Abstract
The use of corticosteroids has been controversial in viral pneumonia. In most cases, application of methylprednisolone in severe and critical viral pneumonia patients can quickly alleviate the symptoms of dyspnea and prevent disease progression. However, some scholars have confirmed that corticosteroids delayed the body's clearance of the virus. In our retrospective non‐randomized study, 34 patients under 50 years old and diagnosed with coronavirus disease 2019 (COVID‐19) were included. According to the given methylprednisolone treatment (n = 18) or not (n = 16), they were separated into two groups. By comparing the clinical data we concluded that corticosteroids therapy can effectively release COVID‐19 symptoms such as persistent fever and difficult in breathing, improve oxygenation, and prevent disease progression. However, it can prolong the negative conversion of nucleic acids.
Keywords: COVID‐19, CT image lesion, methylprednisolone, treatment, virus nucleic acid
Highlights
1. The use of glucocorticoids prolongs the time required for COVID‐19 patients to turn negative consecutively for nucleic acids test.
2. The use of glucocorticoids in critical and severe COVID‐19 typology patients can prevent the progression of this disease, especially to promote the absorption of CT lesion images
3. We confirm the safety and necessity of glucocorticoids applications in severe and critical COVID‐19 patients.
1. INTRODUCTION
In Yichang city, Hubei province, the first outbreak of coronavirus disease 2019 (COVID‐19) commenced on 24 January 2020 in the Yichang Third People's Hospital. With 931 persons becoming infected and the increasing number of COVID‐19 patients, the medical resources of the Yichang Third People's Hospital were in short supply. In this case, Jiangnan District of Yichang Central People's Hospital has been put into use urgently, and nearly 30% of COVID‐19 confirmed patients in Yichang have been treated in our hospital. Until date, this highly contagious respiratory disease has caused infections worldwide, 211 countries have found confirmed patients until 29 April 2020. 1 Unfortunately, there is no specific drug for this disease at present. As one of the drugs for the treatment of interstitial pneumonia, corticosteroid have been studied in many epidemic viral pneumonia. Arabi et al 2 found that corticosteroid therapy can relieve respiratory symptoms in middle east respiratory syndrome (MERS) patients, and this treatment was not related to mortality, but related to MERS coronavirus RNA clearance replacement. Peiris et al 3 reported that corticosteroid treatment in early stage of severe acute respiratory syndrome was associated with a higher subsequent plasma viral load. Brun‐Buisson 4 analyzed the clinical impact of corticosteroids therapy on 208 H1N1 influenza‐related acute respiratory distress syndrome (ARDS) patients and found no beneficial effect. During the progression of viral pneumonia, an outbreak of inflammation may cause ARDS. Despite the controversial role of corticosteroids, they are often used as a supportive approach to minimize inflammatory response in viral pneumonia. 5 , 6 As a type of corticosteroid, methylprednisolone is widely used in clinics because of its large distribution, high concentration, and long retention time in alveolar epithelial cells. 7 However, although there are concerns about whether the use of corticosteroids will slow down the clearance of the coronavirus 8 and increase the possibility of opportunistic infections 9 , in severe cases, especially in critically ill COVID‐19 patients, the use of corticosteroids seems to be indispensable. 10 , 11 , 12
2. MATERIALS AND METHODS
A retrospective comparison study was performed on COVID‐19 patients under 50 years old who were admitted between 30 January and 20 February 2020. Regardless of whether the patient has a positive nucleic acid test result before hospital admission, we re‐tested the nasopharyngeal swabs for COVID‐19 on the first day of admission. All patients were tested positive for new coronavirus nucleic acid in nasopharyngeal swabs by real‐time fluorescent reverse transcription polymerase chain reaction. Among them, 8 patients developed persistent high fever (body temperature >39°C) for three consecutive days, or progressed in computed tomography (CT) imaging within 2 days (ie, the number of affected lung segments increased by 50%, or the range of the original affected lung segments increased by 50%) or progressed into hypoxia and difficulty breathing, so they received methylprednisolone treatment, the initial dose was calculated based on his body weight (1‐2 mg/kg/d) and this dose were gradually halved every 3 days, the total glucocorticoid treating course ranged from 5 to 10 days. In addition, all the 34 patients have received antiviral therapy (200 mg of arbidol three times a day, 400 mg/100 mg of lopinavir and ritonavir twice daily) for 7 to 10 days. In the end, all these patients recovered smoothly and were discharged according to the standard, that is, the symptoms were significantly relieved, the CT imaging lesions were obviously absorbed, and the two consecutive nucleic acid detection were negative. Most important, there was no death. Based on whether they were treated with methylprednisolone or not, they were divided into two groups, the basic information and disease typology are described in Table 1.
Table 1.
Clinical demographics of COVID‐19 confirmed cases in the methylprednisolone treatment and nontreatment group
| Methylprednisolone treatment group | Non‐methylprednisolone treatment group | |
|---|---|---|
| No. of patients | 18 | 16 |
| Age, y | 38.22 ± 8.95 | 33.75 ± 7.80 |
| Female | 7 | 5 |
| Male | 11 | 11 |
| Comorbidities | 3 hypertension | 1 hypertension |
| Typology | 6 moderate, 12 severe | 12 moderate, 4 severe |
| PaO2/FiO2 (P/F) | 236.61 ± 47.13 | 358.19 ± 38.82 |
| Pneumonia severity index (PSI) | 4.44 ± 11.99 | −3.75 ± 5.00 |
| Nuclei acid negative period | 29.11 ± 6.61* | 24.44 ± 5.21* |
| Number of pulmonary segments with lesion before treatment | 6.78 ± 3.62 N.S | 5.13 ± 4.10 N.S |
| Number of pulmonary segments with lesion after 20‐d treatment | 6.22 ± 4.68 N.S | 2.63 ± 2.87 N.S |
Typology of COVID‐19: Mild: the clinical symptoms are mild and no pneumonia manifestations can be found in imaging. Moderate: patients have symptoms such as fever and respiratory tract symptoms, etc, and pneumonia manifestations can be seen in imaging. Severe: Adults who meet any of the following criteria: respiratory rate ≥30 breaths/min; oxygen saturation ≤93% at a rest state; arterial partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) ≤300 mm Hg. Patients with >50% lesions progression within 24 to 48 h in lung imaging should be treated as severe cases. Critical: meeting any of the following criteria: occurrence of respiratory failure requiring mechanical ventilation; presence of shock; other organ failure that requires monitoring and treatment in the ICU. Critical cases are further divided into early, middle, and late stages according to the oxygenation index and compliance of respiratory system. 13
*P < .05.
CT images, 20 days before and after treatment, of the two groups were compared. Good lung CT lesion image absorption is defined as the number of affected lung segments is reduced by half, or the CT density of lesion images is significantly reduced. The time of viral nucleic acid negative conversion is calculated as the time interval between the two consecutive negative detections of new coronavirus nucleic acid and the onset of illness (Table 1).
Statistical analyses were performed using the SPSS 21.0 (IBM). Data were shown as mean values ± standard deviation (SD). The two‐tailed Student t test was used to compare the two groups of means in this study, P values were determined to measure the significance. P values <.001 were considered extremely significant (expressed as ***), <.01 were considered very significant (expressed as **), <.05 were considered significant (expressed as *), ≥.05 were considered not significant (abbreviated as N.S) (Table 2).
Table 2.
Characteristics of CT imaging changes, 20 d before and after treatment, in the methylprednisolone treatment and non‐methylprednisolone treatment group
| Time point | Ground glass opacity | Cloudy | Interstitial changes and fibrous stripes | Consolidation |
|---|---|---|---|---|
| Methylprednisolone treatment group (n = 18) | ||||
| 20 d Before treatment | 10 | 0 | 7 | 6 |
| 20 d After treatment | 4 | 11 | 3 | 2 |
| Non‐methylprednisolone treatment group (n = 16) | ||||
| 20 d Before treated | 12 | 0 | 8 | 3 |
| 20 d After treated | 2 | 10 | 2 | 0 |
3. RESULTS
We compared the changes of CT imaging, 20 days before and after treatment, and found that most of the lung lesions were presented as follows: ground glass opacity, cloudy, interstitial changes, fibrous stripes, and consolidation (Figure 1). After 20 days of antiviral treatment, four cases in the non‐methylprednisolone treatment group completely absorbed the lung lesion on CT image, 12 cases partially absorbed the lung lesion (Figure 2A,B). In the methylprednisolone treatment group, compared with CT image 20 days before antiviral and methylprednisolone treatment, 2 cases completely absorbed the lung lesion on CT image, 14 cases partially absorbed the lung lesion, and 2 cases enlarged the lung lesion (Figure 2C,D). However, there was no statistical difference in the total number of pulmonary segments with lesion before and after 20 days of treatment in both the groups. The time needed for viral nucleic acid negative conversion in the non‐methylprednisolone treatment group (24.44 ± 5.21) was shorter than that in the methylprednisolone treatment group (29.11 ± 6.61, P = .03). Fifteen patients in the methylprednisolone treatment group developed hypoxia before treatment (ie, the percentage of finger oxygen saturation without oxygen inhalation was less than or equal to 97%). However, after these patients were discharged and isolated for 14 days, the blood oxygen saturation returned to normal levels (Figure 3).
Figure 1.
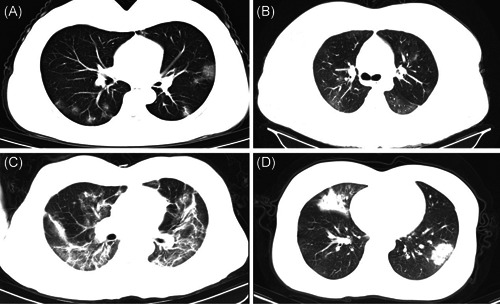
Features of lung lesions on CT images of COVID‐19 confirmed cases. A, Ground glass opacity. B, Cloudy. C, Interstitial changes and fibrous stripes. D, Consolidation
Figure 2.

Lung lesion on CT image before and after 20 days of treatment. A, Lung CT image before treatment of non‐methylprednisolone treatment group group. B, Lung CT image after 20 days treatment of non‐methylprednisolone treatment group. C, Lung CT image before treatment of methylprednisolone treatment group. D, Lung CT image after 20 days treatment of methylprednisolone treatment group
Figure 3.

Changes in oxygen saturation in methylprednisolone treatment group at different time point
4. DISCUSSION
COVID‐19 is an animal‐derived virus, 14 bats may be the host of the virus and intermediate hosts may include pangolins. 15 To date, 237 cases of COVID‐19 diagnosed patients in Yichang City, Hubei Province have been treated in our hospital. The age of all the COVID‐19 patients treated in our hospital range from 0.5 to 87 years old, and there is no significant difference in the number of infections based on their gender, which indicates that the entire population is susceptible to COVID‐19, regardless of gender or age. Unfortunately, there are still no specific drug to treat COVID‐19 at present. According to the diagnosis and treatment guideline issued by the Chinese National Health Council on 3 March 2020, corticosteroids are recommended for severe, especially critically ill patients, 13 which can effectively alleviate the symptoms of patients such as dyspnea and high fever, but also can prevent patients from rapid progress in lung imaging. However, due to the well‐known reasons, corticosteroids are highly controversial in the treatment of viral pneumonia. Some scholars believe that corticosteroids can slow down the clearance of the virus, 8 and long‐term use of corticosteroids will also cause a series of such as opportunistic fungal infection, femoral head necrosis, etc. 16
In our study, we found that the time required for nucleic acid negative conversion in the corticosteroids treatment group was longer than that in the non‐corticosteroids treatment group, which is consistent with the opinion that the use of corticosteroids can slow down the clearance of the virus. 4 , 12 , 17 But more importantly, although the proportion of severe and critical typology patients in the methylprednisolone treatment group is higher than that in the non‐methylprednisolone treatment group, the percentage of good CT imaging lung lesion absorption in methylprednisolone treatment group (88.9%) is comparable with that of the non‐corticosteroids treatment group (100%), and oxygen‐deficiency of the hypoxic patients are gradually improved in the corticosteroids treatment group. Despite the fact that two severe patients in methylprednisolone treatment group had larger lung lesions after 20 days of treatment, they did not turn into critical cases and both of them recovered and were successfully discharged with no complications.
On the basis of the result of retrospective analysis of clinical data, we concluded that corticosteroids are a safe and effective treatment for COVID‐19. In the early stage of the disease, the use of corticosteroids may affect the clearance of the virus, but in the period of rapid disease progression, the use of corticosteroids can inhibit the further deterioration of the disease and create opportunities for treatment. From the perspective of the treatment for critical and severe patients in our hospital, corticosteroids plays a very important role in saving patients' lives and rapidly suppressing the progress of the disease. Considering that the majority typology of COVID‐19 patients under 50 years old are moderate typology, the use of corticosteroids requires individualized selection. Corticosteroids need to be used promptly when disease progression changes rapidly and may develop into severe or critical cases. If there is insufficient evidence, it is best to avoid the use of corticosteroids, as nucleic acid negative transformation takes longer after treatment, which may be due to slower virus removal.
5. CONCLUSION
This study has limitations because it is not a randomized study, and the population included in the study are all under the age of 50, which cannot represent the characteristics of all COVID‐19 patients, furthermore, we only used one type of corticosteroid in our study. which cannot represents the efficacy of all glucocorticoid drugs. Due to the urgency, it is difficult to conduct rigorous large‐scale clinical trials in a short period of time. Although corticosteroids therapy is widely controversial in viral pneumonia, it is safe and effective in clinical applications. Therefore, larger randomized studies are needed to confirm our preliminary results.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
YG and ZJ collected and analyzed the data, and prepared the manuscript. SC contributed to the collecting and interpretation of radiological materials. GX and LG were involved in the patient management and organization. BG designed the study and reviewed the manuscript.
Gong Y, Guan L, Jin Z, Chen S, Xiang G, Gao B. Effects of methylprednisolone use on viral genomic nucleic acid negative conversion and CT imaging lesion absorption in COVID‐19 patients under 50 years old. J Med Virol. 2020;92:2551–2555. 10.1002/jmv.26052
REFERENCES
- 1. People's daily 2020.
- 2. Arabi YM, Mandourah Y, Al‐Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757‐767. [DOI] [PubMed] [Google Scholar]
- 3. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS‐associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brun‐Buisson C, Richard JC, Mercat A, Thiébaut AC, Brochard L. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2011;183(9):1200‐1206. [DOI] [PubMed] [Google Scholar]
- 5. Liu H, Li J, Chen M, Su J. Glucocorticoid treatment of suspected organizing pneumonia after H7N9 infection: a case report. Medicine. 2019;98(34):e16839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ronchetti S, Migliorati G, Bruscoli S, Riccardi C. Defining the role of glucocorticoids in inflammation. Clin Sci (Lond). 2018;132(14):1529‐1543. [DOI] [PubMed] [Google Scholar]
- 7. Tu G‐W, Shi Y, Zheng Y‐J, et al. Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. J Transl Med. 2017;15(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chai Z, Zhang X, Dobbins AL, et al. Optimization of dexamethasone administration for maintaining global transduction efficacy of adeno‐associated virus serotype 9. Hum Gene Ther. 2019;30(7):829‐840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid‐induced side effects: A comprehensive review: Infectious complications and vaccination recommendations. J Am Acad Dermatol. 2017;76(2):191‐198. [DOI] [PubMed] [Google Scholar]
- 10. Zhang W, Zhao Y, Zhang F, et al. The use of anti‐inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID‐19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou W, Liu Y, Tian D, et al. Potential benefits of precise corticosteroids therapy for severe 2019‐nCoV pneumonia. Signal Transduct Target Ther. 2020;5(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ye Z, Wang Y, Colunga‐Lozano LE, et al. Efficacy and safety of corticosteroids in COVID‐19 based on evidence for COVID‐19, other coronavirus infections, influenza, community‐acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta‐analysis. Can Med Assoc J. 2020:cmaj.200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.新型冠状病毒肺炎诊疗方案(试行第六版). 天津中医药 2020:1‐5.
- 14. Ji W, Wang W, Zhao X, Zai J, Li X. Cross‐species transmission of the newly identified coronavirus 2019‐nCoV. J Med Virol. 2020;92(4):433‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu P, Chen W, Chen J‐P. Viral metagenomics revealed sendai virus and coronavirus infection of malayan pangolins (Manis javanica). Viruses. 2019;11(11):979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanaka H, Hosono O, Kobayashi H, Yoshikawa N, Matsumiya R. Glucocorticoids. Nihon Rinsho. 2013;71(7):1261‐1265. [PubMed] [Google Scholar]
- 17. Booth CM. Clinical features and short‐term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801‐2809. [DOI] [PubMed] [Google Scholar]


