Abstract
Severe pneumonia which shares several of the features of acute respiratory distress syndrome (ARDS) is the main cause of morbidity and mortality in Coronavirus disease 19 (Covid‐19) for which there is no effective treatment, so far. ARDS is caused and sustained by an uncontrolled inflammatory activation characterized by a massive release of cytokines (cytokine storm), diffuse lung oedema, inflammatory cell infiltration, and disseminated coagulation. Macrophage and T lymphocyte dysfunction plays a central role in this syndrome. In several experimental in vitro and in vivo models, many of these pathophysiological changes are triggered by stimulation of the P2X7 receptor. We hypothesize that this receptor might be an ideal candidate to target in Covid‐19‐associated severe pneumonia.
Linked Articles
This article is part of a themed issue on The Pharmacology of COVID‐19. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.21/issuetoc
Abbreviations
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- BALF
bronchoalveolar lavage fluid
- Covid‐19
Coronavirus disease 19
- DAMP
damage‐associated molecular pattern
- MAS
macrophage activation syndrome
- NLRP3
NACHT, LRR and PYD domain‐containing protein 3
- pmeLUC
plasma membrane luciferase
- PRR
pattern recognition receptor
- Sars‐Cov
severe acute respiratory syndrome‐Coronavirus
- sJIA
systemic juvenile idiopathic arthritis
- SLE
systemic lupus erythematosus
- TIMP‐1
tissue inhibitor of metalloproteinase‐1
An effective treatment for Coronavirus disease 19 (Covid‐19) is still lacking, although anecdotal evidence suggests that IL‐6 blockade is beneficial (Mehta et al., 2020), and blockers of other pro‐inflammatory cytokines such as IL‐1β are being considered in Covid‐19 patients showing hyperinflammation (Monteagudo, Boothby, & Gertner, 2020). In such a dramatic situation, it might be necessary to explore unconventional, but rational, therapeutic options, particularly in the light of the unsatisfactory results achieved with current therapies such as hydroxychloroquine (Magagnoli et al., 2020). There is evidence that severely ill Covid‐19 patients develop a cytokine storm syndrome causing severe pneumonia that fulfils some but not all of the Berlin criteria of acute respiratory distress syndrome (ARDS) (ARDS Definition Task Force, 2012), because in about 50% of cases, severe hypoxemia is associated with near normal respiratory system compliance (Gattinoni, Chiumello, et al., 2020). This might be due to the loss of control over lung perfusion and hypoxic vasoconstriction, in Covid‐19 pneumonia, which may cause extensive ventilation/perfusion mismatching (Gattinoni, Coppola, et al., 2020). Pneumonia, the main cause of morbidity and mortality in Covid‐19 patients (Mehta et al., 2020; Ruan et al., 2020), is thought to be due to uncontrolled, virus‐mediated, direct activation of lung macrophages in a process resembling the macrophage activation syndrome (MAS) observed in some rheumatological diseases such as systemic juvenile idiopathic arthritis (sJIA), adult onset Still's disease, and systemic lupus erythematosus (SLE), although it is debatable whether hyperinflammation in Covid‐19 should be considered MAS sensu strictu (Crayne, Albeituni, Nichols, & Cron, 2019; Henderson et al., 2020). Covid‐19 patients show massive infiltration by inflammatory cells (neutrophils and monocytes/macrophages) in their lungs and increased blood levels of IL‐1β, IL‐6, IL‐2, IL‐7, TNF‐α, and several other pro‐inflammatory cytokines and chemokines (Guo et al., 2020). While the main target of coronaviruses are lung epithelial cells, macrophages and dendritic cells are also infected, where these viruses cause abortive infection and sustained activation, that in turn drive hyperinflammation (Fehr & Perlman, 2015). Thus, macrophage inhibition is likely to be a crucial component of the prevention of the extensive lung injury caused by severe acute respiratory syndrome‐Coronavirus‐2 (Sars‐Cov‐2). Consequently, a promising target receptor to down‐modulate macrophage responses might be the P2X7 receptor.
The P2X7 receptor is a plasma membrane receptor gated by extracellular ATP, the earliest and most ubiquitous damage‐associated molecular pattern (DAMP) released at all inflammatory sites, the lungs included (Di Virgilio, Sarti, & Coutinho‐Silva, 2020; Tolle & Standiford, 2013). Very interestingly, increased ATP levels are also found in bronchoalveolar lavage fluid (BALF) from patients with ARDS or from mice with LPS‐induced acute lung injury (ALI) (Cicko et al., 2018). Increased extracellular ATP levels have also been directly demonstrated in vivo in the lungs of mice following inhalation of LPS, using the bioluminescent luciferase probe, plasma membrane luciferase (pmeLUC) (Cicko et al., 2018). The cell surface receptor used by SARS‐Cov‐2 to infect cells, ACE2, is also expressed on monocytes and macrophages, the cell types that express the P2X7 receptor to the highest level. Thus, it is conceivable that mononuclear phagocytes are hyperstimulated at the same time by the virus and by extracellular ATP via the P2X7 receptors.
The P2X7 receptor has a central role in inflammation as it is one of the most potent stimulators of the NACHT, LRR and PYD domain‐containing protein 3 (NLRP3) inflammasome and therefore of caspase‐1 activation and IL‐1β and IL‐18 release (Di Virgilio, Dal Ben, Sarti, Giuliani, & Falzoni, 2017) (Figure 1). There is evidence that the NLRP3 inflammasome can be also directly activated by SARS‐Cov, the aetiological agent of SARS epidemic in 2002, via a Ca2+‐dependent mechanism (Nieto‐Torres et al., 2015), although the role of Ca2+ in the activation of this inflammasome subtype is debated (Di Virgilio, 2013). There is, as yet, no report of a direct stimulation of the NLRP3 inflammasome by SARS‐Cov‐2, but it cannot be excluded that NLRP3 could be synergistically stimulated by viral proteins from SARS‐Cov‐2 and the P2X7 receptor. Stimulation of P2X7 receptors also promotes release of other cytokines and chemokines, for example, IL‐6, TNF‐α, CCL2, IL‐8, CCL3 and CXCL2, of pro‐fibrotic factors such as TGF‐β, and extracellular matrix remodelling factors, for example, metalloproteinase‐9 and tissue inhibitor of metalloproteinase (TIMP)‐1 (Di Virgilio et al., 2017; Riteau et al., 2010). In models of bleomycin‐ or silica‐induced lung fibrosis, P2X7 receptor‐deficient mice showed markedly reduced lung inflammation (Monção‐Ribeiro et al., 2014; Riteau et al., 2010). This suggests that P2X7 receptor antagonists might be of benefit even for those Covid‐19 patients with less severe pneumonia, who do not require admission to Intensive Care Units but will later develop lung fibrosis.
FIGURE 1.
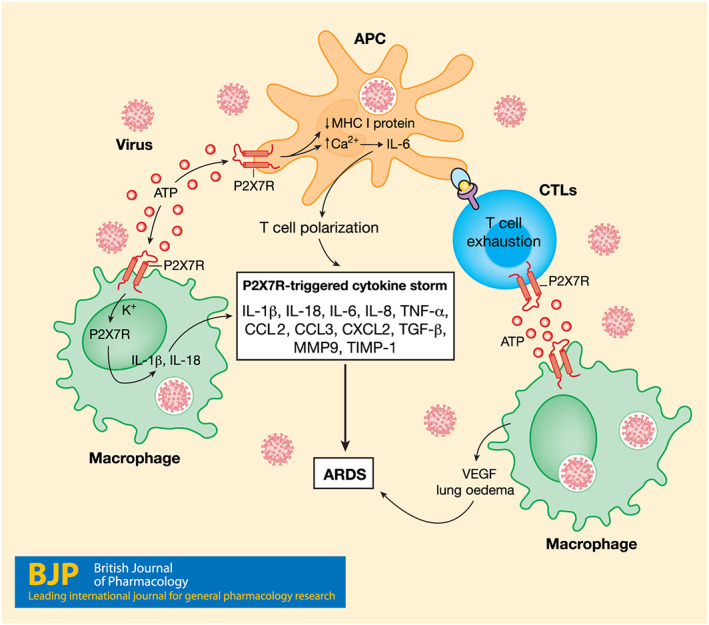
Central role of the P2X7 receptor (P2X7R) in Sars‐Cov‐2‐dependent lung inflammation. Sars‐Cov‐2 infects lung mononuclear phagocytes, apart from the epithelial cells, triggering massive ATP release which feeds back on the P2X7 receptors expressed on macrophages and antigen‐presenting cells (APCs). Activation of P2X7 receptors down‐modulates MHC‐I expression and at the same time promotes IL‐6 release, NLRP3‐mediated IL‐1β and IL‐18 release, VEGF secretion, and thus lung oedema. Release of several other cytokines and chemokines is also promoted by P2X7 receptor activation. Stimulation of P2X7 receptors on APC‐stimulated T lymphocytes (cytotoxic T lymphocytes; CTLs) causes exhaustion and T cell polarization. The combined effect of all these processes promotes the pathophysiological changes typical of ARDS
The P2X7 receptor is a potent trigger of ROS production. Therefore, its overactivation, at sites of severe inflammation, may inhibit lymphocyte functions by impairing mitochondrial metabolism, as has been shown in monocytes during sepsis (Martínez‐García et al., 2019). Dysfunctional mitochondria are a feature of exhausted T lymphocytes (Desdín‐Micó, Soto‐Heredero, & Mittelbrunn, 2018). Although no specific data are available in Covid‐19, reduced T cell function, as documented by the increased expression of T lymphocyte exhaustion markers, is a feature of coronavirus‐induced lung infection (Diao et al.,2020 2020). It is known that cytotoxic T lymphocytes and NK cells lose their ability to lyse virus‐infected cells in MAS and this loss is a major cause of the sustained cytokine release, driving the cytokine storm typical of this syndrome and of Covid‐19 as well (Crayne et al., 2019). In addition, a key feature of ARDS is the extensive pulmonary oedema, largely dependent on release of VEGF. Although the pathogenic role of VEGF in ARDS is controversial, blood VEGF levels are reported to be increased in Covid‐19 patients, and this has prompted a clinical study aimed at testing the effects of bevacizumab administration (NCT04275414). While mostly known for its pro‐inflammatory activity, the P2X7 receptor is also a potent inducer of VEGF release and of neo‐angiogenesis in vivo and, accordingly, blockade of P2X7 receptors inhibited VEGF‐dependent increase in vascular permeability (Clapp et al., 2019). Thus, targeting the P2X7 receptor might prove beneficial to fight the early exudative phase in ARDS. Furthermore, thromboembolic complications are common among critically ill Covid‐19 patients (Tang, Li, et al., 2020), which has led to assessment of the protective effect of heparin administration (Tang, Bai, et al., 2020). Targeting P2X7 receptors might also be beneficial in treating thromboembolism, as stimulation of these receptors promotes a massive release of tissue factor (Baroni et al., 2007; Furlan‐Freguia, Marchese, Gruber, Ruggeri, & Ruf, 2011).
A commonly used experimental model for ARDS is the intratracheal application of LPS in mice. This causes ALI, an acute lung inflammation resembling human ARDS (Cicko et al., 2018). ALI is induced by inhalation of LPS, which might not faithfully mimic virus‐triggered pneumonia since LPS mainly targets plasma membrane pattern recognition receptors (PRRs), while SARS‐CoV‐2 targets intracellular receptors. However, when LPS reaches critical concentrations, as, for example, in sepsis, it can aberrantly localize in the cytoplasm, where it can activate NLRP3 via a non‐canonical pathway (Kayagaki et al., 2011). Early work showed that pharmacological blockade of P2X7 receptors, or its genetic ablation, substantially reduced inflammatory cell infiltration, cytokine levels, and lung damage in acute inflammatory response in ALI (Cicko et al., 2018; Wang et al., 2015). P2X7 receptor deficiency also reduced alveolar macrophage death and pro‐IL‐1α release in the lungs of LPS‐treated mice (Dagvadorj et al., 2015). The P2X7 receptor has also been proposed as an inflammatory biomarker (Giuliani et al., 2019), and its monitoring in Covid‐19 by recently developed radiopharmaceuticals has been recently suggested (Juengling, Maldonado, Wuest, & Schindler, 2020). On the basis of these convergent observations, we hypothesize that the P2X7 receptor could be an ideal candidate receptor for pharmacological targeting in Covid‐19‐associated ARDS.
Low MW compounds targeting the P2X7 receptor have been developed by most pharmaceutical companies and have undergone extensive Phase I clinical trials revealing an excellent safety profile (Arulkumaran, Unwin, & Tam, 2011). However, their therapeutic efficacy in a number of chronic inflammatory diseases investigated in Phase II trials turned out to be limited, leading most companies to stop or outsource clinical research on P2X7 receptors, with the exception of Johnson & Johnson, who in late 2019 started a new Phase II clinical trial to test the efficacy of P2X7 receptor blockade in depression (Cully, 2020). The therapeutic effects of P2X7 receptor blockade has never been tested in a disease condition characterized by uncontrolled hyperinflammation, as in Covid‐19.
We propose that administration of P2X7 receptor antagonists might be beneficial in patients with severe pneumonia, and more so in patients admitted to Intensive Care Units who need intubation, with the possible exclusion of patients responding to standard therapies, or with severe hepatic or renal failure. The aim will be to dampen hyperinflammation, to decrease the possible untoward effects of ventilation and to prevent development of lung fibrosis. We are aware that much should be done to find the appropriate treatment regimens as P2X7 receptor antagonists are only available for oral administration, so far. This might be an inefficient route of delivery, although other drugs tentatively used to treat Covid‐19, such as hydrochloroquine or colchicine, are administered by this route. It is clear that faster acting routes of administration should be explored.
Identifying a marker of receptor engagement will be crucial. An obvious marker to monitor P2X7 receptor blockade would be blood IL‐1β levels, but this might be inappropriate in this situation and a marker more directly linked to the P2X7 receptor would be more reliable. The P2X7 receptor can be shed into the blood in several inflammatory conditions or in cancer, in a process promoted by stimulation of the P2X7 receptor itself, i.e., P2X7 receptor stimulation triggers P2X7 receptor release (Giuliani et al., 2019). Thus, measurement of P2X7 receptor levels in the blood, which can be easily performed, could be used to assess the extent of P2X7 receptor blockade. Targeting the P2X7 receptor would have the advantage of acting upstream of tocilizumab and anakinra, thus possibly preventing the release of other factors responsible for lung damage, such as ROS. In addition, P2X7 receptor blockade would also inhibit VEGF release and therefore prevent lung oedema. On the basis of these considerations, we think that P2X7 receptor antagonists should be given a chance, at least for the compassionate treatment of Covid‐19 patients with rapidly evolving ARDS.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, et al., 2019, 2019; Alexander, Mathie et al., 2019).
CONFLICT OF INTEREST
F.D.V. is a member of the Scientific Advisory Board of Biosceptre Ltd, a UK‐based Biotech involved in the development of P2X7‐targeted antibodies.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Natural Product Research, Design and Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
ACKNOWLEDGEMENTS
F.D.V. was supported by the Italian Association for Cancer Research (Associazione Italiana per la Ricerca sul Cancro [AIRC]) Grants IG 13025, IG 18581, and IG 22883; the Ministry of Education of Italy (Ministero dell'Istruzione, dell'Università e della Ricerca) Grant 20178YTNWC; and funds from the University of Ferrara. M.R. was supported by the Ministry of Education of Italy Grant 20178YTNWC and funds from the University of Padova. Y.T. was supported by the Project First‐Class Disciplines Development of Chengdu University of Traditional Chinese Medicine (CZYHW1901) and Science and Technology Program of Sichuan Province of China (2019YFH0108).
Di Virgilio F, Tang Y, Sarti AC, Rossato M. A rationale for targeting the P2X7 receptor in Coronavirus disease 19. Br J Pharmacol. 2020;177:4990–4994. 10.1111/bph.15138
REFERENCES
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARDS Definition Task Force . (2012). Acute respiratory distress syndrome: The Berlin definition. JAMA, 307, 2526–2533. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- Arulkumaran, N. , Unwin, R. J. , & Tam, F. W. (2011). A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert Opinion on Investigational Drugs, 20, 897–915. 10.1517/13543784.2011.578068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni, M. , Pizzirani, C. , Pinotti, M. , Ferrari, D. , Adinolfi, E. , Calzavarini, S. , … di Virgilio, F. (2007). Stimulation of P2 (P2X7) receptors in human dendritic cells induces the release of tissue factor‐bearing microparticles. The FASEB Journal, 21, 1926–1933. 10.1096/fj.06-7238com [DOI] [PubMed] [Google Scholar]
- Cicko, S. , Köhler, T. C. , Ayata, C. K. , Müller, T. , Ehrat, N. , Meyer, A. , … Idzko, M. (2018). Extracellular ATP is a danger signal activating P2X7 receptor in a LPS mediated inflammation (ARDS/ALI). Oncotarget, 9, 30635–30648. 10.18632/oncotarget.25761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp, C. , Diaz‐Lezama, N. , Adan‐Castro, E. , Ramirez‐Hernandez, G. , Moreno‐Carranza, B. , Sarti, A. C. , … di Virgilio, F. (2019). Pharmacological blockade of the P2X7 receptor reverses retinal damage in a rat model of type 1 diabetes. Acta Diabetologica, 56, 1031–1036. 10.1007/s00592-019-01343-4 [DOI] [PubMed] [Google Scholar]
- Crayne, C. B. , Albeituni, S. , Nichols, K. E. , & Cron, R. Q. (2019). The immunology of macrophage activation syndrome. Frontiers in Immunology, 10, 119 10.3389/fimmu.2019.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully, M. (2020). Can anti‐inflammatory strategies light up the dim depression pipeline? Nature Reviews. Drug Discovery, 19, 224–225. 10.1038/d41573-020-00049-5 [DOI] [PubMed] [Google Scholar]
- Dagvadorj, J. , Shimada, K. , Chen, S. , Jones, H. D. , Tumurkhuu, G. , Zhang, W. , … Arditi, M. (2015). Lipopolysaccharide induces alveolar macrophage necrosis via CD14 and the P2X7 receptor leading to interleukin‐1α release. Immunity, 42, 640–653. 10.1016/j.immuni.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desdín‐Micó, G. , Soto‐Heredero, G. , & Mittelbrunn, M. (2018). Mitochondrial activity in T cells. Mitochondrion, 41, 51–57. 10.1016/j.mito.2017.10.006 [DOI] [PubMed] [Google Scholar]
- Di Virgilio, F. (2013). The therapeutic potential of modifying inflammasomes and NOD‐like receptors. Pharmacological Reviews, 65, 872–905. 10.1124/pr.112.006171 [DOI] [PubMed] [Google Scholar]
- Di Virgilio, F. , Dal Ben, D. , Sarti, A. C. , Giuliani, A. L. , & Falzoni, S. (2017). The P2X7 receptor in infection and inflammation. Immunity, 47, 15–31. 10.1016/j.immuni.2017.06.020 [DOI] [PubMed] [Google Scholar]
- Di Virgilio, F. , Sarti, A. C. , & Coutinho‐Silva, R. (2020). Purinergic signalling, DAMPs and inflammation. American Journal of Physiology. Cell Physiology, 318, C832–C835. 10.1152/ajpcell.00053.2020 [DOI] [PubMed] [Google Scholar]
- Diao, B. , Wang, C. , Tan, Y. , Chen, X. , Liu, Y. , Ning, L. , … Chen, Y. (2020). Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID‐19). Frontiers in Immunology, 11, 827 10.1101/2020.02.18.20024364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr, A. R. , & Perlman, S. (2015). Coronaviruses: An overview of their replication and pathogenesis. Methods in Molecular Biology, 1282, 1–23. 10.1007/978-1-4939-2438-7_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan‐Freguia, C. , Marchese, P. , Gruber, A. , Ruggeri, Z. M. , & Ruf, W. (2011). P2X7 receptor signaling contributes to tissue factor‐dependent thrombosis in mice. The Journal of Clinical Investigation, 121, 2932–2944. 10.1172/JCI46129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni, L. , Chiumello, D. , Caironi, P. , Busana, M. , Romitti, F. , Brazzi, L. , & Camporota, L. (2020). COVID‐19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care MedicineOnline ahead of print, 46, 1099–1102. 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni, L. , Coppola, S. , Cressoni, M. , Busana, M. , Rossi, S. , & Chiumello, D. (2020). Covid‐19 does not lead to a “typical” acute respiratory distress syndrome. American Journal of Respiratory and Critical Care MedicineOnline ahead of print, 201, 1299–1300. 10.1164/rccm.202003-0817LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani, A. L. , Berchan, M. , Sanz, J. M. , Passaro, A. , Pizzicotti, S. , Vultaggio‐Poma, V. , … di Virgilio, F. (2019). The P2X7 receptor is shed into circulation: Correlation with C‐reactive protein levels. Frontiers in Immunology, 10, 793 10.3389/fimmu.2019.00793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y. R. , Cao, Q. D. , Hong, Z. S. , Tan, Y. Y. , Chen, S. D. , Jin, H. J. , … Yan, Y. (2020). The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak—An update on the status. Military Medical Research, 7, 11 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res., 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, L. A. , Canna, S. W. , Schulert, G. S. , Volpi, S. , Lee, P. Y. , Kernan, K. F. , … Nigrovic, P. A. (2020). On the alert for cytokine storm: Immunopathology in COVID‐19. Arthritis & Rhematology[Epub ahead of print], 72, 1059–1063. 10.1002/art.41285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengling, F. D. , Maldonado, A. , Wuest, F. , & Schindler, T. H. (2020). The role of nuclear medicine for COVID‐19—Time to act now. Journal of Nuclear Medicine, 781–782. 10.2967/jnumed.120.246611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki, N. , Warming, S. , Lamkanfi, M. , Vande Walle, L. , Louie, S. , Dong, J. , … Dixit, V. M. (2011). Non‐canonical inflammasome activation targets caspase‐11. Nature, 479, 117–121. 10.1038/nature10558 [DOI] [PubMed] [Google Scholar]
- Magagnoli, J. , Narendran, S. , Pereira, F. , Cummings, T. , Hardin, J. W. , Sutton, S. S. , & Ambati, J. (2020). Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid‐19 medRxiv preprint. 10.1101/2020.04.16.20065920 [DOI] [PMC free article] [PubMed]
- Martínez‐García, J. J. , Martínez‐Banaclocha, H. , Angosto‐Bazarra, D. , de Torre‐Minguela, C. , Baroja‐Mazo, A. , Alarcón‐Vila, C. , … Pelegrin, P. (2019). P2X7 receptor induces mitochondrial failure in monocytes and compromises NLRP3 inflammasome activation during sepsis. Nature Communications, 10, 2711 10.1038/s41467-019-10626-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, P. , McAuley, D. F. , Brown, M. , Sanchez, E. , Tattersall, R. S. , & Manson, J. J. (2020). COVID‐19: Consider cytokine storm syndromes and immunosuppression. Lancet, 395, 1033–1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monção‐Ribeiro, L. C. , Faffe, D. S. , Santana, P. T. , Vieira, F. S. , da Graça, C. L. , Marques‐da‐Silva, C. , … Coutinho‐Silva, R. (2014). P2X7 receptor modulates inflammatory and functional pulmonary changes induced by silica. PLoS ONE, 9, e110185 10.1371/journal.pone.0110185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteagudo, L. A. , Boothby, A. , & Gertner, E. (2020). Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatology[Epub ahead of print], 2, 276–282. 10.1002/acr2.11135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04275414 . https://clinicaltrials.gov/ct2/results?cond=&term=NCT04275414.
- Nieto‐Torres, J. L. , Verdiá‐Báguena, C. , Jimenez‐Guardeño, J. M. , Regla‐Nava, J. A. , Castaño‐Rodriguez, C. , Fernandez‐Delgado, R. , … Enjuanes, L. (2015). Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology, 485, 330–339. 10.1016/j.virol.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riteau, N. , Gasse, P. , Fauconnier, L. , Gombault, A. , Couegnat, M. , Fick, L. , … Couillin, I. (2010). Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. American Journal of Respiratory and Critical Care Medicine, 182, 774–783. 10.1164/rccm.201003-0359OC [DOI] [PubMed] [Google Scholar]
- Ruan, Q. , Yang, K. , Wang, W. , Jiang, L. , & Song, J. (2020). Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine[Epub aheadof print], 46, 846–848. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, N. , Bai, H. , Chen, X. , Gong, J. , Li, D. , & Sun, Z. (2020). Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of Thrombosis and Haemostasis[Epub ahead of print], 18, 1094–1099. 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, N. , Li, D. , Wang, X. , & Sun, Z. (2020). Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis, 18, 844–847. 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolle, L. B. , & Standiford, T. J. (2013). Danger‐associated molecular patterns (DAMPs) in acute lung injury. The Journal of Pathology, 229, 145–156. 10.1002/path.4124 [DOI] [PubMed] [Google Scholar]
- Wang, S. , Zhao, J. , Wang, H. , Liang, Y. , Yang, N. , & Huang, Y. (2015). Blockage of P2X7 attenuates acute lung injury in mice by inhibiting NLRP3 inflammasome. International Immunopharmacology, 27, 38–45. 10.1016/j.intimp.2015.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]


