Abstract
Introduction
The aim of this study is to report our clinical experience in the management of pregnant women infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) during the first 30 days of the coronavirus disease (COVID‐19) pandemic.
Material and methods
We reviewed clinical data from the first 60 pregnant women with COVID‐19 whose care was managed at Puerta de Hierro University Hospital, Madrid, Spain from 14 March to 14 April 2020. Demographic data, clinical findings, laboratory test results, imaging findings, treatment received, and outcomes were collected. An analysis of variance (Kruskal‐Wallis test) was performed to compare the medians of laboratory parameters. Fisher's exact test was used to evaluate categorical variables. A correspondence analysis was used to explore associations between variables.
Results
A total of 60 pregnant women were diagnosed with COVID‐19. The most common symptoms were fever and cough (75.5% each) followed by dyspnea (37.8%). Forty‐one women (68.6%) required hospital admission (18 because of disease worsening and 23 for delivery) of whom 21 women (35%) underwent pharmacological treatment, including hydroxychloroquine, antivirals, antibiotics, and tocilizumab. No renal or cardiac failures or maternal deaths were reported. Lymphopenia (50%), thrombocytopenia (25%), and elevated C‐reactive protein (CRP) (59%) were observed in the early stages of the disease. Median CRP, d‐dimer, and the neutrophil/lymphocyte ratio were elevated. High CRP and D‐dimer levels were the parameters most frequently associated with severe pneumonia. The neutrophil/lymphocyte ratio was found to be the most sensitive marker for disease improvement (relative risk 6.65; 95% CI 4.1‐5.9). During the study period, 18 of the women (78%) delivered vaginally. All newborns tested negative for SARS‐CoV‐2 and none of them were infected during breastfeeding. No SARS‐CoV‐2 was detected in placental tissue.
Conclusions
Most of the pregnant women with COVID‐19 had a favorable clinical course. However, one‐third of them developed pneumonia, of whom 5% presented a critical clinical status. CRP and D‐dimer levels positively correlated with severe pneumonia and the neutrophil/lymphocyte ratio decreased as the patients improved clinically. Seventy‐eight percent of the women had a vaginal delivery. No vertical or horizontal transmissions were diagnosed in the neonates during labor or breastfeeding.
Keywords: breastfeeding, coronavirus 2, coronavirus disease‐2019, labor, newborn, pregnancy, severe acute respiratory syndrome , severe acute respiratory syndrome coronavirus 2, vertical transmission
Abbreviations
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- HELLP
hemolysis, elevated liver enzymes, low platelets
- ICU
intensive care unit
- LDH
lactate dehydrogenase
- NLR
neutrophils/lymphocytes ratio
- RT‐PCR
reverse transcription polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Key Message.
Thirty percent of pregnant women with COVID‐19 presented with pneumonia. Increased C‐reactive protein and D‐dimer levels, increased neutrophil/lymphocyte ratios, and lymphopenia were associated with worse outcomes. Vaginal delivery appears to be safe as no neonates were infected at birth.
1. INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is an RNA virus responsible for the 2019 coronavirus disease (COVID‐19). 1 COVID‐19 can present with common cold‐like symptoms to a more severe disease, such as pneumonia and severe acute respiratory syndrome, and may cause multiple organ failure and death.
In the 3 months since the World Health Organization's worldwide Public Health emergency declaration (first COVID‐19 case was reported on 31 December 2019), 2 , 3 more than 3 207 543 confirmed cases and 227 379 deaths were reported globally, including 212 917 cases and 24 275 deaths in Spain (as of 30 April 2020). 4 Progression of COVID‐19 has been exponential; the first Spanish case was confirmed on 1 February 2020 (Canary Islands), followed by a second case 10 days later (on the peninsula). Since then, an exponential growth of cases has continued.
The global management of more than 3 million COVID‐19 patients within a relatively short period has provided important data on epidemiological characteristics, viral transmission mechanisms, clinical symptoms, and diagnosis as well as prevention and treatment of the disease. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 However, knowledge regarding clinical course and management of pregnant women with COVID‐19 is still limited. The published literature to date indicates that pregnant women may be more vulnerable to COVID‐19. The disease may be associated with an increased risk of premature rupture of membranes, preterm delivery, fetal tachycardia, and fetal distress. Lymphocytopenia and elevated C‐reactive protein (CRP) levels were observed in the majority of pregnant women with COVID‐19. 12 , 13 A recently published systematic review suggested that there is a higher risk of severe maternal morbidity and perinatal death associated with COVID‐19 infection, although maternal‐fetal transmission was not detected. 14
The aim of our study was to describe our experience in the clinical management of 60 COVID‐19‐positive pregnant women who were attended to in our hospital during the first month of the epidemic in Spain.
2. MATERIAL AND METHODS
Data were collected from the first 60 pregnant women with COVID‐19 who were treated at Puerta de Hierro University Hospital Madrid, Spain from 14 March to 14 April 2020.
Demographic variables collected were maternal age, type of exposure, gestational age, parity, and information on the course of the pregnancy. In addition, the following clinical data were extracted from the medical records: symptoms, pneumonia diagnosis, CURB65 (Confusion, Urea, Respiratory rate, Blood pressure, age ≥65 years) score for pneumonia severity (shown in Figure 1), hospital admission, respiratory co‐infections, type of delivery, and treatment.
FIGURE 1.
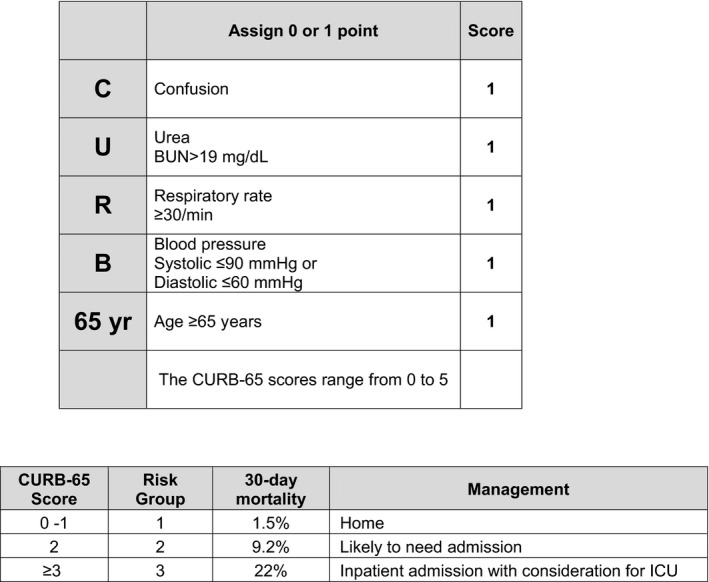
Pneumonia risk score CURB‐65. BUN, blood urea nitrogen; ICU, intensive care unit
All women were seen in the Obstetrics Emergency Room either because they were showing clinical symptoms (eg, fever, cough, and respiratory distress) or because they were in labor. COVID‐19 diagnosis was confirmed by a quantitative reverse transcription polymerase chain reaction (RT‐PCR) test for SARS‐CoV‐2 on nasopharyngeal swabs. All COVID‐19 patients were classified as: (a) Stage I, or early infection phase, if the patient was asymptomatic or if symptoms were fever, cough, diarrhea, or headache. (b) Stage II, or lung disease phase, if patient presented dyspnea; (c) Stage III, or hyperinflammatory phase, if sepsis, shock, or cardiac failure was diagnosed.
Laboratory tests included a complete blood count, complete coagulation, renal and liver function tests, D‐dimer, CRP, lactate dehydrogenase (LDH), creatine kinase, troponin I, ferritin, and interleukin‐6 levels. Moreover, women with symptoms underwent chest radiography, obstetric ultrasound, and fetal heart rate monitoring (if >23 weeks pregnant). For those prescribed hydroxychloroquine, an electrocardiogram (normal QTc interval = 460) was also performed.
2.1. Hospital admission
Pregnant women with COVID‐19 were admitted to the hospital either because they were in labor or because they presented symptoms or signs of disease complications (eg, persistent fever, dyspnea, radiological diagnosis of pneumonia, or oxygen saturations <95%). The severity of pneumonia was classified following a radiography‐based score in which each of the five lung lobes was assessed for degree of involvement and classified as normal: 0%, mild pneumonia: 1%‐25%, moderate pneumonia: 26%‐50%, or severe pneumonia: >50%. 15
2.2. Treatment for pregnant women with COVID‐19
The protocols implemented at our hospital were as follows: (a) mainly symptomatic treatment for asymptomatic patients: rest at home and 1 g of paracetamol every 8 hours, as needed, for fever or general discomfort; (b) Protocol 1 for pregnant women with comorbidities and/or symptoms of upper respiratory tract infection; (c) Protocol 2 for women with mild pneumonia; (d) Protocol 3 for women with severe pneumonia and acute respiratory distress syndrome or for women with poor clinical progression. The description of protocols 1, 2, and 3 is given in Figure 2. Protocols 2 and 3 required hospital admission and oxygen therapy.
FIGURE 2.
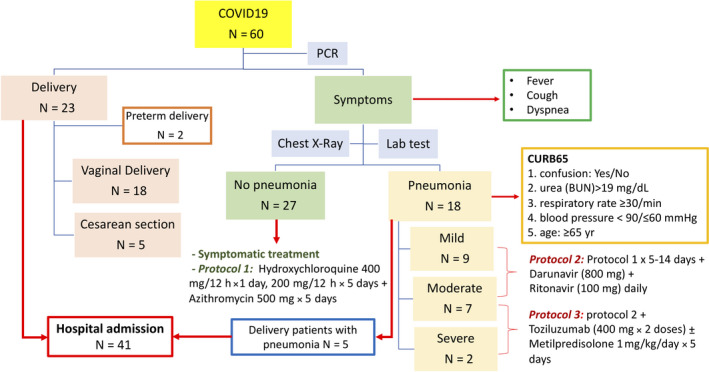
Flowchart of pregnant women with COVID 19 [Color figure can be viewed at wileyonlinelibrary.com]
2.3. Childbirth
Vaginal delivery or cesarean section was indicated according to the protocols of the Spanish Society of Gynecology and Obstetrics. Additionally, cesarean sections were indicated for maternal benefit if a severe health disorder, such as SARS, appeared. Delivery care was performed by obstetricians and all delivery staff followed the precautions and recommendations on infection prevention and control. All newborns were tested for SARS‐CoV‐2 in the first 2 hours after delivery by quantitative RT‐PCR on samples from the respiratory tract (nasopharyngeal swab). In cases where the newborns had respiratory distress syndrome, the SARS‐CoV‐2 test was repeated 24 hours later. Samples of six placentas were also tested for SARS‐CoV‐2 by quantitative RT‐PCR.
2.4. Statistical analyses
Medians and ranges of all the variables were calculated and an analysis of variance (Kruskal‐Wallis test) was performed to compare the medians of the laboratory parameters obtained on the first and last days of assessment. The Fisher's exact test and the relative risk were used to evaluate associations between variables and the health status of pregnant women with COVID‐19; P‐values <.05 were considered significant.
A correspondence analysis was used to explore associations between CRP, D‐Dimer, and LDH levels, and lymphopenia and the progression of the disease in women with mild, moderate, and severe pneumonia. This analysis was also used to establish the value of the goodness‐of‐fit statistics and assign order to unordered variables. The statistical analysis was performed using R version 3.6.3 software (R Core Team, 2020).
2.5. Ethical approval
Approval was given by the hospital's Research Ethics Committee (reference number: PI 78/20; date of approval: 14 April 2020) and an Informed Consent was signed by all patients.
3. RESULTS
A total of 60 pregnant women were confirmed to have COVID‐19 by an RT‐PCR test (cobas® SARS‐CoV‐2 by Roche Diagnostics) for SARS‐CoV‐2 on nasopharyngeal swabs. The demographic characteristics of the study population are shown in Table 1. More than one third of the women were infected at home or by relatives. The median maternal age was 34 years (range 22‐43 years). The median gestational age was 32 weeks (range 5‐41 weeks).
TABLE 1.
Demographic characteristics of COVID 19 in pregnant women
| Overall | N (%) | |
|---|---|---|
| Maternal age | 60 | |
| <30 y | 11 (18.3%) | |
| 30‐34 y | 24 (40.0%) | |
| 35‐40 y | 22 (36.7%) | |
| >40 y | 3 (5.0%) | |
| Exposure type | 60 | |
| Infected at household or by relatives | 21 (35.0%) | |
| Infected at workplace | 6 (10.0%) | |
| Healthcare professionals | 4 | |
| Other professions | 2 | |
| Mixed: family/workplace | 1 (1.7%) | |
| Unknown | 32 (53.3%) | |
| Gestational age | 60 | |
| 1st trimester (1‐12 weeks) | 10 (16.7%) | |
| 2nd trimester (13‐26 weeks) | 16 (26.7%) | |
| 3rd trimester (27‐41 weeks) | 34 (56.6%) | |
| Parity | 60 | |
| Primiparous | 27 (45.0%) | |
| Multiparous | 33 (55.0%) | |
| Plurality | 60 | |
| Singleton | 60 (100%) | |
| Multiple | 0 (0%) | |
| Pregnancy follow up | 60 | |
| Normal | 49 (81.7%) | |
| Abnormal | 11 (18.3%) | |
| FGR/FSGA | 3 | |
| RPB | 3 | |
| Preeclampsia | 3 | |
| DVT | 2 |
Abbreviations: DVT, deep venous thrombosis; FGR, fetal growth restriction (fetal weight <3rd centile for gestational age); FSGA, fetus small‐for‐gestational‐age (fetal weight <10th centile for gestational age); N, number; RPB, risk of preterm birth, y, year.
During the first 5 days of illness, women were asymptomatic but already contagious. Subsequently, 25% remained asymptomatic, 70% developed mild or moderate symptoms, and 5% developed severe to critical symptoms. The most common symptoms among our patients were fever and cough. Dyspnea was present in 17 of them (37.8%). Hospital admissions were necessary in 41 patients (68.3%): 18 as the result of COVID‐19 complications and 23 for deliveries. A total of 10 women needed oxygen support (10%), via nasal prongs for eight women and high‐flow oxygen mask with reservoir bag for two women. Eighteen women (30%) were diagnosed with pneumonia based on a chest radiograph. Three women were evaluated by the intensive care unit (ICU), and one of them required ICU admission. During this period, there were no maternal deaths. Clinical findings are shown in Table 2.
TABLE 2.
Descriptive analyses of clinical findings
| Overall | ||
|---|---|---|
| Symptoms | 60 | N (%) |
| Asymptomatic COVID‐19 | 15 (25.0%) | |
| Symptomatic COVID‐19 | 45 (75.0%) | |
| Fever | 34 (75.5%) | |
| Cough | 34 (75.5%) | |
| Dyspnea | 17 (37.8%) | |
| COVID‐19 stages | 60 | N (%) |
| Stage I or early infection phase | 42 (70.0%) | |
| Stage II or lung disease phase | 15 (25.0%) | |
| Stage III or hyperinflammatory phase | 3 (5.0%) | |
| CURB65 scale | ||
| 0 | 57 (95.0%) | |
| 1 | 3 (5.0%) | |
| ≥2 | 0 (0%) | |
| Pregnant women with COVID‐19 | 60 | N (%) |
| Without pneumonia | 42 (70.0%) | |
| With pneumonia | 18 (30.0%) | |
| Mild | 9 (50.0%) | |
| Moderate | 7 (38.9%) | |
| Severe | 2 (11.1%) | |
| Respiratory co‐infection | 5 (27.8%) | |
| Hospital admissions | 41 | Median (range) |
| COVID‐19 length of stay (days). N = 18 | 3 (1‐13) | |
| Delivery length of stay (days). N = 23 | 3 (1‐9) | |
| Delivery | 60 | N (%) |
| Overall | 23 (38.3%) | |
| Spontaneous vaginal delivery | 14 (60.9%) | |
| Instrumental delivery | 4 (17.4%) | |
| Cesarean section | 5 (21.7%) | |
| Premature delivery | 2 (8.7%) | |
| Vertical transmission to newborn | 0 (0%) | |
| Treatment | 60 | N (%) |
| Symptomatic | 39 (65.0%) | |
| Hydroxychloroquine | 10 (16.7%) | |
| Hydroxychloroquine + lopinavir + ritonavir | 3 (5.0%) | |
| Hydroxychloroquine + darunavir + ritonavir | 3 (5.0%) | |
| Hydroxychloroquine + darunavir + ritonavir + tocilizumab | 2 (3.3%) | |
| Hydroxychloroquine + darunavir + cobicistat | 3 (5.0%) |
The most common laboratory findings in pregnant women with COVID‐19 are summarized in Tables 3 and 4. In the study, the median of CRP and D‐dimer levels, and the neutrophil/lymphocyte ratio (NLR) were elevated. The cut‐off value for each variable in severe pneumonia was as follows: CRP >60 mg/L, D‐dimer >1.9 μg/mL, and lymphocytes <0.9 × 10E3/µL. Of all the women with persisting and severe symptoms, three (5%) had high ferritin and interleukin‐6 levels. None of the individuals were diagnosed with renal or cardiac failure.
TABLE 3.
Changes in laboratory test variables in pregnant women with COVID‐19 during the course of disease
| Total | First day at hospital | Last day at hospital | |||||
|---|---|---|---|---|---|---|---|
| Laboratory variables (measure, normal range) | Median | Range | Median | Range | Median | Range | P |
| Sato 2 (<95%) | 96 | 87‐99 | 97 | 93‐99 | 95 | 91‐100 | .325 |
| AST (U/L, 6‐40) | 27 | 14‐86 | 25 | 13‐58 | 25 | 14‐54 | .876 |
| ALT (U/L, 6‐40) | 15.8 | 8‐108 | 16 | 8‐37 | 17 | 7‐124 | .305 |
| CRP (mg/L, 0.1‐10) | 17.8 | 0.9‐147.4 | 15.9 | 0.9‐118.7 | 17 | 1.8‐147.4 | .873 |
| LDH (U/L, 120‐246) | 188.5 | 134‐437 | 178.5 | 134‐345 | 189 | 120‐352 | .708 |
| Lymphocytes (x10E3/µL, 1.2‐4) | 1.3 | 0.4‐2.5 | 1.2 | 0.6‐2.4 | 1.5 | 0.9‐2.4 | .118 |
| Neutrophils (x10E3/µL, 1.5‐7.5) | 5.6 | 1.2‐10.3 | 5.4 | 2.5‐8.2 | 4.2 | 1.2‐5.9 | .205 |
| N/L ratio (<3) | 4.6 | 1.3‐14.4 | 4.5 | 2.4‐10.2 | 2.6 | 1.3‐5.7 | .009 |
| D‐dimer (μg/mL, 0.1‐0.5) | 1.0 | 0.2‐7.8 | 1.0 | 0.2‐1.9 | 1.3 | 0.3‐2.3 | .834 |
| Platelets (×10E3/µL, 150‐400) | 218 | 100‐466 | 193.5 | 109‐320 | 206 | 147‐382 | .217 |
| Laboratory variables (measure, normal range) | Mild pneumonia | Moderate pneumonia | Severe pneumonia | P | |||
| Median | Range | Median | Range | Median | Range | ||
| Sato 2 (<95%) | 97 | 91‐100 | 96 | 85‐99 | 95 | 87‐99 | .63 |
| AST (U/L, 6‐40) | 36.5 | 25‐62 | 34.3 | 14‐83 | 41 | 20‐86 | .972 |
| ALT (U/L, 6‐40) | 29.5 | 15‐49 | 26 | 8‐108 | 24 | 13‐75 | .747 |
| CRP (mg/L, 0.1‐10) | 23.0 | 4.5‐44.6 | 23.7 | 3.6‐118.7 | 60 | 1.8‐147.4 | .435 |
| LDH (U/L, 120‐246) | 180 | 154‐437 | 210.3 | 154‐379 | 225.5 | 161‐297 | .256 |
| Lymphocytes (×10E3/µL, 1.2‐4) | 1.2 | 0.6‐2 | 1.4 | 0.4‐1.8 | 0.9 | 0.5‐2.4 | .434 |
| Neutrophils (×10E3/µL, 1.5‐7.5) | 3.9 | 2.4‐6.9 | 5.6 | 1.4‐10.1 | 6.2 | 2.4‐10.3 | .024 |
| N/L ratio (<3) | 3.7 | 1.3‐10.2 | 5.2 | 1.7‐14.4 | 6.8 | 2.5‐13.9 | .056 |
| D‐dimer (μg/mL, 0.1‐0.5) | 0.9 | 0.5‐2.2 | 0.9 | 0.4‐1.8 | 1.9 | 0.4‐7.8 | .841 |
| Platelets (×10E3/μL, 150‐400) | 196 | 164‐288 | 233 | 100‐466 | 240 | 132‐321 | .507 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C‐reactive protein; LDH, lactate dehydrogenase; N/L, neutrophil to lymphocyte ratio; Sato 2, oxygen saturation.
TABLE 4.
Distribution of laboratory test variables in pregnant women with COVID‐19
| Laboratory variables (measure, normal range) | Total | First day at hospital | Last day at hospital | P | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Sato 2 (<95%) | 5/19 | 26 | 1/15 | 7 | 3/14 | 21 | .336 |
| ↑AST (U/L, 6‐40) | 4/20 | 20 | 1/17 | 6 | 3/15 | 20 | .445 |
| ↑ALT (U/L, 6‐40) | 1/20 | 5 | 0/17 | 0 | 2/15 | 13 | .371 |
| ↑CRP (mg/L, 0.1‐10) | 15/20 | 75 | 10/17 | 59 | 10/13 | 77 | .575 |
| ↑LDH (U/L, 120‐246) | 4/20 | 20 | 2/14 | 14 | 3/14 | 21 | 1 |
| ↓Lymphocytes (x10E3/microL, 1.2‐4) | 8/20 | 40 | 10/20 | 50 | 4/14 | 29 | .535 |
| ↑Neutrophils (×10E3/μL, 1.5‐7.5) | 2/20 | 10 | 3/20 | 15 | 0/14 | 0 | .420 |
| ↑N/L ratio (<3) | 17/20 | 85 | 19/20 | 95 | 4/14 | 29 | 2.69e‐05 |
| ↑D‐dimer (μg/mL, 0.1‐0.5) | 18/19 | 95 | 15/16 | 96 | 9/12 | 75 | .281 |
| ↓Platelets (×10E3/μL, 150‐400) | 3/20 | 15 | 5/20 | 25 | 0/14 | 0 | .239 |
| Laboratory variables (measure, normal range) | Mild pneumonia | Moderate pneumonia | Severe pneumonia | P | |||
| N | % | N | % | N | % | ||
| Sato 2 (<95%) | 0/4 | 0 | 1/6 | 17 | 1/2 | 50 | .409 |
| ↑AST (U/L, 6‐40) | 1/4 | 25 | 2/6 | 33 | 1/2 | 50 | 1 |
| ↑ALT (U/L, 6‐40) | 0/4 | 0 | 1/6 | 17 | 0/2 | 0 | 1 |
| ↑CRP (mg/L, 0.1‐10) | 3/4 | 75 | 5/6 | 83 | 2/2 | 100 | 1 |
| ↑LDH (U/L, 120‐246) | 0/4 | 0 | 2/6 | 33 | 1/2 | 50 | 0.346 |
| ↓Lymphocytes (×10E3/microL, 1.2‐4) | 2/4 | 50 | 2/6 | 33 | 2/2 | 100 | 0.481 |
| ↑Neutrophils (×10E3/microL, 1.5 −7.5) | 0/4 | 0 | 2/6 | 33 | 0/2 | 0 | 0.636 |
| ↑N/L ratio (<3) | 2/4 | 50 | 6/6 | 100 | 2/2 | 100 | 0.106 |
| ↑D‐dimer (μg/mL, 0.1‐0.5) | 4/4 | 100 | 6/6 | 100 | 2/2 | 100 | 1 |
| ↓Platelets (×10E3/microL, 150‐400) | 0/4 | 0 | 1/6 | 17 | 0/2 | 0 | 1 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C‐reactive protein; LDH, lactate dehydrogenase; N/L, neutrophil to lymphocyte ratio; Sato 2, oxygen saturation.
Oxygen saturation, liver function, CRP and LDH levels, lymphopenia, NLR, dimer‐D levels, and thrombocytopenia were correlated with the worsening of COVID‐19 (Tables 3 and 4).
Symptomatic treatment was required for 39 women (65%), and experimental treatment was administered in 21 women (35%); protocols and distributions of treatments are summarized in Figure 2 and Table 2. In addition, five women required antibiotics for respiratory co‐infections (Pneumococcus, Haemophilus influenzae, methicillin‐resistant Staphylococcus aureus, Mycoplasma pneumoniae), and 25 women were treated with low‐molecular‐weight heparin for thromboprophylaxis.
During the study period, 23 women with COVID‐19 delivered in our hospital; five of them had cesarean section (one for maternal respiratory failure at 34+2 weeks of gestation with uterine activity and breech presentation, two for non‐progression of labor, one for induction failure, and one because of hemolysis, elevated liver enzymes, low platelets [HELLP] syndrome) and 18 had vaginal deliveries. Placental tissue from six women was sent to the Microbiology Unit; SARS‐CoV‐2 was not identified in any of the placental samples. Two of the 23 deliveries were preterm. The clinical course in the puerperium was uncomplicated for 22 women; only one woman (with HELLP syndrome) required ICU admission. The twenty‐three newborns tested negative for SARS‐CoV‐2 by RT‐PCR nasopharyngeal swabs. Twenty‐one of the 23 neonates were breastfed and two of them needed admission to the Neonatal Intensive Care Unit: one because of a respiratory distress syndrome and the other for hemolytic anemia.
The statistical analysis demonstrated that continuous decreases of NLR were shown to be the most sensitive marker for disease improvement (relative risk 6.65; 95% CI 4.1‐5.9). Furthermore, a multivariate analysis with a correspondence analysis revealed an association between CRP and D‐dimer levels with severe pneumonia (P < .000). Likewise, elevated neutrophil counts were associated with severe pneumonia (P = .024).
4. DISCUSSION
According to the evolution of our patients, we describe COVID‐19 as a three‐phase disease.
The early infection phase is a viral response phase, where the most common symptoms are fever, cough, diarrhea, or headache. Seventy percent of our patients were asymptomatic or had mild symptoms at the time of diagnosis; they only received symptomatic treatment and the disease did not progress further in any of these cases. Lymphopenia (50%) and thrombocytopenia (25%) were observed in the early stages of disease, and close to 60% of patients had increased CRP (Table 4).
In the lung disease phase, SARS‐CoV‐2 can cause massive damage to the liver and renal tissues which results from an excessive release of cytokines that, in turn, induces a severe pro‐inflammatory response in the lungs, which frequently causes dyspnea and hypoxemia. In our study, 38% of our patients showed dyspnea and 40% of symptomatic patients presented with pneumonia. Moderate or severe pro‐inflammatory response in the lung accounts for 15% of them (Table 2). Transitory hepatic disorder was present in 25%‐50% of pregnant women with pneumonia (Table 3). During the observation period, lymphopenia (40%), and increased NLR (85%), CRP (75%), and D‐dimer (95%) levels were present in pregnant women with COVID‐19, which is consistent with the published medical data. 12 , 13 , 14 Moreover, our study demonstrated NLR decrease as a potential marker of patient improvement.
The hyperinflammatory phase occurs when an excessive immune and inflammatory response arises. As we previously stated above, critical status in pregnant women with COVID‐19 accounts for 5%. 14 Interleukin‐6 and ferritin levels were significantly elevated during the critical infection phase. However, no patients developed hepatic, renal, or cardiac failure in our cohort. In general terms, respiratory viral infections increase the risk of bacterial infections leading to a more severe respiratory disease. In our cohort, the most severe cases had neutrophilia and a concurrent bacterial sepsis (one with pneumococcus and one with methicillin‐resistant Staphylococcus aureus) or were associated with HELLP syndrome. For this reason, we believe that pregnant women with COVID‐19 with pneumonia should be investigated for the early diagnosis and treatment of possible bacterial respiratory co‐infections.
Data from China suggest that the clinical course of SARS CoV‐2‐infected pregnant women is similar to that of the general population. 16 , 17 , 18 In our experience, the normal course of pregnancy was altered in 18% of COVID‐19‐positive women. In our cohort, 5% had preeclampsia, 5% fetal growth restriction, 5% preterm birth, and 3% coagulopathy.
The incidence of preeclampsia in the non‐COVID‐19 pregnant population is reported to be 3.4%‐4.6%. 19 , 20 One of our patients developed HELLP syndrome and two had preeclampsia. There could be an association between COVID‐19 and preeclampsia as it has been described that SARS‐CoV‐2 uses the angiotensin‐converting enzyme 2 receptor for cell entry. 21
Coronavirus infection is often complicated by coagulopathy, which is associated with a high mortality rate. Additionally, high D‐dimer levels are a sign of coagulopathy and indicates poor prognosis. 22 , 23 , 24 As in non‐pregnant women, D‐dimer may be increased in pregnant and puerperal women with coronavirus infection. In fact, our study showed that D‐dimer increases in those patients with severe clinical features. Given the increased thromboembolic risk during pregnancy and puerperium, consideration should be given to prescribing thromboprophylaxis to these patients. Based on our experience and several observations, 25 we have developed a protocol to initiate low‐molecular‐weight heparin at prophylactic dose for at least 10 days after delivery. We recommend increasing low‐molecular‐weight heparin to a therapeutic dose for 6 weeks after delivery in those women with higher thromboembolic risk.
Finally, the scarce evidence published until now suggests the lack of vertical transmission, as SARS‐CoV‐2 was not detected in the placenta, amniotic fluid or neonate samples immediately after birth. 16 , 26 , 27 However, cesarean sections were performed in almost all previously published cases. 14 At our hospital, 18 of 23 COVID‐19‐positive women had a vaginal delivery and the delivery care followed the World Health Organization recommendations. In all of our cases, neonates tested negative, irrespective of the mode of delivery. Therefore, we hypothesize that there is no vertical transmission through the birth canal.
The Breastfeeding Committee at Puerta de Hierro University Hospital approved breastfeeding of newborns by mothers with COVID‐19, provided that adequate protection measures were taken and World Health Organization and United Nations International Children's Emergency Fund recommendations were followed. 28 , 29 We did not diagnose COVID‐19 in any of the breastfed infants.
The present study has several limitations resulting from its retrospective design and small sample size. This study was carried out in the first 4 weeks of an epidemic that has caused devastating consequences in our country. The scarcity of diagnostic tests, limited knowledge about the disease, the limited access to antiretroviral drugs, and restricted hospital capacity for admission and intensive care could have affected the clinical outcomes. Therefore, our results should be interpreted with caution and their generalizability may be limited.
5. CONCLUSION
In 70% of our cases, the clinical course of COVID‐19 in the pregnant women was mild. Only 30% of the women had pneumonia, 5% of whom developed a critical condition. High CRP and D‐dimer levels correlated with severe pneumonia, whereas an NLR decrease suggested a favorable outcome for pregnant women. Vaginal delivery appears to be safe, as 78% of our patients had vaginal delivery and none of the newborns were infected.
6. ACKNOWLEDGMENTS
We are very grateful to Concepción Payares Herrera for advice about English translation and improve the quality of the manuscript.
Pereira A, Cruz‐Melguizo S, Adrien M, Fuentes L, Marin E, Perez‐Medina T. Clinical course of coronavirus disease‐2019 in pregnancy. Acta Obstet Gynecol Scand. 2020;99:839–847. 10.1111/aogs.13921
REFERENCES
- 1. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019‐nCoV). Jan 30, 2020. https://www.who.int/news‐room/detail/30‐01‐2020‐statement‐on‐the‐second‐meeting‐of‐the‐international‐health‐regulations‐(2005)‐emergency‐committee‐regarding‐the‐outbreak‐of‐novel‐coronavirus‐(2019‐ncov) (accessed Feb 29, 2020)
- 3. Wuhan Municipal Health Commission . Report on current pneumonia epidemic situation in the city. (In Chinese.) Dec 31, 2019. http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989 (accessed Feb 29, 2020)
- 4. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov‐China/situacionActual.htm. (accessed March 21, 2020)
- 5. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382:1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu F, Zhao SU, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019‐nCoV) in Wuhan, China. J Med Virol. 2020;92:441‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu P, Hao X, Lau EHY, et al. Real‐time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill. 2020;25:2000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu H. Drug treatment options for the 2019‐new coronavirus (2019‐nCoV). Biosci Trends. 2020;16:69‐71. [DOI] [PubMed] [Google Scholar]
- 12. Liang H, Acharya G. Novel corona virus disease (COVID‐19) in pregnancy: what clinical recommendations to follow? Acta Obstet Gynecol Scand. 2020;99:439‐442. [DOI] [PubMed] [Google Scholar]
- 13. Poon LC, Yang H, Kapur A, et al. Global interim guidance on coronavirus disease 2019 (COVID‐19) during pregnancy and puerperium from FIGO and allied partners: Information for healthcare professionals. Int J Gynecol Obstet. 2020;149:273‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID‐19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99:823‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong HYF, Lam HYS, Fong A‐T, et al. Frequency and distribution of chest radiographic findings in COVID‐19 positive patients. Radiology. 2019. 10.1148/radiol.2020201160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu N, Li W, Kang Q, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID‐19 in Wuhan, China: a retrospective, single‐centre, descriptive study. Lancet Infect Dis. 2020;20:559‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with Coronavirus Disease (COVID‐19) pneumonia: a preliminary analysis. Am J Roentgenol. 2020;8:1‐6. [DOI] [PubMed] [Google Scholar]
- 19. Abalos E, Cuesta C, Grosso AL, et al. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1‐7. [DOI] [PubMed] [Google Scholar]
- 20. Ananth CV, Keyes KM, Wapner RJ. Pre‐eclampsia rates in the United States, 1980–2010: age‐period‐cohort analysis. BMJ. 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;2020:844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thachil J. The versatile heparin in COVID‐19. J Thromb Haemost. 2020;18:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Y, Zhao R, Zheng S, et al. Lack of vertical transmission of severe acute respiratory syndrome Coronavirus 2, China. Emerg Infect Dis. 2020;26:1335‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwartz DA. An analysis of 38 pregnant women with COVID‐19, their newborn infants, and maternal‐fetal transmission of SARS‐CoV‐2: Maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020. 10.5858/arpa.2020-0901-SA [DOI] [PubMed] [Google Scholar]
- 28. WHO, UNICEF . Protecting, promoting, and supporting breastfeeding in facilities providing maternity and newborn services: the revised Baby‐friendly Hospital Initiative 2018 Implementation guidance. World Health Organization, Geneva, Switzerland: Department of Nutrition for Health and Development World Health Organization; 2018. https://www.who.int/nutrition/publications/infantfeeding/bfhi‐implementation/en/. Accessed June 4, 2020. [Google Scholar]
- 29. UNICEF . https://www.unicef.org/stories/novel‐coronavirus‐outbreak‐what‐parents‐should‐know. 2019. Accessed on March 24, 2020.


