Abstract
Background
Objective data on chemosensitive disorders during COVID‐19 are lacking in the Literature.
Methods
Multicenter cohort study that involved four Italian hospitals. Three hundred and forty‐five COVID‐19 patients underwent objective chemosensitive evaluation.
Results
Chemosensitive disorders self‐reported by 256 patients (74.2%) but the 30.1% of the 89 patients who did not report dysfunctions proved objectively hyposmic. Twenty‐five percentage of patients were seen serious long‐lasting complaints. All asymptomatic patients had a slight lowering of the olfactory threshold. No significant correlations were found between the presence and severity of chemosensitive disorders and the severity of the clinical course. On the contrary, there is a significant correlation between the duration of the olfactory and gustatory symptoms and the development of severe COVID‐19.
Conclusions
Patients under‐report the frequency of chemosensitive disorders. Contrary to recent reports, such objective testing refutes the proposal that the presence of olfactory and gustatory dysfunction may predict a milder course, but instead suggests that those with more severe disease neglect such symptoms in the setting of severe respiratory disease.
Keywords: ageusia, anosmia, COVID‐19, smell, taste
1. INTRODUCTION
Both its high pathogenicity and easy person‐to‐person transmission have allowed SARS‐CoV‐2 to spread rapidly worldwide. 1 Moreover, asymptomatic and paucisymptomatic patients, which represent up to four fifths of cases, facilitate silent transmission. 2 The identification and isolation of these patients is therefore essential to slow down the chain of infection. To achieve this goal, it is mandatory to establish what the most frequent signs and symptoms may be in the early stages of the disease or in paucisymptomatic patients.
Olfactory and gustatory disturbances were reported as a rare clinical finding in Chinese case series on COVID‐19, affecting only 5% of the patients. 3 On the contrary, since the first outbreaks of the epidemic in Europe, we realized that these disorders were reported by many more COVID‐19 patients. 4 In the past few weeks, several authors have reported similar findings.5, 6, 7, 8, 9, 10 In particular, these symptoms seem to be common in the early stages of the disease and in paucisymptomatic patients. 11 However, all these reports are anamnestic or observational while the literature lacks objective studies that evaluate the gustatory and olfactory function in these patients. Objective evaluation with validated, repeatable and standardized tests is crucial to establish exactly what the frequency, extent, clinical characteristics of these disorders are and then monitor their course over time. Moreover, understanding mechanisms of sensorineural olfactory and gustatory loss with SARS‐CoV‐2 infections might provide novel insights into aspects of viral pathogenesis.
Psychophysical tests represent one of the cornerstone in the evaluation of chemosensitive functions. 12 Using this test methodology, we recently published a preliminary study on a restricted series of hospitalized patients objectively assessed at the University Hospital of Sassari. 13 However, the majority of tests currently reported in the literature can only be administered in person during the patient's visit and have limited availability. This limitation does not allow assessment of all cases in home quarantine, excluding from the objective evaluation the majority of patients in the initial stages of the disease, paucisymptomatic or with mild and moderate forms of COVID‐19. To overcome this limitation, we have recently proposed and validated a new psychophysical test that can be self‐administered by the patient at home. 14 The scoring system of this new test, can produce scores on a scale of values equal to that provided by the operator‐administered test. For this reason, the results of the two tests are directly comparable.
In this study, we report and analyze the results of a large Italian multicenter study that objectively investigated chemoreceptive disorders in COVID‐19 patients.
2. MATERIALS AND METHODS
The multicenter study was conducted in four Italian hospitals: Sassari University Hospital, Salerno University Hospital, San Paolo Hospital in Milan, Bellaria‐Maggiore Hospital in Bologna.
The olfactory and gustatory functions were objectively evaluated in two groups of patients:
Group quarantine: home quarantined health care workers, with nasopharyngeal swab positive for SARS‐CoV‐2 infection.
Group hospitalized: hospitalized patients with nasopharyngeal swab positive for SARS‐CoV‐2 infection.
The exclusion criteria for both groups, included: patients under 18 years of age, psychiatric or neurological diseases, assisted ventilation or other causes of poor compliance, previous trauma, surgery or radiotherapy in the oral and nasal cavities, pre‐existing test or smell dysfunctions, history of allergic rhinitis or chronic rhinosinusitis.
All patients provided informed consent for participation in the study. The evaluation protocol was approved by an independent ethics committee (No. 378‐2020‐OSS‐AUSLBO).
Preliminarily, some anamnestic data were collected for all patients in both groups: gender, age, comorbidity or conditions that could be cause for exclusion, which symptoms were seen and when these symptoms started, when positivity to the first nasopharyngeal swab was confirmed. Following the criteria proposed by Tian et al 15 subjects were divided into four groups of clinical severity: asymptomatic, mild (mild symptoms, no radiological evidence of pneumonia), moderate (radiological evidence of pneumonia without dyspnea or respiratory failure), and severe (radiological evidence of pneumonia with dyspnea and respiratory failure). All patients were carefully investigated for any previous or current presence of chemosensitive disorders during the SARS‐CoV‐2 infection.
2.1. Group quarantined functional assessment
The patient cohort was composed of health personnel (doctors, paramedics, nurses, auxiliary staff) in home quarantine, after positivity for SARS‐CoV‐2 confirmed to the nasopharyngeal swab. This group of patients was composed both of cases in which the swab was performed following the onset of suspicious symptoms, and of asymptomatic patients with positivity detected incidentally to the routine swab.
The patients were contacted by phone, the operator explained the study methodology and asked the subjects to collect all the materials necessary for its correct execution, thus contacting the operator again to perform the telephone test. The olfactory threshold was determined using nine solutions with increasing concentration of denatured ethyl‐alcohol. The olfactory discriminative capability was instead tested by means of seven groups of odorants, for each of which the patient expressed an evaluation from 0 (no discrimination) to 10 (normal discrimination). Finally, the gustatory function was assessed for each of the primary tastes by means of solutions prepared by the patient. 16 The evaluation methodology and the scoring system have been previously described in detail in the paper in which this test has been validated. 14
2.2. Group hospitalized functional assessment
Both the olfactory threshold and the odor discriminative ability were assessed. All tests were conducted in a quiet room. Olfactory function assessment was carried out by means of the Connecticut Chemosensory Clinical Research Center orthonasal olfaction test (CCCRC).17, 18 The CCCRC is a simple, validated and widely used test, that includes a butanol threshold assessment and a 10‐items odor identification test using common odors. The same standardized and validated test that was used for the group quarantine, which investigates the ability to perceive four primary tastes (sweet, salty, sour and bitter), was performed to evaluate the gustatory function. The evaluation methodology and the scoring system have been previously described in detail. 13
2.3. Data analysis
The two tests provide standardized data on the same evaluation scale of the olfactory and gustatory function. For this reason, the data of the two groups were then analyzed together to obtain comparable information based on the stage of the disease and the characteristics of the patients. Statistical analysis was performed using SPSS 26.0 (IBM, Armonk, New York). Categorical variables are reported in numerals and percentages of the total. Descriptive statistics for quantitative variables are given as the mean ± SD (SD). The statistical analysis of differences in olfactory and gustatory function, between population subgroups, was performed using Mann‐Whitney U test and Kruskal‐Wallis H test. Fisher's exact test was used to evaluate frequency differences. The level of statistical significance was set at P ≤ .05 with a 95% confidence interval.
3. RESULTS
Three hundred and forty‐five COVID‐19 patients (146 males, 199 females, mean age 48.5 years old) who met the inclusion criteria were enrolled for the study the University Hospital of Sassari, University Hospital “San Paolo” of Milan, University Hospital of Salerno and Bellaria‐Maggiore Hospital of Bologna.
The study cohort was composed of 161 patients in home quarantine, assigned to the quarantine group and evaluated remotely, and 184 hospitalized patients, assigned to the hospitalized group and evaluated directly by the operators in the hospital. Table 1 summarizes patient general characteristics and clinical features (Table 1). The study included patients in all stages of clinical severity of the disease: 10 (2.9%) asymptomatic, 168 (48.7%) mild disease, 140 (40.6%) moderate disease, and 27 (7.8%) severe disease.
TABLE 1.
General and clinical features of the study population
| No. of patients | |
|---|---|
| Group quarantine | 161 (46.7%) |
| Group hospitalized | 184 (53.3%) |
| Total | 345 |
| Gender | |
| Male | 146 (42.3%) |
| Female | 199 (57.7%) |
| Age (years) | 48.5 ± 12.8 (range 23‐88) |
| Days from COVID‐19 symptoms onset | 14.8 ± 7.4 (range 2‐35) |
| Days from positive swab | 9.9 ± 5.8 (range 1‐28) |
| Clinical stage | |
| Asymptomatic | 10 (2.9%) |
| Mild | 168 (48.7%) |
| Moderate | 140 (40.6%) |
| Severe | 27 (7.8%) |
Chemosensitive dysfunctions during COVID‐19 have been self‐reported by 256 patients (74.2% of the study population). 79.3% of these patients reported combined chemosensitive disturbances, 8.6% isolated olfactory disorders and 12.1% isolated taste disorders. At the time of the test, the disorder was self‐reported as completely regressed in 31.3% of the patients with regard to the sense of smell and in 50.4% for the taste. The objective results derived from the olfactory and gustatory assessments of the two study groups were analyzed together. 14 A framework summary of the data obtained is reported in Table 2.
TABLE 2.
Chemosensitive self‐reported and objective findings
| Objective evaluation results | No. of patients (%) | Fisher's exact test |
|---|---|---|
| Olfactory function | ||
| Normal | 104 (30.1%) | |
| Mild hyposmia | 76 (22%) | |
| Moderate hyposmia | 59 (17.1%) | P = .000 |
| Severe hyposmia | 45 (13%) | |
| Anosmia | 61 (17.7%) | |
| Gustatory function | ||
| Normal | 190 (55.1%) | |
| Mild hypogeusia | 78 (22.6%) | |
| Moderate hypogeusia | 25 (7.2%) | |
| Severe hypogeusia | 16 (4.6%) | P = .000 |
| Ageusia | 36 (10.4%) | |
| Referred chemosensory dysfunctions | ||
| Olfactory and taste disorders | 203 (58.8%) | |
| Isolated olfactory disorder | 22 (6.4%) | |
| Objective normal | 17 (77.3%) | |
| Objective mild hypogeusia | 4 (18.2%) | P = .024 |
| Objective moderate hypogeusia | 1 (4.5%) | |
| Isolated taste disorder | 31 (9%) | |
| Objective normal olfaction | 21 (67.7%) | |
| Objective mild hyposmia | 10 (32.3%) | P = .000 |
| Total | 256 (74.2%) | P = .000 |
| No chemosensitive disorders | 89 (25.8%) | |
| Objective normal taste | 89 (100%) | P = 1.000 |
| Objective normal olfaction | 62 (69.7%) | |
| Objective mild hyposmia | 27 (30.3%) | P = .000 |
| Referred chemosensory recovery | ||
| Olfactory recovery (N = 225) | 70 (31.1%) | |
| Objective normal | 21 (30%) | |
| Objective mild hyposmia | 39 (55.7%) | P = .000 |
| Objective moderate hyposmia | 10 (14.2%) | |
| Taste recovery (N = 234) | 118 (50.4%) | |
| Objective normal | 84 (71.2%) | |
| Objective mild hypogeusia | 29 (24.6%) | P = .000 |
| Objective moderate hypogeusia | 5 (4.2%) | |
| Chemosensitive symptom duration (N = 256) | ||
| ≤7 days | 191 (74.6%) | |
| >7 days | 65 (25.4%) | P = .000 |
3.1. Olfactory function evaluation results
Sixty‐five percentage of patients reported having had olfactory disorders during the infection (Table 2). However, the evaluation of patients who reported isolated taste disorders or no dysfunction, revealed the presence of mild hyposmia in an additional 10.7% of patients (P = .000). Furthermore, 70% of patients who reported complete resolution, proved hyposmic to objective test (P = .000). The analysis of the study population subgroups, selected according to the disease duration, showed a high frequency of olfactory disorders throughout the observation period, ranging between 77.4% (day 1‐4) and 69.2% (day 25‐35) (Figure 1). In the early stages, severe dysfunctions (ie, anosmia or severe hyposmia) affected 70.9% of patients. Starting from day 10, most of the dysfunctions were instead represented by mild and moderate hyposmias (Figure 1). The average olfactory score improved rapidly in the first 10 days, reaching moderate hyposmia values that were maintained for the rest of the observation period.
FIGURE 1.
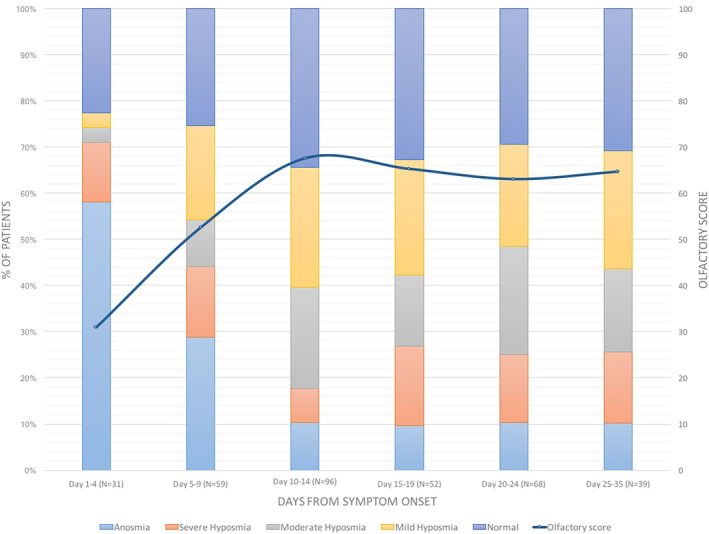
Olfactory disorders: sub‐group analysis [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Gustatory function evaluation results
Overall, 234 patients (67.8%) reported having or having had gustatory disorders during SARS‐CoV‐2 infection (Table 2). At the time of the examination, based on the taste scores obtained, ageusia was detected in 36 cases (10.4%), 119 patients were seen mild, moderate, or severe hypogeusia (34.5%) while in 190 cases (55.1%) the gustatory function was normal. Residual hypogeusia was detected in 28.8% of patients who reported complete taste recovery (Table 2). The study of gustatory function in groups of patients in different stages of the disease, revealed a significant reduction of taste disorders starting on day 10 to 14, with an average taste score that returns, in the third week, to substantially normal values (Figure 2).
FIGURE 2.
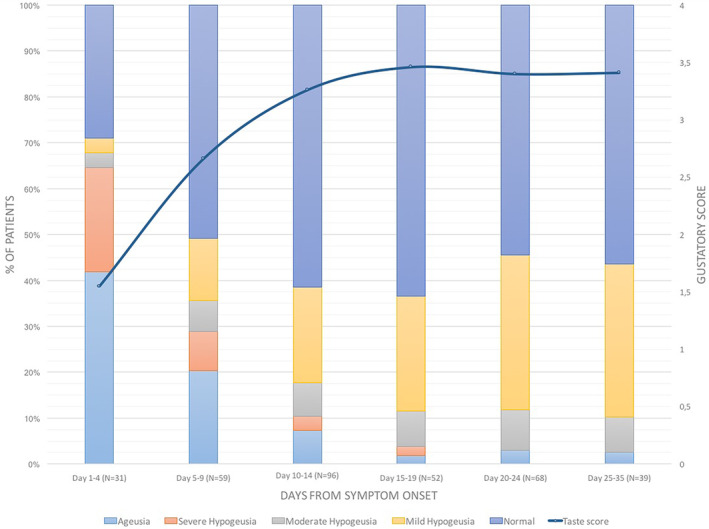
Gustatory disorders: sub‐group analysis [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Statistical analysis results
No significant correlation was found between the gustatory and olfactory scores and the gender or age of the patients (Table 3). Both the olfactory (P = .000) and gustatory (P = .001) scores showed statistically significant differences between subpopulations selected on the duration of the disease. A more detailed analysis showed that the improvement in the scores is significant between the first and second week (both olfaction and taste P = .000) of disease, but not between the second and third (olfaction P = .119, taste P = .632). No statistically significant correlation was found between the COVID‐19 severity and the presence or extent of chemosensitive disorders (Table 4). On the contrary, a duration of chemosensitive symptoms greater than 7 days, showed a statistically significant correlation (P = .000) with the development of moderate (relative risk 1.12) and severe (relative risk 2.33) COVID‐19. The latter analysis was performed only on patients with a disease duration of more than 14 days, as the first 2 weeks coincides with the higher risk period for respiratory deterioration. 1
TABLE 3.
General statistical analysis results
| No. of patients | Olfactory score, mean ± SD | Taste score, mean ± SD | |||
|---|---|---|---|---|---|
| Gender | |||||
| Male | 146 (42.3%) | 58.9 ± 32.6 | 2.9 ± 1.4 | ||
| Female | 199 (57.7%) | 60.9 ± 31.7 | 3.1 ± 1.2 | ||
| Mann‐Whitney U‐test | P = .543 | P = .085 | |||
| Age | |||||
| ≤50 years | 204 (59.1%) | 61 ± 33.1 | 3 ± 1.3 | ||
| >50 years | 141 (40.9%) | 58.7 ± 30.6 | 3.1 ± 1.2 | ||
| Mann‐Whitney U‐test | P = .333 | P = .856 | |||
| Days from symptoms onset | Mann‐Whitney U test | ||||
| Olfaction | Taste | ||||
| 1‐7 days | 70 (20.3%) | 37.3 ± 36.4 | 1.9 ± 1.7 | P = .000 | P = .000 |
| 8‐14 days | 116 (33.6%) | 68.4 ± 28.6 | 3.2 ± 1.2 | ||
| P = .119 | P = .632 | ||||
| >14 days | 159 (46.1%) | 64.1 ± 27.7 | 3.4 ± 0.8 | ||
| Kruskal‐Wallis H test | P = .000 | P = .000 | |||
TABLE 4.
Clinical statistical analysis results
| COVID‐19 severity | No. of patients | Olfactory score, mean ± SD | Taste score, mean ± SD | Mann‐Whitney U test | |
|---|---|---|---|---|---|
| Olfaction | Taste | ||||
| Mild disease | 168 (48.7%) | 54.5 ± 35.3 | 2.8 ± 1.6 | P = .052 | P = .103 |
| Moderate disease | 115 (33.3%) | 64.5 ± 27.2 | 3.3 ± 1 | ||
| P = .575 | P = .619 | ||||
| Severe disease | 52 (15.1%) | 61.3 ± 27.9 | 3.3 ± 0.9 | ||
| Kruskal‐Wallis H test | P = .154 | P = .112 | |||
| Type of chemosensitive disorder | Mild disease (N = 168) | Moderate disease (N = 115) | Severe disease (N = 52) | Fisher's exact test |
|---|---|---|---|---|
| Combined chemosensitive disorders (N = 203) | 100 (59.5%) | 71 (61.7%) | 32 (61.5%) | P = .941 |
| Taste disorder (N = 234) | 118 (70.2%) | 82 (71.3%) | 34 (65.4%) | P = .730 |
| Olfactory disorder (N = 225) | 112 (66.6%) | 77 (67%) | 36 (69.2%) | P = .974 |
| Duration of chemosensitive symptoms (N = 119)* | Mild disease (N = 20) | Moderate disease (N = 69) | Severe disease (N = 30) | Fisher's exact test |
|---|---|---|---|---|
| ≤7 days | 16 (80%) | 48 (69.6) | 9 (30%) | P = .000 |
| >7 days | 4 (20%) | 21 (30.4%) | 21 (70%) | |
| Relative risk | RR 1.12 | RR 2.33 |
4. DISCUSSION
Chemosensitive disorders are recently emerging as highly prevalent symptoms during COVID‐19.4, 5, 6, 7, 8, 9, 10 The studies published in the last few weeks are mostly anamnestic and do not provide an objective quantification of the problem.4, 5, 6, 7, 8, 9, 10, 11 The studies that subject patients to psychophysical tests are few and based on the analysis of small case series and of patients in late stages of the infection.13, 19 The lack of objective data quantifying the extent of the problem, has meant that in many countries chemosensitive disorders have not yet been included in the COVID‐19 guidelines.
The analysis of our case history confirmed a high incidence of chemosensitive disorders during SARS‐CoV‐2 infection. 74.2% (256 subjects) of patients reported experiencing or having experienced a chemosensitive disorder during the course of the infection. This data is consistent with what has been found in other anamnestic studies.6, 9, 10 However, the objective analysis of patients who did not report taste and smell disturbances, showed mild hyposmia in 30.3% of cases. Moreover, patients with subjectively reported isolated dysfunctions of taste or smell, on psychophysical tests presented with associated hyposmia or hypogeusia in 32.3% (P = .000) and 22.7% (P = .024) of the cases, respectively. Anamnestic and interview studies may therefore underestimate the frequency of these disorders.
The clinical onset of chemosensitive disorders occurs characteristically in the very early stages of the symptomatic infection, generally in the first 3 days. Interestingly, chemosensitive symptoms were the first symptom of COVID‐19 in 29.2% of patients and the only one in 9.5% of the cases. According previous studies,4, 5, 6, 7, 8, 9, 10, 11, 13, 14, 19 no correlation was found between olfactory and gustatory disorders and nasal obstruction or rhinitis symptoms, present only in 7.8% of evaluated patients.
The possibility to perform psychophysical tests remotely, made it possible to evaluate home‐quarantined patients in very early stages of infection, when chemoreceptive symptoms were most prevalent. In the first 4 days from the clinical onset, the frequency of severe chemosensitive disorders was very high, reaching a rate of 71% for anosmia and severe hyposmia (Figure 1) and 64.5% for ageusia and severe hypoageusia (Figure 2).
As reported by patients, chemosensitivity recovery occurred in less than 7 days in 74.6% of cases (P = .000). However, the evaluation of these patients revealed residual mild or moderate hyposmia in 69.9% (P = .000) and mild or moderate hypogeusia in 28.8% (P = .000) of cases. This difference, already found in the preparatory evaluations for this study13, 14 could be related to the presence of a mild previous disturbance, or to the fact that the patient had noticed such a great improvement, compared to the condition of ageusia and/or anosmia that he had suffered, to consider his current chemoreceptive capacity as normal. Intercepting these residual disturbances is impossible with the studies based on interviews published so far which, also for this reason, underestimate the extent of these symptoms. From the analysis of the data of this case series, the average taste and smell scores have gradually improved in the first 15 days, reaching average values that do not vary significantly in the subsequent observation periods (Figures 1 and 2). The recovery of the gustatory function was more effective and at 15 days the average score returned to the normal range (Figure 2). In contrast, the average olfactory score improved significantly in the first 2 weeks without returning to normal values, but always remaining in the range of hyposmia, even in the group of patients evaluated in the third and fourth week from the clinical onset (Figure 1). The statistical analysis confirmed the significance of the correlation between gustatory and olfactory scores and the duration from the beginning of the disease (Table 3). It is important to underline that the frequency of severe anosmia and hyposmia was also significant in the groups of patients evaluated in the third and fourth week (Figure 1). Obviously, there is potential for later recovery but the implication of 25% long term anosmia and severe hyposmia, given the high incidence of infection globally, means that there will be a significant number of patients with long term morbidity. It has implications in that we should be looking to trial potential treatments in this cohort, starting from day 14 onwards, which also coincides with the end of high‐risk period for respiratory deterioration.
Moreover, the clinical findings of this study allow us to orient ourselves among the pathogenetic hypotheses that we have recently proposed. 20 The general tendency to an albeit partial remission and the relative infrequency of other neurological symptoms, tends to exclude a pathogenesis linked to the invasion of the central nervous system through the olfactory pathway and subsequent neuronal death, as hypothesized by several authors,9, 10 at least in the majority of patients. A recent experimental study demonstrates that in mouse and in human olfactory mucosa, COVID‐19 virus links angiotensin‐2 converting enzyme (ACE2) receptors which are expressed in support cell, stem cell and perivascular cell of the olfactory epithelium, but neither in sensory and olfactory bulbar neurons. 21 The infection of these supporting cells may than affect negatively the function of olfactory neurons. Moreover, the infection of basal cells, precursors of the olfactory epithelium receptors, could block or slow down sensory cell turnover which normally lasts 28 to 30 days. 22 This hypothesis would justify the detection of a high frequency of olfactory disorders 3 to 4 weeks after clinical onset. The full functional recovery could be reached later, more than a month not from the beginning of the disease but from the end of the infection.
As regard taste dysfunction pathogenesis, it is well known that ACE2‐inhibitors can induce ageusia with a complex mechanism which involves G‐protein‐coupled protein and sodium channel present in the taste buds. 23 The SARS‐CoV‐2, infecting the cells and binding these receptors, could inactivate these latter, blocking the transformation of chemical gustatory signals into action potential and consequently the sensory perception of taste. The rapid recovery of gustatory disorders found in COVID‐19 patients, can be linked to the rapid turnover of the taste receptor cells which is only 7 to 10 days. 24
The results of the chemosensory evaluation in the 10 asymptomatic patients of this case series are very important to hypothesize the usefulness of gustatory or olfactory deficits as screening markers for SARS‐CoV‐2 infection. Based on a series of 154 COVID‐19 patients, Wee et al 25 concluded that self‐reported olfactory and taste disorders had 95.5% specificity and 22.7% sensitivity as a screening criterion for COVID‐19. The olfactory threshold, widely increased also in the paucisymptomatic patients of our series, could represent a criterion that would increase the sensitivity of chemoreceptive disorders as screening for SARS‐CoV‐2 infection. Moreover, all asymptomatic patients in our series presented an olfactory threshold at the lower limits of the norm (ie, they perceived the dilution N = 6). All patients in this cohort were in home quarantine and the test performed remotely tends to slightly overestimate the olfactory threshold which is then compensated by a more critical assessment of the discriminative ability compared to the CCCRC test. 14 If evaluated by the operator, these patients would have presented a slightly hyposmic threshold. In these days, we are testing the olfactory threshold on all the staff of the University Hospital of Sassari before the execution of routine control nasopharyngeal swabs. The results of this study will give us more precise information regarding the validity of chemosensitive disorders as a screening marker. However, on the basis of the results obtained from this case series, we can conclude that the sudden reduction in taste and/or smell, even if not associated with any other symptom, must be considered strongly suspected for an ongoing SARS‐CoV‐2 infection.
The significance of chemosensitive disorders as a prognostic factor remains controversial. Analyzing self‐reported data from 169 COVID‐19 patients, Yan et al 26 concluded that olfactory loss is associated with a milder clinical course. However, in studies reliant on self‐reported loss, chemosensitive disorders may simply be overlooked or forgotten in the setting of severe disease, ventilatory support, and prolonged recovery. On the basis of the objective results of our study there is no correlation between the frequency and extent of chemosensitive disorders and the severity of the disease (Table 4). For this reason, we can conclude that the chemosensitive symptoms during COVID‐19 should not be overlooked as they are not signs of milder forms. In fact, on the contrary, a statistically significant correlation was found between the self‐reported duration of the chemosensitive disturbances and the clinical picture severity. Patients who reported a symptom duration of more than 7 days, have a 2.33 times greater risk of developing severe forms of COVID‐19 (Table 4).
5. CONCLUSIONS
This study provides further evidence of a high prevalence of self‐reported disturbance of smell and taste in association with confirmed COVID‐19 infection.
Moreover, this is the first study to report application of a home smell and taste test alongside convention psychophysical testing to determine the severity of olfactory and gustatory dysfunction in a significant number of patients. This highlights that patients under‐report the frequency of chemosensitive disorders, Contrary to recent reports, such objective testing refutes the proposal that the presence of olfactory and gustatory dysfunction may predict a milder course, but instead suggests that those with more severe disease neglect such symptoms in the setting of severe respiratory disease.
CONFLICT OF INTEREST
None of the authors has a financial interest in any of the products, devices or drugs mentioned in this manuscript.
Vaira LA, Hopkins C, Salzano G, et al. Olfactory and gustatory function impairment in COVID‐19 patients: Italian objective multicenter‐study. Head & Neck. 2020;42:1560–1569. 10.1002/hed.26269
REFERENCES
- 1. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Day M. Covid‐19: four fifths of cases are asymptomatic, China figures indicate. BMJ. 2020. 10.1136/bmj.m1375. [DOI] [PubMed] [Google Scholar]
- 3. Mao L, Wang M, Chen S, et al. Neurological manifestations of hospitalized patients with COVID‐19 in Wuhan, China: a retrospective case series study. JAMA Neurol. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vaira LA, Salzano G, Deiana G, De Riu G. Ageusia and anosmia: common findings in COVID‐19 patients. Laryngoscope. 2020. 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xydakis MS, Dehgani‐Mobaraki P, Holbrook EH, et al. Smell and taste dysfunction in patients with COVID‐19. Lancet Infect Dis. 2020. 10.1016/S1473-3099(20)30293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS‐CoV‐2 infection. JAMA. 2020. 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID‐19 pandemic. Rhinology. 2020. 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- 8. Russel B, Moss C, Rigg A, et al. Anosmia and ageusia are emerging as symptoms in patients with COVID‐19: what does the current evidence say? Ecancermedicalscience. 2020;14. 10.3332/ecancer.2020.ed98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klopfenstein T, Kadiane‐Oussou NJ, Toko L, et al. Features of anosmia in COVID‐19 patients. Med Mal Infect. 2020. 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020. 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaira LA, Salzano G, De Riu G. The importance of olfactory and gustatory disorders as early symptoms of coronavirus disease (COVID‐19). Br J Oral Maxillofac Surg. 2020. 10.1016/j.bjoms.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dory RL. Psychopysical testing of smell and taste function. Handb Clin Neurol. 2019;164:229‐246. [DOI] [PubMed] [Google Scholar]
- 13. Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID‐19 patients: a single‐center experience on 72 cases. Head Neck. 2020. 10.1002/HED.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaira LA, Salzano G, Petrocelli M, Deiana G, Salzano FA, De Riu G. Validation of a self‐administered olfactory and gustatory test for the remotely evaluation of COVID‐19 patients in home quarantine. Head Neck. 2020. 10.1002/hed.26228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tian S, Hu N, Lou J, et al. Characteristics of COVID‐19 infection in Beijing. J Infect. 2020;80:401‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Massarelli O, Vaira LA, Biglio A, Gobbi R, Dell'aversana Orabona G, De Riu G. Sensory recovery of myomucosal flap oral cavity reconstructions. Head Neck. 2018;40:467‐474. [DOI] [PubMed] [Google Scholar]
- 17. Cain WS, Gent JF, Goodspeed RB, Leonard G. Evaluation of olfactory dysfunction in the Connecticut chemosensory clinical research center. Laryngoscope. 1988;98:83‐88. [DOI] [PubMed] [Google Scholar]
- 18. Veyseller B, Ozucer B, Karaaltin AB, et al. Connecticut (CCCRC) olfactory test: normative values in 426 healthy volunteers. Indian J Otolaryngol Head Neck Surg. 2014;66:31‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moein ST, Hahemian SMR, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol. 2020. 10.1111/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vaira LA, Salzano G, Fois AF, Piombino P, De Riu G. Potential pathogenesis of ageusia and anosmia in COVID‐19 patients. Int Forum Allergy Rhinol. 2020. 10.1002/alr.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brann DH, Tsukahara T, Weinreb C, et al. Non‐neuronal expression of SARS‐CoV‐2 entry genes in the olfactory system suggests mechanisms underlying COVID‐19‐associated anosmia. BioRxiv. 2020. 10.1101/2020.03.25.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graziadei PPC, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1‐18. [DOI] [PubMed] [Google Scholar]
- 23. Suliburska J, Duda G, Pupek‐Musialik D. The influence of hypotensive drugs on the taste sensitivity in patients with primary hypertension. Acta Pol Pharm. 2012;60:121‐127. [PubMed] [Google Scholar]
- 24. Oakley B, Riddle DR. Receptor cell regeneration and connectivity in olfaction and taste. Exp Neurol. 1992;115:50‐54. [DOI] [PubMed] [Google Scholar]
- 25. Wee LE, Chan YFZ, Teo NWY, et al. The role of self‐reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID‐19. Eur Arch Otorhinolaryngol. 2020. 10.1007/s00405-020-05965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yan CH, Faraji FF, Divya PP, Ostrander BT, DeConde AS. Self‐reported olfactory loss associates with outpatient clinical course in COVID‐19. Int Forum Allergy Rhinol. 2020. 10.1002/alr.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]


