Abstract
Coronavirus disease 2019 (COVID‐19), the disease resulting from infection by a novel coronavirus, SARS‐Cov2, has rapidly spread since November 2019 leading to a global pandemic. SARS‐Cov2 has infected over four million people and caused over 290,000 deaths worldwide. Although most cases are mild, a subset of patients develop a severe and atypical presentation of acute respiratory distress syndrome (ARDS) that is characterised by a cytokine release storm (CRS). Paradoxically, treatment with anti‐inflammatory agents and immune regulators has been associated with worsening of ARDS. We hypothesize that the intrinsic circadian clock of the lung and the immune system may regulate individual components of CRS, and thus, chronotherapy may be used to effectively manage ARDS in COVID‐19 patients.
LINKED ARTICLES
This article is part of a themed issue on The Pharmacology of COVID‐19. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.21/issuetoc
Abbreviations
- ACTH
adrenocorticotropic hormone
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- Bmal1
brain and muscle ARNT‐like 1
- CIITA
class II, major histocompatibility complex, trans activator
- COVID‐19
coronavirus disease 2019
- CRS
cytokine release storm
- G‐CSF
granulocyte colony‐stimulating factor
- GM‐CSF
granulocyte macrophage colony‐stimulating factor
- HPA
hypothalamic–pituitary–adrenal
- IAV
influenza A virus
- NK
natural killer
- NSAIDS
nonsteroidal anti‐inflammatory drug(s)
- Per2
period circadian regulator 2
- SARS‐Cov
severe acute respiratory syndrome coronavirus
- SCN
suprachiasmatic nuclei
- TCR
T‐cell receptor
- TLR4
toll‐like receptor 4
- VILI
ventilator induced lung injury
1. INTRODUCTION
Since December 2019, SARS‐Cov2 has spread rapidly leading to a global pandemic of COVID‐19 (Guo et al., 2020). Although COVID‐19 is mild in the majority of cases, a subset of patients quickly develop acute respiratory distress syndrome (ARDS), a clinical presentation of acute lung injury (ALI), that leads to respiratory failure requiring mechanical ventilation (Fung, Yuen, Ye, Chan, & Jin, 2020). This unprecedented crisis is equal only in magnitude to the 1918 influenza pandemic (Taubenberger & Morens, 2006). Regrettably, despite important medical and technological advancements since then, our approach to treating patients with acute lung injury following influenza or SARS‐Cov2 infection remain palliative at best, with no proven pharmacological therapies (Mehta et al., 2020).
A central challenge for the development of therapies that target ARDS is the myriad of pro‐inflammatory mediators that are released during ARDS (Conti et al., 2020). This response in COVID‐19‐induced ARDS has been termed as a cytokine release storm (CRS) (Mehta et al., 2020; Ruan, Yang, Wang, Jiang, & Song, 2020). The CRS seen in severe cases of COVID‐19 include high numbers neutrophils and low levels of lymphocytes, as well as elevated serum levels of IL‐1β, IL‐2, IL‐6, IL‐8, IL‐9, IL‐10, IL‐17, G‐CSF, GM‐CSF, IFNγ, TNFα, CXCL10 (IP10), and CCL2 (MCP1) (Huang et al., 2020; Ruan et al., 2020). Thus, understanding the mechanisms that modulate the release of these pro‐inflammatory mediators in ALI is paramount to developing effective strategies to treat ARDS.
There is an outstanding paradox that COVID‐19 causes a CRS that is associated with increased lethality, although reports suggest that anti‐inflammatory drugs such as ibuprofen could aggravate the progression of disease. Further, recent studies in respiratory infections have shown that while anti‐inflammatory agents could alleviate symptoms, they can also promote increased viral shedding (Walsh et al., 2016). Even though this possibility has not been confirmed with COVID‐19, it is very plausible that this is the reason why NSAIDS could be detrimental. In this sense, ideal management of COVID‐19 would entail a reduction of harmful inflammatory mediators that damage the host but maintain expression of key mediators that target the virus.
An emerging avenue of therapeutic development involves the circadian clock, the biological timer that has been shown to control the rhythmic expression and release of many cytokines in inflammatory settings (Labrecque & Cermakian, 2015; Thompson, Walmsley, & Whyte, 2014). However, despite the known effects of the circadian clock in lung diseases such as asthma (Clark, 1987), how the circadian clock influences the progression of ALI remains largely unknown.
Studying the circadian rhythm of lung injury, secondary to ventilation therapy (ventilator induced lung injury, VILI), is a current concern for COVID‐19. Circadian rhythm disruption was seen in a rat model of VILI with high and low tidal volumes by studying the expression of Bmal1, clock, Per2, and REV‐ERBα mRNA expression. REV‐ERBα was found to have an important role in VILI and inflammation. That is, circadian rhythm disorder in inflammation response may be a novel pathogenesis of VILI (Li, Wang, Hu, & Tan, 2013). Club cells have been also found to have a role in lung circadian rhythm. Selective ablation of these cells resulted in the loss of circadian rhythm in lung slices, further demonstrating the importance of this cell type in maintaining pulmonary circadian rhythm in one murine and human lung tissue study (Gibbs et al., 2009).
The immune system displays circadian rhythms, for instance at the beginning of daily activity, there is increased expression of pro‐inflammatory mediators such as IL‐1β, IL‐6, IL‐12, TLR9 and TLR4, CCL2, CXCL1, and CCL5, as well as macrophage and leukocyte activity, which leads to potential damage in injured tissues. By contrast, anti‐inflammatory mediators and other growth or angiogenesis factors, such as the VEGF, peak during the resting phase (Curtis, Bellet, Sassone‐Corsi, & O'Neill, 2014; Koyanagi et al., 2003; Liu et al., 2006; Nakamura et al., 2016; Scheiermann, Gibbs, Ince, & Loudon, 2018; Vgontzas et al., 2005). Moreover, in both CD8 and CD4 T cells, the cytotoxic activity against viral antigens reached the highest levels during the resting phase (Bollinger et al., 2011; Nobis et al., 2019) while the cytotoxic activity of natural killer (NK) cells was greatest at the beginning of the active part of the day (Logan et al., 2012).
The lungs also have an intrinsic circadian clock which plays a key role in inflammation and leukocyte migration in the lungs, as well as in many lung diseases including viral pneumonia (Nosal, Ehlers, & Haspel, 2019; Sundar, Yao, Sellix, & Rahman, 2015).
Circadian rhythms in viral respiratory illness have been so far examined in mice for parainfluenza and influenza A viruses (IAV), which cause bronchiolitis and pneumonia in humans, respectively. With either viruses, acute inflammatory responses, but not the peak viral load, vary with the time of inoculation in wild type mice (Ehlers et al., 2018; Sengupta et al., 2019). Similarly, deletion of the clock gene Bmal1 worsens acute lung injury and lung inflammation in response to parainfluenza or IAV, suggesting that circadian clocks may play a role (immunological or otherwise) in the resolution of viral pneumonia. Additionally, there is strong evidence from animal models that the circadian regulation within the lung is important in the likelihood of developing chronic lung disease such as pulmonary fibrosis in the aftermath of the infections.
A key regulator of the circadian clock is the hypothalamic–pituitary–adrenal (HPA) axis (Tsang, Astiz, Friedrichs, & Oster, 2016). The suprachiasmatic nuclei (SCN) neurons in the hypothalamus is the central clock that modulates the peripheral clocks via the HPA axis, through the adrenocorticotropic hormone (ACTH), the sympathetic nervous system (SNS), and the subsequent rhythmic release of hormones (glucocorticoids, adrenaline and noradrenaline) from the adrenal glands. This ultimately regulates the rhythm of the internal clocks genes found in each cell such as Bmal1 and Per2 (Scheiermann, Kunisaki, & Frenette, 2013).
Interestingly, autopsies from patients who died in the SARS epidemic of 2003 revealed the presence of SARS‐Cov in the adrenal glands concomitant with degeneration and necrosis (Pal, 2020). Further, SARS‐Cov expresses amino acid sequences with homology to the host ACTH hormone (Wheatland, 2004). Thus, antibodies produced by the host to target the virus may also attack the host ACTH, resulting in cortisol insufficiency (Wheatland, 2004). Also, a potential mechanism that results in dysregulation of the circadian clock following SARS‐Cov2 infection may be through its effect on dampening the HPA axis and ACTH (Pal, 2020) as well clock gene alterations due to mechanical ventilation (Li et al., 2013). Present studies are underway to measure serum cortisol and ACTH levels that will help elucidate the mechanisms leading to circadian clock dysfunction in severe COVID‐19 cases (Pal, 2020).
Moreover, the time of the day in which a viral infection occurs affects survival. For instance, infections at the beginning of the activity phase are more fatal than infections that occur at the beginning of the resting phase (Sengupta et al., 2019). Evidence indicates an essential role of the circadian rhythm of NK cells underlying this temporal pattern. These temporal patterns may have important implications for shift workers, where ablated cortisol levels (Kudielka, Buchtal, Uhde, & Wust, 2007) and higher incidences of cardiovascular complications are reported (Vyas et al., 2012). Therefore, the time of exposure and viral load of SARS‐Cov2 infection may result in worsening effects in shift workers, in particular healthcare workers where up to 20% of the responding health care workers were infected in Italy (Lancet, 2020).
Due to circadian variations of the immune system and the lungs, the effect of immune modulators and anti‐inflammatory agents on the activity of cells and cytokines in injured tissues also depend on the time of the day in which these medications are taken (Al‐Waeli et al., 2020). It is plausible that a proper circadian timing of anti‐inflammatory drugs (chronotherapy) can target the detrimental inflammatory cascade in COVID‐ 19 patients without interfering with the fight of the immune system against the virus. For instance, the circadian time of drug administration will differentially affect various cytokines involved in viral immunity and COVID‐19, including among others CXCL10, IL‐1β, IL‐4, IL‐8, IL‐10, TCR, INF‐α, CIITA, TNFα, and TLR as well as the activity of T cells (CD8), NK cells, and B cells (Al‐Waeli et al., 2020; Canan et al., 2014). In line with these findings, a recent study on a bone injury model, showed that cytokines and clock genes could be effectively regulated by the timing of NSAIDs administration (Al‐Waeli et al., 2020). More importantly, the cytokines that were influenced by timed administration of drugs coincide with those involved in the CRS in COVID 19 (IL‐1 β, IL‐8, IL‐10R, IL‐6R, and TNFα), as well as the activity of immune cells involved in the anti‐viral immune response (CD8, NK, and B cells).
Based on the known circadian peak (point of culmination of an oscillatory function) and circadian through (lowest value of an oscillatory function) for known detrimental (Table 1) or beneficial (Table 2) inflammatory mediators identified in COVID‐19 patients, treatment could be optimised for chronotherapy. Chronotherapy include both chronopharmacokinetics and chronopharmacodynamics where circadian oscillations in the expression levels of the drug and target are taken into consideration to maximise drug efficacy (Levi & Schibler, 2007).
TABLE 1.
Detrimental inflammatory mediators in the cytokine release storm (CRS) in COVID‐19
| PEAK | Trough | Reference | |
|---|---|---|---|
| IL‐1β | Bedtime | Early morning | (Einhorn, Majeska, Rush, Levine, & Horowitz, 1995) |
| IL‐2 | 1200 h | (Young et al., 1995) | |
| IL‐6 |
First peak 1900 h Second peak 0500 h |
(Vgontzas et al., 2005) | |
| IL‐8 |
First peak 1000 h Second peak 2100 h |
(Rahman et al., 2015) | |
| IL‐10 a |
First peak 0730 h Second peak 1930 h |
(Young et al., 1995) | |
| G‐CSF | 2200 h | (Jilma et al., 1999) | |
| GM‐CSF |
First peak 1330 h Second peak 1930–2330 h |
(Young et al., 1995), (Rahman et al., 2015; Young et al., 1995), | |
| TNFα |
First peak 0730 h Second peak 1200–1330 h |
(Young et al., 1995); (Young et al., 1995; Zabel, Linnemann, & Schlaak, 1993) | |
| CCL2 | 0200 h | (Rahman et al., 2015) |
Involved both in the “cytokine storm” and in the anti‐viral response.
TABLE 2.
Beneficial inflammatory mediators in the cytokine release storm (CRS) in COVID‐19
| Peak | Trough | Reference | |
|---|---|---|---|
| B cells | 0200–0300 h | 1100 h | (Born, Lange, Hansen, Molle, & Fehm, 1997) |
| T cells: Naive, central memory, effector memory CD4+ and CD8+ T cells | 0200 h | 1400 h | (Dimitrov et al., 2009) |
| Effector CD8+ T cells | 1400 h | 0200 h | (Dimitrov et al., 2009) |
| NK cells | 1100–1400 h | 0200 h | (Born et al., 1997) |
| IL‐10 a | First peak 0730 hSecond peak 1930 h | (Young et al., 1995) | |
| IFNγ | 0000–0300 h | 0800–1100 h | (Petrovsky & Harrison, 1998; Petrovsky, McNair, & Harrison, 1998) |
Involved both in the cytokine storm and in the anti‐viral response.
2. CONCLUSIONS
Thus, adjusting the timing of the day in which the medications are given to result in highest drug levels at the time point when detrimental inflammatory mediators reach their Peak (Figure 1). This would mean that afternoon is the preferred time window for drug administration whereas intake at night should be avoided. This is particularly important when administering immune modulators where a single dose is usually given. Furthermore, the goal of chronotherapy in COVID‐19 is to avoid reaching steady‐state drug levels; as in the case of anti‐inflammatory therapy, these would dampen the inflammatory response directed towards the virus.
FIGURE 1.
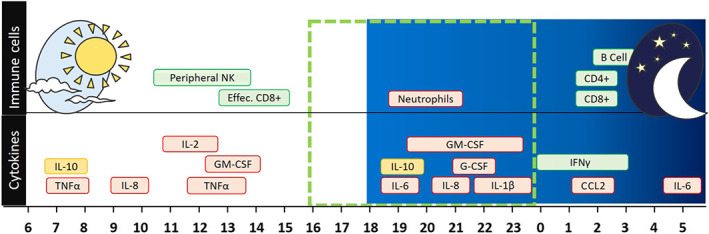
Diagram showing , over 24 h, the peak of cytokines and immune responses involved in the cytokine release storm (CRS) of COVID‐19. Peak detrimental inflammatory mediators (red) and beneficial adaptive immune response against viral infections (green) are shown during a 24‐h time period. The dotted line and the red clock indicate the period between 4:00 p.m. and midnight in which the detrimental effects of CRS outweighs the beneficial effect of the adaptive immune response. This period of time could be the ideal target for anti‐inflammatory treatments. On the other hand, the green clocks indicate the periods of anti‐viral activity of the immune system; these are the periods in which anti‐inflammatory therapy should be avoided
2.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Cidlowski et al., 2019; Alexander, Fabbro et al., 2019; Alexander, Kelly et al., 2019).
CONFLICT OF INTEREST
There are no competing insterests to delcare.
ACKOWLEDGEMENTS
This study was funded by American Heart Association Innovative Project Award 18IPA34170220 to H.K.‐Q. The Welch Foundation AU‐1971‐20180324 to S‐H. Y. and AU‐1731‐20190330 to Z.C., and Canadian Institute for Health Research CIHR PJT‐168875 and the Canada Research Chair program to F.T.
Tamimi F, Abusamak M, Akkanti B, Chen Z, Yoo S‐H, Karmouty‐Quintana H. The case for chronotherapy in Covid‐19‐induced acute respiratory distress syndrome. Br J Pharmacol. 2020;177:4845–4850. 10.1111/bph.15140
Contributor Information
Faleh Tamimi, Email: faleh.tamimimarino@mcgill.ca, Email: fmarino@edu.qu.qa.
Seung‐Hee Yoo, Email: seung-hee.yoo@uth.tmc.edu.
Harry Karmouty‐Quintana, Email: harry.karmouty@uth.tmc.edu.
REFERENCES
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Nuclear hormone receptors. British Journal of Pharmacology, 176, S229–S246. 10.1111/bph.14750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Faccenda, E. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Other Protein Targets. British Journal of Pharmacology, 176, S1–S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Waeli, H. , Nicolau, B. , Stone, L. , Nada, L. A. , Gao, Q. , Abdallah, M. , … Tamimi, F. (2020). Chronotherapy of non‐steroidal anti‐inflammatory drugs may enhance postoperative recovery. Scientific Reports, 10, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger, T. , Leutz, A. , Leliavski, A. , Skrum, L. , Kovac, J. , Bonacina, L. , … Solbach, W. (2011). Circadian clocks in mouse and human CD4+ T cells. PLoS ONE, 6, e29801 10.1371/journal.pone.0029801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born, J. , Lange, T. , Hansen, K. , Molle, M. , & Fehm, H. L. (1997). Effects of sleep and circadian rhythm on human circulating immune cells. Journal of Immunology, 158, 4454–4464. [PubMed] [Google Scholar]
- Canan, C. H. , Gokhale, N. S. , Carruthers, B. , Lafuse, W. P. , Schlesinger, L. S. , Torrelles, J. B. , & Turner, J. (2014). Characterization of lung inflammation and its impact on macrophage function in aging. Journal of Leukocyte Biology, 96, 473–480. 10.1189/jlb.4A0214-093RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, T. (1987). Diurnal rhythm of asthma. Chest, 91, 137S–141S. 10.1378/chest.91.6_Supplement.137S [DOI] [PubMed] [Google Scholar]
- Conti, P. , Ronconi, G. , Caraffa, A. , Gallenga, C. , Ross, R. , Frydas, I. , & Kritas, S. K. (2020). Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by COVID‐19: Anti‐inflammatory strategies. Journal of Biological Regulators and Homeostatic Agents, 34. [DOI] [PubMed] [Google Scholar]
- Curtis, A. M. , Bellet, M. M. , Sassone‐Corsi, P. , & O'Neill, L. A. (2014). Circadian clock proteins and immunity. Immunity, 40, 178–186. 10.1016/j.immuni.2014.02.002 [DOI] [PubMed] [Google Scholar]
- Dimitrov, S. , Benedict, C. , Heutling, D. , Westermann, J. , Born, J. , & Lange, T. (2009). Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood, the Journal of the American Society of Hematology, 113, 5134–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers, A. , Xie, W. , Agapov, E. , Brown, S. , Steinberg, D. , Tidwell, R. , … Haspel, J. A. (2018). BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunology, 11, 97–111. 10.1038/mi.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn, T. A. , Majeska, R. J. , Rush, E. B. , Levine, P. M. , & Horowitz, M. C. (1995). The expression of cytokine activity by fracture callus. Journal of Bone and Mineral Research, 10, 1272–1281. [DOI] [PubMed] [Google Scholar]
- Fung, S.‐Y. , Yuen, K.‐S. , Ye, Z.‐W. , Chan, C.‐P. , & Jin, D.‐Y. (2020). A tug‐of‐war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: Lessons from other pathogenic viruses. Emerging Microbes & Infections, 9, 558–570. 10.1080/22221751.2020.1736644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, J. E. , Beesley, S. , Plumb, J. , Singh, D. , Farrow, S. , Ray, D. W. , & Loudon, A. S. I. (2009). Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology, 150, 268–276. 10.1210/en.2008-0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y. R. , Cao, Q. D. , Hong, Z. S. , Tan, Y. Y. , Chen, S. D. , Jin, H. J. , … Yan, Y. (2020). The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak—An update on the status. Military Medical Research, 7, 11 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res., 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395, 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilma, B. , Hergovich, N. , Stohlawetz, P. , Eichler, H. G. , Bauer, P. , & Wagner, O. (1999). Circadian variation of granulocyte colony stimulating factor levels in man. British Journal of Haematology, 106, 368–370. 10.1046/j.1365-2141.1999.01543.x [DOI] [PubMed] [Google Scholar]
- Koyanagi, S. , Kuramoto, Y. , Nakagawa, H. , Aramaki, H. , Ohdo, S. , Soeda, S. , & Shimeno, H. (2003). A molecular mechanism regulating circadian expression of vascular endothelial growth factor in tumor cells. Cancer Research, 63, 7277–7283. [PubMed] [Google Scholar]
- Kudielka, B. M. , Buchtal, J. , Uhde, A. , & Wust, S. (2007). Circadian cortisol profiles and psychological self‐reports in shift workers with and without recent change in the shift rotation system. Biological Psychology, 74, 92–103. 10.1016/j.biopsycho.2006.08.008 [DOI] [PubMed] [Google Scholar]
- Labrecque, N. , & Cermakian, N. (2015). Circadian clocks in the immune system. Journal of Biological Rhythms, 30, 277–290. 10.1177/0748730415577723 [DOI] [PubMed] [Google Scholar]
- Lancet, T. (2020). COVID‐19: Protecting health‐care workers. Lancet (London, England), 395, 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi, F. , & Schibler, U. (2007). Circadian rhythms: Mechanisms and therapeutic implications. Annual Review of Pharmacology and Toxicology, 47, 593–628. 10.1146/annurev.pharmtox.47.120505.105208 [DOI] [PubMed] [Google Scholar]
- Li, H. , Wang, C. , Hu, J. , & Tan, J. (2013). A study on circadian rhythm disorder of rat lung tissue caused by mechanical ventilation induced lung injury. International Immunopharmacology, 249–254. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Mankani, G. , Shi, X. , Meyer, M. , Cunningham‐Runddles, S. , Ma, X. , & Sun, Z. S. (2006). The circadian clock period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide‐induced endotoxic shock. Infection and Immunity, 74, 4750–4756. 10.1128/IAI.00287-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, R. W. , Zhang, C. , Murugan, S. , O'Connell, S. , Levitt, D. , Rosenwasser, A. M. , & Sarkar, D. K. (2012). Chronic shift‐lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. The Journal of Immunology, 188, 2583–2591. 10.4049/jimmunol.1102715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, P. , McAuley, D. F. , Brown, M. , Sanchez, E. , Tattersall, R. S. , & Manson, J. J. (2020). COVID‐19: Consider cytokine storm syndromes and immunosuppression. The Lancet, 395, 1033–1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, Y. , Nakano, N. , Ishimaru, K. , Ando, N. , Katoh, R. , Suzuki‐Inoue, K. , … Nakao, A. (2016). Inhibition of IgE‐mediated allergic reactions by pharmacologically targeting the circadian clock. Journal of Allergy and Clinical Immunology, 137, 1226–1235. 10.1016/j.jaci.2015.08.052 [DOI] [PubMed] [Google Scholar]
- Nobis, C. C. , Laramée, G. D. , Kervezee, L. , De Sousa, D. M. , Labrecque, N. , & Cermakian, N. (2019). The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proceedings of the National Academy of Sciences, 116, 20077–20086. 10.1073/pnas.1905080116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosal, C. , Ehlers, A. , & Haspel, J. A. (2019). Why lungs keep time: Circadian rhythms and lung immunity. Annual Review of Physiology, 82, 391–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal, R. (2020). COVID‐19, hypothalamo‐pituitary‐adrenal axis and clinical implications. Endocrine, 68, 251–252. 10.1007/s12020-020-02325-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky, N. , & Harrison, L. C. (1998). The chronobiology of human cytokine production. International Reviews of Immunology, 16, 635–649. 10.3109/08830189809043012 [DOI] [PubMed] [Google Scholar]
- Petrovsky, N. , McNair, P. , & Harrison, L. C. (1998). Diurnal rhythms of pro‐inflammatory cytokines: Regulation by plasma cortisol and therapeutic implications. Cytokine, 10, 307–312. 10.1006/cyto.1997.0289 [DOI] [PubMed] [Google Scholar]
- Rahman, S. A. , Castanon‐Cervantes, O. , Scheer, F. A. , Shea, S. A. , Czeisler, C. A. , Davidson, A. J. , & Lockley, S. W. (2015). Endogenous circadian regulation of pro‐inflammatory cytokines and chemokines in the presence of bacterial lipopolysaccharide in humans. Brain, Behavior, and Immunity, 47, 4–13. 10.1016/j.bbi.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan, Q. , Yang, K. , Wang, W. , Jiang, L. , & Song, J. (2020). Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine, 46(5), 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann, C. , Gibbs, J. , Ince, L. , & Loudon, A. (2018). Clocking in to immunity. Nature Reviews Immunology, 18, 423–437. 10.1038/s41577-018-0008-4 [DOI] [PubMed] [Google Scholar]
- Scheiermann, C. , Kunisaki, Y. , & Frenette, P. S. (2013). Circadian control of the immune system. Nature Reviews Immunology, 13, 190–198. 10.1038/nri3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta, S. , Tang, S. Y. , Devine, J. C. , Anderson, S. T. , Nayak, S. , Zhang, S. L. , … Ga, F. G. (2019). Circadian control of lung inflammation in influenza infection. Nature Communications, 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar, I. K. , Yao, H. , Sellix, M. T. , & Rahman, I. (2015). Circadian molecular clock in lung pathophysiology. American Journal of Physiology Lung Cellular and Molecular Physiology, 309, L1056–L1075. 10.1152/ajplung.00152.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger, J. K. , & Morens, D. M. (2006). 1918 Influenza: the mother of all pandemics. Emerging Infectious Diseases, 12, 15–22. 10.3201/eid1209.05-0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, A. A. , Walmsley, S. R. , & Whyte, M. K. (2014). A local circadian clock calls time on lung inflammation. Nature Medicine, 20, 809–811. 10.1038/nm.3649 [DOI] [PubMed] [Google Scholar]
- Tsang, A. H. , Astiz, M. , Friedrichs, M. , & Oster, H. (2016). Endocrine regulation of circadian physiology. The Journal of Endocrinology, 230, R1–r11. 10.1530/JOE-16-0051 [DOI] [PubMed] [Google Scholar]
- Vgontzas, A. N. , Bixler, E. O. , Lin, H. M. , Prolo, P. , Trakada, G. , & Chrousos, G. P. (2005). IL‐6 and its circadian secretion in humans. Neuroimmunomodulation, 12, 131–140. 10.1159/000084844 [DOI] [PubMed] [Google Scholar]
- Vyas, M. V. , Garg, A. X. , Iansavichus, A. V. , Costella, J. , Donner, A. , Laugsand, L. E. , … Hackam, D. G. (2012). Shift work and vascular events: Systematic review and meta‐analysis. BMJ (Clinical Research Ed), 345, e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, P. , Behrens, N. , Chaigneau, F. R. C. , McEligot, H. , Agrawal, K. , Newman, J. W. , … Gershwin, L. J. (2016). A randomized placebo controlled trial of ibuprofen for respiratory syncytial virus infection in a bovine model. PLoS ONE, 11(4), e0152913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatland, R. (2004). Molecular mimicry of ACTH in SARS—Implications for corticosteroid treatment and prophylaxis. Medical Hypotheses, 63, 855–862. 10.1016/j.mehy.2004.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, M. R. I. , Matthews, J. P. , Kanabrocki, E. L. , Sothern, R. B. , Roitman‐Johnson, B. , & Scheving, L. E. (1995). Circadian rhythmometry of serum interleukin‐2, interleukin‐10, tumor necrosis factor‐α, and granulocyte‐macrophage colony‐stimulating factor in men. Chronobiology International, 12, 19–27. 10.3109/07420529509064496 [DOI] [PubMed] [Google Scholar]
- Zabel, P. , Linnemann, K. , & Schlaak, M. (1993). Circadian rhythm in cytokines. Immunität Und Infektion, 21(Suppl 1), 38–40. [PubMed] [Google Scholar]


