MDD reported higher levels of palpitations, restlessness, flushing, sweating, tremors, and anxiety than healthy comparisons during a sympathomimetic challenge with yohimbine (Heninger et al. 1988). On the other hand, despite similar physiological responses, patients with depression were more tolerant than panic patients to symptoms induced by CO2 inhalation (Gorman et al. 2001). Attenuated insula activation has also been observed in depression. Avery et al. (2014) found decreased activation in the mid-insula during an interoceptive attention task, with insula-limbic system dysconnectivity predicting depression severity. In a heartbeat counting study, depressed patients showed reduced activity in the anterior insula, and this signal correlated with depression severity (Wiebking et al. 2010). In our proposed model of active inference, interoceptive processing dysfunctions can be viewed as the consequence of allostatic load due to the persistence of prediction errors that arise from a mismatch between the internal model and the interoceptive afferences. However, such dysfunctions could also be due to context rigidity, that is, a failure to adjust the model in response to a different context. These two possibilities will need to be further investigated within the experimental framework proposed above.
Somatic symptoms.
Despite the exaggerated symptom reports characterizing somatic symptom disorder (SSD), interoceptive awareness seems to be quite poor in SSD. SSD patients endorse higher dyspnea based on contextual environmental cues and show poor task performance (Bogaerts et al. 2008), and individuals reporting high amounts of physical symptoms have a tendency to overestimate the intensity of inspiratory breathing loads (Petersen et al. 2015). Patients with psychosomatic disorders score lower on heartbeat counting tasks than healthy controls (Mussgay et al. 1999). Medical outpatients complaining of palpitations show low resting heartbeat detection accuracy despite their overstated experiences (Barsky et al. 1994). At the same time, these patients have poorer interoceptive distress tolerance, evidenced by greater anxiety and physical discomfort during an exercise treadmill test (Barsky et al. 1998). The high psychiatric comorbidity and low interoceptive tolerance in these patients suggest that panic and somatic symptom disorders share some overlapping phenotypic dysfunction in neural circuitry that underlies the heightened attention toward, and negative interpretation of, interoceptive signals. Therefore, these dysfunctions are best understood as the result of a persistent discrepancy between a model that generates strong expectations of an aversive interoceptive percept and evidence that is at odds with this expectation, together with an inability of the prediction error to adjust the model.
Anorexia nervosa.
If changes in observed body state can be routinely felt, it is also possible to feel changes in the predicted body state, so long as the prediction triggers a somatic error. To highlight this point, we examined interoceptive processing in anorexia nervosa (AN) by administering double-blinded infusions of either saline or isoproterenol, a peripherally acting beta-adrenergic agonist akin to adrenaline (Khalsa et al. 2015). The general notion was to perturb the observed body state systematically under different contexts, either shortly before or after a calorically dense meal. In general, AN patients reported feeling isoproterenol-induced sensations at the same rate as healthy comparison participants, suggesting preserved interoception for changes in their observed body state. However, there was one important exception. During saline infusions, which did not alter the observed body state, the patients reported significantly heightened sensations of palpitations and dyspnea, and they disproportionately localized heartbeat sensations to the chest (Khalsa et al. 2018). This presence of interoceptive sensation without visceral change suggests that AN patients were actually feeling cardiorespiratory visceral illusions. Moreover, these illusions were only present during the premeal state, a time period that is known to trigger strong feelings of fear and anxiety in patients with AN. It seems possible that such visceral illusions permeate many aspects of these patients’ lives, leading to maladaptive corrective actions that often include a profound avoidance of food. At the neural level, AN patients also show exaggerated neural responses in the absence of stimulation. For example, recovered AN patients showed hyperactivation in the insular cortex during the anticipation and resolution of restricted breathing but, surprisingly, not during the loads themselves (Berner et al. 2018). This sustained discrepancy into recovery provides preliminary evidence of the presence of visceral illusions at the neural level as well. Taken together, these findings support the hypothesis that AN patients may have context-specific (e.g., hunger state) hyperprecise priors that generate strong expectations about visceral sensations. Importantly, it may be the selection of particular actions in response to these hyperprecise priors (e.g., starvation to avoid the actual sensing of visceral sensations) that forms one core feature of the psychopathology.
Anxiety sensitivity.
Corrective actions are not always adaptive and can readily perpetuate psychopathology, as evidenced by the slippery slope of anxiety sensitivity. The trait of anxiety sensitivity refers to an individual’s fear of experiencing anxiety-related symptoms and sensations, especially those arising from within the body (e.g., heart palpitations or dyspnea); such fear is a core dimensional construct underlying the initiation and maintenance of pathological anxiety (Taylor 2014). Individuals with high levels of anxiety sensitivity often believe that these sensations can lead to adverse consequences such as death, insanity, or social rejection. Such catastrophic misinterpretations make anxiety sensitivity an anxiety amplifier; people with high levels of anxiety sensitivity are quickly alarmed by anxiety-related sensations, and this further intensifies their anxiety and reinforces their avoidance. For this reason, anxiety sensitivity has been referred to as a fundamental fear that is distinct from derivative ones, such that the fear of anxiety can provide a motive for avoiding any stimulus likely to incite anxious symptoms (McNally 2002). Consequently, most patients with pathological anxiety also have heightened anxiety sensitivity, including patients with panic disorder, agoraphobia, posttraumatic stress disorder (PTSD), generalized anxiety disorder, and social anxiety disorder as well as depression and substance addiction (Naragon-Gainey 2010, Olatunji & Wolitzky-Taylor 2009).
Recent evidence suggests that reducing anxiety sensitivity may be important for the prevention and treatment of anxiety across diagnostic categories. Prospective studies have shown that anxiety sensitivity is a strong predictor for the onset of mood and anxiety disorders and the development of spontaneous panic attacks (Taylor 2014, Telch et al. 2012), whereas longitudinal studies have shown that individuals with high anxiety sensitivity have a propensity for greater chronicity of illness and a higher likelihood of experiencing future anxiety symptoms (Naragon-Gainey 2010, Schmidt et al. 2006). Controlled studies have demonstrated significant reductions in anxiety sensitivity following successful treatment with psychotherapy (Smits et al. 2008b) or pharmacotherapy (Simon et al. 2004), and several transdiagnostic treatments have been developed to specifically target anxiety sensitivity using different forms of interoceptive exposure (Deacon et al. 2012, Worden et al. 2015). Taken together, this evidence supports the notion that anxiety sensitivity is a fundamental driver of anxiety, and treatments that target anxiety sensitivity have the potential of helping patients overcome anxiety irrespective of their specific anxiety diagnosis (Boswell et al. 2013).
In the context of this framework, anxiety sensitivity can be conceptualized as manifesting from two primary disturbances: (a) hyperprecise priors that generate exaggerated body predictions whenever exposed to sensory signals that have become associated with anxiety, and (b) the persistent triggering of abnormally large somatic errors by sensory signals (either real or imagined) irrespective of context; that is, context rigidity. These sensitized sensory triggers lead to somatic error, followed by corrective action that upregulates the observed body state to be more in line with the predicted body state. However, by upregulating the very visceral signals one is sensitized to, the process can quickly escalate into a vicious cycle that culminates in the avoidance of all sensory cues associated with the experience of anxiety. Although avoidance of perceived triggers quickly reduces the somatic error, the relief is only temporary, and the vicious cycle returns whenever the trigger is once again encountered. Because the ultimate goal of the corrective action is to minimize somatic error as quickly and efficiently as possible, whether or not the corrective action is adaptive or maladaptive is not considered during the regulatory process. For this reason, avoidance and withdrawal behavior is often the action of choice because it rapidly reduces somatic error. As the error is reduced, the avoidance behavior is reinforced, which helps to explain why avoidance is the primary behavioral manifestation of anxiety and the symptom that elicits the most impairment in life functioning.
INTEROCEPTIVE INTERVENTIONS
I now proceed to urge the vital point of my whole theory, which is this: If we fancy some strong emotion, and then try to abstract from our consciousness of it all the feelings of its bodily symptoms, we find we have nothing left behind, no “mind-stuff’ out of which the emotion can be constituted, and that a cold and neutral state of intellectual perception is all that remains.... Emotion dissociated from all bodily feeling is inconceivable.
—William James, The Principles of Psychology
If the body is the nexus by which therapies can directly alter interoceptive states, then it follows that body-based therapies should provide a more direct entry point by which to manipulate the interoceptive system and correct somatic error. These practices provide the brain with an intense inflow of salient and unambiguous bottom-up sensory inputs, albeit across different interoceptive channels depending on the intervention, ultimately forcing a shift (or potentially a reset) in the brain’s mapping of the observed body state.
Based on our framework, exposure-based therapies principally target avoidance behaviors by experientially teaching individuals how to approach the very things in life they have been avoiding. This requires placing the person in situations likely to exacerbate somatic error, at least temporarily, but the exposure to the aversive experience forces the brain to develop a new adaptive model. In other words, with each new context that individuals are exposed to, they have an opportunity to adaptively adjust their prior expectations with new evidence. For example, a hyperprecise expectation that sensing the heartbeat can lead to a heart attack may be modulated toward alternative noncatastrophic expectations if the individuals sense their heartbeat in the absence of heart attack symptoms. This happens primarily via inhibitory fear learning, which includes standard mechanisms such as habituation and desensitization, but importantly, it seems to rely on the successful learning of new information via expectancy violations (e.g., by changing one’s expectation of harm when encountering an avoided situation) (Craske et al. 2008).
Exposure therapy focuses on exposing patients to a range of different anxiety-inducing situations, contexts, memories, and thoughts. For any given individual, especially individuals with high levels of anxiety sensitivity, there could exist a myriad of different contexts and stimuli that can trigger feelings of anxiety, making it impossible to expose one to all possibilities. If it is true that context rigidity is part of anxious psychopathology within an active inference framework, then systematically changing expectations in one context may facilitate using the adjusted model in another context. Interoceptive exposure therapy capitalizes on the fact that priors focused on interoceptive afferences may generalize more easily than exteroceptive stimuli (which can differ dramatically from one context to the next). Anxiety sensitivity, the target of most interoceptive exposure interventions, has historically been conceptualized as distress in response to symptoms of fear and anxiety (e.g., elevated heart rate and other indicators of sympathetic arousal). However, this sensitivity to the physical symptoms of anxiety may be part of a broader aversion to any physical symptom that has been conditioned to an aversive emotional state. This broader construct can be reframed as somatic sensitivity, and it incorporates all forms of somatic sensations that have been aversively conditioned (Domschkeet al. 2010). Interoceptive exposure serves to weaken this conditioned association through extinction learning, whereby instead of erasing previously learned associations (e.g., between physical sensations and aversive emotional outcomes), these same physical sensations acquire new, competing associations (e.g., they are safe, tolerable, and do not always lead to an aversive outcome) that serve to inhibit the original conditioned association (Boettcher et al. 2016).
The most effective interoceptive exposures closely reproduce the specific sensations associated with a given patient’s emotional distress. For example, interoceptive exposure for panic disorder uses strategies that direct patients to attend to their feared sensations and challenge their catastrophic cognitions so that the sensations of physiological arousal no longer provoke panic and avoidance behavior (Stewart & Watt 2008). To date, the majority of interoceptive exposures have focused on reproducing cardiovascular sensations (e.g., racing heart), respiratory sensations (e.g., shortness of breath), or vestibular sensations (e.g., dizziness) using simple and controllable provocation procedures (e.g., running in place to achieve a racing heart, breathing through a straw to achieve shortness of breath, spinning in circles to achieve dizziness). Beyond treating panic disorder with agoraphobia (Craske et al. 1997, Meuret et al. 2018), interoceptive exposure techniques have been successfully applied to other conditions (Khoury et al. 2018), including PTSD (Wald & Taylor 2008), social anxiety (Dixon et al. 2015), eating disorders (Boswell et al. 2015), substance use disorders (Zvolensky et al. 2003), and somatic symptom disorders like irritable bowel syndrome (Craske et al. 2011).
Mindfulness techniques represent a different approach to processing aversive interoceptive sensations, which may help minimize somatic errors by shifting attention away from the predicted body state and toward the observed body state (Farb et al. 2015). In the ideal setting, body predictions and somatic error signals may naturally dissipate as the mind attempts to function with low precision priors that trigger fewer regulatory responses, allowing the entire predictive model to be driven by incoming sensory input from the present moment in time. Despite promising results early on (Hofmann et al. 2010), recent meta-analyses have found rather small effects for mindfulness-based interventions in clinically anxious populations, with many patients finding it difficult to sustain their attention on present-moment interoceptive sensations (Goyal et al. 2014). Mindfulness has fared better for depression, showing medium to large effects for preventing relapse (Goldberg et al. 2018), although it is unclear how much of these effects are being driven by mindfulness per se or by the cognitive component of the therapy.
Our group has recently started investigating Floatation-REST (Reduced Environmental Stimulation Therapy), an intervention that attenuates exteroceptive sensory input to the nervous system through the act of floating supine in a pool of water saturated with Epsom salt. The float experience is calibrated so that sensory signals from visual, auditory, olfactory, gustatory, thermal, tactile, vestibular, gravitational, and proprioceptive channels are minimized, as is most movement and speech. The effect of this profound sensory reduction appears to be one of heightened interoceptive awareness and physiological relaxation (Feinstein et al. 2018a). Initial studies have shown that Floatation-REST leads to lower levels of chronic anxiety in generalized anxiety disorder (Jonsson & Kjellgren 2016) as well as lower levels of acute anxiety in patients with heightened anxiety sensitivity (Feinstein et al. 2018b). By attenuating all external sensory input to the brain, environmentally triggered predictions are minimized, creating a unique circumstance in which predictive models have very little incoming sensory information. This reduced sensory environment may provide an anxious and hypervigilant nervous system with a rare respite from the daily barrage of external triggers and stressors that it has been sensitized to over the years. Moreover, a reduction in muscle tension, especially in the upper and lower back, was one of the most prominent effects we have found so far in our float research (Feinstein et al. 2018a,b); such reduction could be playing an important positive role, given that a recent study found that musculoskeletal pain and tension is the most commonly reported somatic symptom across all types of depressive and anxiety disorders (Bekhuis et al. 2015). We consider it possible that the float environment may help facilitate access to mindful states, because it seems to provide a robust enhancement of interoceptive awareness for cardiorespiratory sensations, helping individuals to focus their attention on the observed body state and on present-moment sensations such as the breath (Feinstein et al. 2018a). In anxious patients, this interoceptive enhancement may weaken the bond between visceral sensations and anxiety, especially when these sensations are experienced against a backdrop of reduced muscle tension and physiological relaxation. Ultimately, this may lead to long-term reductions in anxiety sensitivity and to the formation of a new predictive model, one that links the experience of visceral sensations with a state of relaxation instead of anxiety (Feinstein et al. 2018a).
Another potentially promising interoceptive approach is whole body hyperthermia (WBH), which involves the selective stimulation of heat-sensitive thermosensory pathways projecting from the skin to subcortical and cortical brain regions to achieve an elevated core body temperature of 38.5°C. A recent randomized sham-controlled trial found that a single session of WBH produced a significant antidepressant effect lasting up to six weeks in depressed patients (Janssen et al. 2016). Although the underlying mechanism is uncertain, the study’s effective sham control suggests that the active antidepressant component was related to the heightened modulation of observed thermosensory and inflammatory signals from the body. From the perspective of somatic error, this intervention also relies heavily on modulation of the current body state, potentially forcing a recalibration of the prior set point. Other interventions involving interoceptive modulations of the observed body state that have been found to potentially benefit mental health include the following: the modulation of muscle tension via Swedish massage (Rapaport et al. 2016), yoga (Jeter et al. 2015), and exercise (Smits et al. 2008a): repeated brief exposures to high doses of CO2 (Wolpe 1987): and cyclic activation of the sympathetic nervous system and suppression of the immune response through cold immersion and CO2 modulation using alternating cycles of hyperventilation followed by breath holding (Kox et al. 2014).
CONCLUSION
Interoception is essential for maintaining a homeostatic balance between the brain and the body in a dynamically changing world. If Darwin’s thesis is true, and the survival of the species depends on the ability to adaptively respond to change, then the active inference framework described here highlights two potential failures of adaptation: hyperprecise priors and context rigidity. Both create an insensitivity to change, leading to persistent somatic error, unsustainable allostatic load, maladaptive corrective actions, and eventually psychopathology. There remain many details to work out. For example, what roles do different brain regions and brainstem nuclei play in this dynamic process? What are the developmental, physiological, and behavioral bases for the proposed interoceptive dysfunctions? How can interventions be targeted to ameliorate these dysfunctions, and does accomplishing this objective improve mental health? It is our hope that the active inference approach to interoceptive psychopathology will provide a framework for answering these questions and advancing our understanding of the role of interoception in mental health.
FUTURE DIRECTIONS.
Investigations of interoceptive psychopathology within an active inference framework should model the influence of hyperprecise priors and context rigidity in generating somatic error.
Somatic error detection and interoceptive awareness can be optimized using homeostatic perturbations and exposure to anxiogenic stimuli that vary in predictability and controllability.
Sustained somatic error dysregulation may result in long-term brain alterations localized to regions involved in the prediction, detection, and regulation of somatic errors.
Body-based interoceptive interventions provide a direct entry point by which to manipulate the interoceptive system and correct somatic error.
ACKNOWLEDGEMENTS:
The authors would like to thank W. Kyle Simmons for early discussions pertaining to this work, and acknowledge the following funding sources: The William K. Warren Foundation, NIGMS P20GM121312 (MPP, JSF, SSK), NCCIH R34AT009889 (JSF), and NIMH K23MH112949 (SSK).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Am. Psychiatr. Assoc. 2013. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: Am. Psychiatr. Publ; 5th ed. [Google Scholar]
- Ardizzi M, Ferri F. 2018. Interoceptive influences on peripersonal space boundary. Cognition 177:79–86 [DOI] [PubMed] [Google Scholar]
- Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. 2014. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biological Psychiatry 76: 258–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK. 2015. Interoceptive predictions in the brain. Nat. Rev. Neurosci 16:419–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsky AJ, Ahern DK, Brener J, Surman OS, Ring C, Dec GW. 1998. Palpitations and cardiac awareness after heart transplantation. Psychosom. Med 60:557–62 [DOI] [PubMed] [Google Scholar]
- Barsky AJ, Cleary PD, Barnett MC, Christiansen CL, Ruskin JN. 1994. The accuracy of symptom reporting by patients complaining of palpitations. Am. J. Med 97:214–21 [DOI] [PubMed] [Google Scholar]
- Bekhuis E, Boschloo L, Rosmalen JG, Schoevers RA. 2015. Differential associations of specific depressive and anxiety disorders with somatic symptoms. J. Psychosom. Res 78:116–22 [DOI] [PubMed] [Google Scholar]
- Benke C, Blumenthal TD, Modess C, Hamm AO, Pane-Farre CA. 2015. Effects of anxiety sensitivity and expectations on the modulation of the startle eyeblink response during a caffeine challenge. Psychopharmacology 232:3403–16 [DOI] [PubMed] [Google Scholar]
- Berner LA, Simmons AN, Wierenga CE, Bischoff-Grethe A, Paulus MP, et al. 2018. Altered interoceptive activation before, during, and after aversive breathing load in women remitted from anorexia nervosa. Psychol. Med 48(1): 142–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher H, Brake CA, Barlow DH. 2016. Origins and outlook of interoceptive exposure. J. Behav. Ther. Exp. Psychiatry 53:41–51 [DOI] [PubMed] [Google Scholar]
- Bogaerts K, Millen A, Li W, De Peuter S, Van Diest I, et al. 2008. High symptom reporters are less interoceptively accurate in a symptom-related context. J. Psychosom. Res 65:417–24 [DOI] [PubMed] [Google Scholar]
- Borg C, Chouchou F, Dayot-Gorlero J, Zimmerman P, Maudoux D, et al. 2018. Pain and emotion as predictive factors of interoception in fibromyalgia. J. Pain Res 11:823–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell JF, Anderson LM, Anderson DA. 2015. Integration of interoceptive exposure in eating disorder treatment. Clin. Psychol. Sci. Pract 22:194–210 [Google Scholar]
- Boswell JF, Farchione TJ, Sauer-Zavala S, Murray HW, Fortune MR, Barlow DH. 2013. Anxiety sensitivity and interoceptive exposure: a transdiagnostic construct and change strategy. Behav. Ther 44:417–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Berner LA, Jones MD, Reilly EE, Cusack A, et al. 2017. Psychometric evaluation and norms for the Multidimensional Assessment of Interoceptive Awareness (MAIA) in a clinical eating disorders sample. Eur. Eating Disord. Rev 25:411–16 [DOI] [PubMed] [Google Scholar]
- Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. 2015. Vagal sensory neuron subtypes that differentially control breathing. Cell 161:622–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Jatlow PI. 1985. Increased anxiogenic effects of caffeine in panic disorders. Arch. Gen. Psychiatry 42:233–43 [DOI] [PubMed] [Google Scholar]
- Craig AD. 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci 3:655–66 [DOI] [PubMed] [Google Scholar]
- Craig AD. 2009. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci 10:59–70 [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. 2008. Optimizing inhibitory learning during exposure therapy. Behav. Res. Ther 46:5–27 [DOI] [PubMed] [Google Scholar]
- Craske MG, Rowe M, Lewin M, Noriega-Dimitri R. 1997. Interoceptive exposure versus breathing retraining within cognitive-behavioural therapy for panic disorder with agoraphobia. Br. J. Clin. Psychol 36(Pt. 1):85–99 [DOI] [PubMed] [Google Scholar]
- Craske MG, Wolitzky-Taylor KB, Labus J, Wu S, Frese M, et al. 2011. A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations. Behav. Res. Ther 49:413–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. 2004. Neural systems supporting interoceptive awareness. Nat. Neurosci 7:189–95 [DOI] [PubMed] [Google Scholar]
- Damasio AR. 1999. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace [Google Scholar]
- Davenport PW, Vovk A. 2009. Cortical and subcortical central neural pathways in respiratory sensations. Respir. Physiol. Neurobiol 167:72–86 [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. 2010. Phasic versus sustained fear in rats and humans: role of the extended amygdala in fear versus anxiety. Neuropsychopharmacology 35:105–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon BJ, Lickel JJ, Possis EA, Abramowitz JS, Mahaffey BG, Wolitzky-Taylor K. 2012. Do cognitive reappraisal and diaphragmatic breathing augment interoceptive exposure for anxiety sensitivity? J. Cogn. Psychother 26:257–69 [Google Scholar]
- Desmedt O, Luminet O, Corneille O. 2018. The heartbeat counting task largely involves non-interoceptive processes: evidence from both the original and an adapted counting task. Biol. Psychol 138:185–88 [DOI] [PubMed] [Google Scholar]
- Dillon DJ, Gorman JM, Liebowitz MR, Fyer AJ, Klein DF. 1987. Measurement of lactate-induced panic and anxiety. Psychiatry Res. 20:97–105 [DOI] [PubMed] [Google Scholar]
- Dixon LJ, Kemp JJ, Farrell NR, Blakey SM, Deacon BJ. 2015. Interoceptive exposure exercises for social anxiety. J. Anxiety Disord 33:25–34 [DOI] [PubMed] [Google Scholar]
- Domschke K, Stevens S, Pfleiderer B, Gerlach AL. 2010. Interoceptive sensitivity in anxiety and anxiety disorders: an overview and integration of neurobiological findings. Clin. Psychol. Rev 30:1–11 [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Ogilvie AD, Lawrence AD. 2007. Heartbeat perception in depression. Behav. Res. Ther 45:1921–30 [DOI] [PubMed] [Google Scholar]
- Dunn BD, Stefanovitch I, Evans D, Oliver C, Hawkins A, Dalgleish T. 2010. Can you feel the beat? Interoceptive awareness is an interactive function of anxiety- and depression-specific symptom dimensions. Behav. Res. Ther 48:1133–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Breuer P. 1992. Increased cardiac awareness in panic disorder. J. Abnorm. Psychol 101:371–82 [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. 2007. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164:1476–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing DL, Manassei M, Gould van Praag C, Philippides AO, Critchley HD, Garfinkel SN. 2017. Sleep and the heart: interoceptive differences linked to poor experiential sleep quality in anxiety and depression. Biol. Psychol 127:163–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb N, Daubenmier J, Price CJ, Gard T, Kerr C, et al. 2015. Interoception, contemplative practice, and health. Front. Psychol 6:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Tranel D. 2016a. A Tale of Survival from the World of Patient SM. New York: Guilford Press [Google Scholar]
- Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, et al. 2013. Fear and panic in humans with bilateral amygdala damage. Nat. Neurosci 16:270–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Khalsa SS, Salomons TV, Prkachin KM, Frey-Law LA, et al. 2016b. Preserved emotional awareness of pain in a patient with extensive bilateral damage to the insula, anterior cingulate, and amygdala. Brain Struct. Funct 221(3):1499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Khalsa SS, Yeh H, Al Zoubi O, Arevian AC, et al. 2018a. The elicitation of relaxation and interoceptive awareness using floatation therapy in individuals with high anxiety sensitivity. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3:555–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Khalsa SS, Yeh H, Wohlrab C, Simmons WK, et al. 2018b. Examining the short-term anxiolytic and antidepressant effect of Floatation-REST. PLOS ONE 13:e0190292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacconi CM, Kouptsova JE, Kohler S. 2017. A role for visceral feedback and interoception in feelings-of-knowing. Conscious Cogn. 53:70–80 [DOI] [PubMed] [Google Scholar]
- Friston K 2008. Hierarchical models in the brain. PLOS Comput. Biol 4:e1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Schwartenbeck P, Fitzgerald T, Moutoussis M, Behrens T, Dolan RJ. 2013. The anatomy of choice: active inference and agency. Front. Hum. Neurosci 7:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Daunizeau J, Kiebel SJ. 2009. Reinforcement learning or active inference? PLOS ONE 4:e6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DJ, Waugh CE, Bhattacharjee K, Thompson RJ, Gotlib IH. 2013. Interoceptive awareness, positive affect, and decision making in major depressive disorder. J. Affect. Disord 151:780–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Manassei MF, Hamilton-Fletcher G, In den Bosch Y, Critchley HD, Engels M. 2016. Interoceptive dimensions across cardiac and respiratory axes. Philos. Trans. R. Soc. B 371(1708):20160014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou E, Mai S, Fernandez KC, Pollatos O. 2018I see neither your fear, nor your sadness—interoception in adolescents. Conscious Cogn. 60:52–61 [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Uyar F, Koushik J, Jennings JR, et al. 2017. A brain phenotype for stressor-evoked blood pressure reactivity. J. Am. Heart Assoc 6(9):e002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Tucker RP, Greene PA, Davidson RJ, Wampold BE, et al. 2018. Mindfulness-based interventions for psychiatric disorders: a systematic review and meta-analysis. Clin. Psychol. Rev 59:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, et al. 2015. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72:305–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, Kent J, Martinez J, Browne S, Coplan J, Papp LA. 2001. Physiological changes during carbon dioxide inhalation in patients with panic disorder, major depression, and premenstrual dysphoric disorder: evidence for a central fear mechanism. Archives of general psychiatry 58: 125–31 [DOI] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, et al. 2014. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern. Med 174:357–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, Fan J. 2013. Anterior insular cortex and emotional awareness. J. Comp. Neurol 521:3371–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurguis GN, Vitton BJ, Uhde TW. 1997. Behavioral, sympathetic and adrenocortical responses to yohimbine in panic disorder patients and normal controls. Psychiatry Res. 71:27–39 [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, et al. 2010. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol. Psychiatry 68:982–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy AB, Meston CM. 2018. Interoception and awareness of physiological sexual arousal in women with sexual arousal concerns. J. Sex Marital Ther 44:398–409 [DOI] [PubMed] [Google Scholar]
- Hassanpour MS, Simmons WK, Feinstein JS, Luo Q, Lapidus RC, et al. 2018. The insular cortex dynamically maps changes in cardiorespiratory interoception. Neuropsychopharmacology 43:426–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heninger GR, Charney DS, Price LH. 1988. α2-adrenergic receptor sensitivity in depression: the plasma MHPG, behavioral, and cardiovascular responses to yohimbine. Arch. Gen. Psychiatry 45:718–26 [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. 1990. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J. Comp. Neurol 293:540–80 [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. 2010. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J. Consult. Clin. Psychol 78:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen CW, Lowry CA, Mehl MR, Allen JJ, Kelly KL, et al. 2016. Whole-body hyperthermia for the treatment of major depressive disorder: a randomized clinical trial. JAMA Psychiatry 73:789–95 [DOI] [PubMed] [Google Scholar]
- Jenkinson PM, Taylor L, Laws KR. 2018. Self-reported interoceptive deficits in eating disorders: a meta-analysis of studies using the eating disorder inventory. J. Psychosom. Res 110:38–45 [DOI] [PubMed] [Google Scholar]
- Jeter PE, Slutsky J, Singh N, Khalsa SBS. 2015. Yoga as a therapeutic intervention: a bibliometric analysis of published research studies from 1967 to 2013. J. Altern. Complement. Med 21:586–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson K, Kjellgren A. 2016. Promising effects of treatment with flotation-REST (restricted environmental stimulation technique) as an intervention for generalized anxiety disorder (GAD): a randomized controlled pilot trial. BMC Complement. Altern. Med 16:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WM, Ryu Y, Lee YS, Wallraven C, Chae Y. 2017. Role of interoceptive accuracy in topographical changes in emotion-induced bodily sensations. PLOS ONE 12:e0183211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, et al. 2018. Interoception and mental health: a roadmap. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3:501–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Craske MG, Li W, Vangala S, Strober M, Feusner JD. 2015. Altered interoceptive awareness in anorexia nervosa: effects of meal anticipation, consumption and bodily arousal. Int. J. Eating Disord 48:889–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Feinstein JS. 2018. The somatic error hypothesis of anxiety In The Interoceptive Mind (pp. 144–164), ed. Tsakiris M, Preester H. New York: Oxford Univ. Press. [Google Scholar]
- Khalsa SS, Feinstein JS, Li W, Feusner JD, Adolphs R, Hurlemann R. 2016. Panic anxiety in humans with bilateral amygdala lesions: pharmacological induction via cardiorespiratory interoceptive pathways. J. Neurosci 36:3559–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Hassanpour MS, Strober M, Craske MG, Arevian AC, Feusner JD. 2018. Interoceptive anxiety and body representation in anorexia nervosa. Front. Psychiatry 9:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Lapidus RC. 2016. Can interoception improve the pragmatic search for biomarkers in psychiatry? Front. Psychiatry 7:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. 2009. The pathways of interoceptive awareness. Nat. Neurosci 12:1494–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury NM, Lutz J, Schuman-Olivier Z. 2018. Interoception in psychiatric disorders: a review of randomized, controlled trials with interoception-based interventions. Harvard Rev. Psychiatry 26:250–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiken LG, Shook NJ, Robins JL, Clore JN. 2018. Association between mindfulness and interoceptive accuracy in patients with diabetes: preliminary evidence from blood glucose estimates. Complement. Ther. Med 36:90–92 [DOI] [PubMed] [Google Scholar]
- Klabunde M, Collado D, Bohon C. 2017. An interoceptive model of bulimia nervosa: a neurobiological systematic review. J. Psychiatr. Res 94:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kox M, van Eijk LT, Zwaag J, van den Wildenberg J, Sweep FC, et al. 2014. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. PNAS 111(20):7379–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak MJ, Cuthbert BN. 2016. The NIMH Research Domain Criteria Initiative: Background, Issues, and Pragmatics. Psychophysiology 53: 286–97 [DOI] [PubMed] [Google Scholar]
- Kunstman JW, Clerkin EM, Palmer K, Peters MT, Dodd DR, Smith AR. 2016. The power within: the experimental manipulation of power interacts with trait BDD symptoms to predict interoceptive accuracy. J. Behav. Ther. Exp. Psychiatry 50:178–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner RJ, Fresco DM. 2016. Interaction effect of brooding rumination and interoceptive awareness on depression and anxiety symptoms. Behav. Res. Ther 85:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler A, Foell J, Bekrater-Bodmann R. 2018. Interoception and its interaction with self, other, and emotion processing: implications for the understanding of psychosocial deficits in borderline personality disorder. Curr. Psychiatry Rep 20:28. [DOI] [PubMed] [Google Scholar]
- Lukowska M, Sznajder M, Wierzchon M. 2018. Error-related cardiac response as information for visibility judgements. Sci. Rep 8:1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AC, Gentsch A, Schroder L, Schutz-Bosbach S. 2018. Cardiac interoceptive learning is modulated by emotional valence perceived from facial expressions. Soc. Cogn. Affect. Neurosci 13(7):677–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Hardy CJD, Russell LL, Clark CN, Dick KM, et al. 2017. Impaired interoceptive accuracy in semantic variant primary progressive aphasia. Front. Neurol 8:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys C, Daunizeau J, Friston KJ, Stephan KE. 2011. A Bayesian foundation for individual learning under uncertainty. Front. Hum. Neurosci 5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. 2003. The concept of allostasis in biology and biomedicine. Horm. Behav 43:2–15 [DOI] [PubMed] [Google Scholar]
- McMorris T, Barwood M, Corbett J. 2018. Central fatigue theory and endurance exercise: toward an interoceptive model. Neurosci. Biobehav. Rev 93:93–107 [DOI] [PubMed] [Google Scholar]
- McNally RJ. 2002. Anxiety sensitivity and panic disorder. Biol. Psychiatry 52:938–46 [DOI] [PubMed] [Google Scholar]
- Meuret AE, Ritz T, Wilhelm FH, Roth WT, Rosenfield D. 2018. Hypoventilation therapy alleviates panic by repeated induction of dyspnea. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3:539–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul CL, Stagg SD, Herbelin B, Aspell JE. 2018. The feeling of me feeling for you: interoception, alexithymia and empathy in autism. J. Autism Dev. Disord 48(9):2953–67 [DOI] [PubMed] [Google Scholar]
- Murphy J, Millgate E, Geary H, Ichijo E, Coll MP, et al. 2018. Knowledge of resting heart rate mediates the relationship between intelligence and the heartbeat counting task. Biol. Psychol 133:1–3 [DOI] [PubMed] [Google Scholar]
- Mussgay L, Klinkenberg N, Ruddel H. 1999. Heart beat perception in patients with depressive, somatoform, and personality disorders. J. Psychophysiol 13:27–36 [Google Scholar]
- Naragon-Gainey K 2010. Meta-analysis of the relations of anxiety sensitivity to the depressive and anxiety disorders. Psychol. Bull 136:128–50 [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Goldschmidt-Clermont PJ. 2012. Heartache and heartbreak—the link between depression and cardiovascular disease. Nat. Rev. Cardiol 9:526–39 [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Wolitzky-Taylor KB. 2009. Anxiety sensitivity and the anxiety disorders: a meta-analytic review and synthesis. Psychol. Bull 135:974–99 [DOI] [PubMed] [Google Scholar]
- Owens AP, Allen M, Ondobaka S, Friston KJ. 2018. Interoceptive inference: from computational neuroscience to clinic. Neurosci. Biobehav. Rev 90:174–83 [DOI] [PubMed] [Google Scholar]
- Palmer CE, Tsakiris M. 2018. Going at the heart of social cognition: Is there a role for interoception in self-other distinction? Curr. Opin. Psychol 24:21–26 [DOI] [PubMed] [Google Scholar]
- Palser ER, Fotopoulou A, Pellicano E, Kilner JM. 2018. The link between interoceptive processing and anxiety in children diagnosed with autism spectrum disorder: extending adult findings into a developmental sample. Biol. Psychol 136:13–21 [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. 2003. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage 19:1439–48 [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. 2006. An insular view of anxiety. Biol. Psychiatry 60:383–87 [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. 2010. Interoception in anxiety and depression. Brain Struct. Funct 214:451–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, McEwen BS, Friston K. 2017. Uncertainty and stress: why it causes diseases and how it is mastered by the brain. Prog. Neurobiol 156:164–88 [DOI] [PubMed] [Google Scholar]
- Petersen S, Van Staeyen K, Vogele C, von Leupoldt A, Van den Bergh O. 2015. Interoception and symptom reporting: disentangling accuracy and bias. Front. Psychol 6:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo G, Barca L, Friston KJ. 2015. Active inference and cognitive-emotional interactions in the brain. Behav. Brain Sci 38:e85. [DOI] [PubMed] [Google Scholar]
- Pfeifer G, Garfinkel SN, Gould van Praag CD, Sahota K, Betka S, Critchley HD. 2017. Feedback from the heart: Emotional learning and memory is controlled by cardiac cycle, interoceptive accuracy and personality. Biol. Psychol 126:19–29 [DOI] [PubMed] [Google Scholar]
- Piech RM, Strelchuk D, Knights J, Hjalmheden JV, Olofsson JK, Aspell JE. 2017. People with higher interoceptive sensitivity are more altruistic, but improving interoception does not increase altruism. Sci. Rep 7:15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl R, Yeragani VK, Balon R, Rainey JM, Lycaki H, et al. 1988. Isoproterenol-induced panic attacks. Biol. Psychiatry 24:891–902 [DOI] [PubMed] [Google Scholar]
- Pollatos O, Traut-Mattausch E, Schandry R. 2009. Differential effects of anxiety and depression on interoceptive accuracy. Depress. Anxiety 26:167–73 [DOI] [PubMed] [Google Scholar]
- Powers AR, Mathys C, Corlett PR. 2017. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science 357:596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport MH, Schettler P, Larson ER, Edwards SA, Dunlop BW, et al. 2016. Acute Swedish massage monotherapy successfully remediates symptoms of generalized anxiety disorder: a proof-of-concept, randomized controlled study. J. Clin. Psychiatry 77:e883–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassovsky Y, Kushner MG. 2003. Carbon dioxide in the study of panic disorder: issues of definition, methodology, and outcome. J. Anxiety Disord 17:1–32 [DOI] [PubMed] [Google Scholar]
- Ricciardi L, Demartini B, Crucianelli L, Krahe C, Edwards MJ, Fotopoulou A. 2016. Interoceptive awareness in patients with functional neurological symptoms. Biol. Psychol 113:68–74 [DOI] [PubMed] [Google Scholar]
- Richter J, Hamm AO, Pane-Farre CA, Gerlach AL, Gloster AT, et al. 2012. Dynamics of defensive reactivity in patients with panic disorder and agoraphobia: implications for the etiology of panic disorder. Biol. Psychiatry 72:512–20 [DOI] [PubMed] [Google Scholar]
- Ring C, Brener J. 2018. Heartbeat counting is unrelated to heartbeat detection: a comparison of methods to quantify interoception. Psychophysiology 55(9):e13084. [DOI] [PubMed] [Google Scholar]
- Rogers ML, Hagan CR, Joiner TE. 2018. Examination of interoception along the suicidality continuum. J. Clin. Psychol 74:1004–16 [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Daw N, Jepma M, Wimmer GE, Wager TD. 2014. Representation of aversive prediction errors in the human periaqueductal gray. Nat. Neurosci 17:1607–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R, Ronchi R, Donz J, Bello-Ruiz J, Herbelin B, et al. 2018. Insula mediates heartbeat related effects on visual consciousness. Cortex 101:87–95 [DOI] [PubMed] [Google Scholar]
- Santangelo G, Vitale C, Baiano C, D'Iorio A, Longo K, et al. 2018. Interoceptive processing deficit: a behavioral marker for subtyping Parkinson's disease. Parkinsonism Relat. Disord 53:64–69 [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Zvolensky MJ, Maner JK. 2006. Anxiety sensitivity: prospective prediction of panic attacks and Axis I pathology. J. Psychiatr. Res 40:691–99 [DOI] [PubMed] [Google Scholar]
- Schulz SM. 2016. Neural correlates of heart-focused interoception: a functional magnetic resonance imaging meta-analysis. Philos. Trans. R. Soc. B 371(1708):20160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunck T, Erb G, Mathis A, Gilles C, Namer IJ, et al. 2006. Functional magnetic resonance imaging characterization of CCK-4-induced panic attack and subsequent anticipatory anxiety. Neuroimage 31:1197–208 [DOI] [PubMed] [Google Scholar]
- Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. 2015. The dorsal posterior insula subserves a fundamental role in human pain. Nat. Neurosci 18:499–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. 2011. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci 12:154–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. 2006. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol. Psychiatry 60:402–9 [DOI] [PubMed] [Google Scholar]
- Simon NM, Otto MW, Smits JA, Nicolaou DC, Reese HE, Pollack MH. 2004. Changes in anxiety sensitivity with pharmacotherapy for panic disorder. J. Psychiatr. Res 38:491–95 [DOI] [PubMed] [Google Scholar]
- Smith A, Forrest L, Velkoff E. 2018. Out of touch: Interoceptive deficits are elevated in suicide attempters with eating disorders. Eating Disord. 26:52–65 [DOI] [PubMed] [Google Scholar]
- Smith R, Thayer JF, Khalsa SS, Lane RD. 2017. The hierarchical basis of neurovisceral integration. Neurosci. Biobehav. Rev 75:274–96 [DOI] [PubMed] [Google Scholar]
- Smits JA, Berry AC, Rosenfield D, Powers MB, Behar E, Otto MW. 2008a. Reducing anxiety sensitivity with exercise. Depress. Anxiety 25:689–99 [DOI] [PubMed] [Google Scholar]
- Smits JA, Berry AC, Tart CD, Powers MB. 2008b. The efficacy of cognitive-behavioral interventions for reducing anxiety sensitivity: a meta-analytic review. Behav. Res. Ther 46:1047–54 [DOI] [PubMed] [Google Scholar]
- Stephan KE, Manjaly ZM, Mathys CD, Weber LA, Paliwal S, et al. 2016. Allostatic self-efficacy: a metacognitive theory of dyshomeostasis-induced fatigue and depression. Front. Hum. Neurosci 10:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P 2014. Homeostasis versus allostasis: implications for brain function and mental disorders. JAMA Psychiatry 71:1192–93 [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Francis HM, Oaten MJ, Schilt R. 2018. Hippocampal dependent neuropsychological tests and their relationship to measures of cardiac and self-report interoception. Brain Cogn. 123:23–29 [DOI] [PubMed] [Google Scholar]
- Stewart SH, Watt MC. 2008. Introduction to the special issue on interoceptive exposure in the treatment of anxiety and related disorders: novel applications and mechanisms of action. J. Cogn. Psychother 22:291–302 [Google Scholar]
- Taylor S 2014. Anxiety Sensitivity: Theory, Research, and Treatment of the Fear of Anxiety. New York: Routledge [Google Scholar]
- Telch MJ, Rosenfield D, Lee HJ, Pai A. 2012. Emotional reactivity to a single inhalation of 35% carbon dioxide and its association with later symptoms of posttraumatic stress disorder and anxiety in soldiers deployed to Iraq. Arch. Gen. Psychiatry 69:1161–68 [DOI] [PubMed] [Google Scholar]
- Tsakiris M 2017. The multisensory basis of the self: from body to identity to others. Q. J. Exp. Psychol 70:597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda S, Tochizawa S, Shibata M, Terasawa Y. 2016. Prospective memory mediated by interoceptive accuracy: a psychophysiological approach. Philos. Trans. R. Soc. B 371(1708):20160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh O, Witthoft M, Petersen S, Brown RJ. 2017. Symptoms and the body: taking the inferential leap. Neurosci. Biobehav. Rev 74:185–203 [DOI] [PubMed] [Google Scholar]
- Villena-Gonzalez M, Moenne-Loccoz C, Lagos RA, Alliende LM, Billeke P, et al. 2017. Attending to the heart is associated with posterior alpha band increase and a reduction in sensitivity to concurrent visual stimuli. Psychophysiology 54:1483–97 [DOI] [PubMed] [Google Scholar]
- Wald J, Taylor S. 2008. Responses to interoceptive exposure in people with posttraumatic stress disorder (PTSD): a preliminary analysis of induced anxiety reactions and trauma memories and their relationship to anxiety sensitivity and PTSD symptom severity. Cogn. Behav. Ther 37:90–100 [DOI] [PubMed] [Google Scholar]
- Wiebking C, Bauer A, de Greck M, Duncan NW, Tempelmann C, Northoff G. 2010. Abnormal body perception and neural activity in the insula in depression: an fMRI study of the depressed “material me.” World J. Biol. Psychiatry 11:538–49 [DOI] [PubMed] [Google Scholar]
- Windmann S, Schonecke OW, Frohlig G, Maldener G. 1999. Dissociating beliefs about heart rates and actual heart rates in patients with cardiac pacemakers. Psychophysiology 36:339–42 [DOI] [PubMed] [Google Scholar]
- Wittkamp MF, Bertsch K, Vogele C, Schulz A. 2018. A latent state-trait analysis of interoceptive accuracy. Psychophysiology 55:e13055. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Simmons AN, Flagan T, Lane SD, Wackermann J, Paulus MP. 2011. Neural substrates of time perception and impulsivity. Brain Res. 1406:43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe J 1987. Carbon dioxide inhalation treatments of neurotic anxiety: an overview. J. Nerv. Ment. Dis 175:129–33 [DOI] [PubMed] [Google Scholar]
- Worden BL, Davis E, Genova M, Tolin DF. 2015. Development of an anxiety sensitivity (AS) intervention for high-AS individuals in substance use disorders treatment. Cogn. Ther. Res 39:343–55 [Google Scholar]
- Yoris A, Abrevaya S, Esteves S, Salamone P, Lori N, et al. 2018. Multilevel convergence of interoceptive impairments in hypertension: new evidence of disrupted body-brain interactions. Hum. Brain Mapp. 39:1563–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoris A, Garcia AM, Traiber L, Santamaria-Garcia H, Martorell M, et al. 2017. The inner world of overactive monitoring: neural markers of interoception in obsessive-compulsive disorder. Psychol. Med 47:1957–70 [DOI] [PubMed] [Google Scholar]
- Zaman J, De Peuter S, Van Diest I, Van den Bergh O, Vlaeyen JW. 2016. Interoceptive cues predicting exteroceptive events. Int. J. Psychophysiol 109:100–6 [DOI] [PubMed] [Google Scholar]
- Zamariola G, Cardini F, Mian E, Serino A, Tsakiris M. 2017. Can you feel the body that you see? On the relationship between interoceptive accuracy and body image. Body Image 20:130–36 [DOI] [PubMed] [Google Scholar]
- Zamariola G, Maurage P, Luminet O, Corneille O. 2018. Interoceptive accuracy scores from the heartbeat counting task are problematic: evidence from simple bivariate correlations. Biol. Psychol 137:12–17 [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Lejuez C, Kahler CW, Brown RA. 2003. Integrating an interoceptive exposure-based smoking cessation program into the cognitive-behavioral treatment of panic disorder: theoretical relevance and case demonstration. Cogn. Behav. Pract 10:347–57 [Google Scholar]