Watch the interview with the author
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ACE2
angiotensin‐converting enzyme 2
- AIH
autoimmune hepatitis
- AUD
alcohol use disorder
- COVID‐19
coronavirus disease 2019
- CT
computed tomography
- ESCMID
European Society of Clinical Microbiology and Infectious Diseases
- HCC
hepatocellular carcinoma
- HSI
hepatic steatosis index
- MAFLD
metabolic‐associated fatty liver disease
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NSAID
nonsteroidal anti‐inflammatory drug
- SOT
solid organ transplant
Because between 1% and 11% of patients with Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection may have chronic liver disease, 1 , 2 understanding interactions between the infection and underlying hepatic disease is essential for clinical management. Both emerging data and clinical experience have revealed the influence of underlying liver disease on coronavirus disease 2019 (COVID‐19) outcomes, as well as the impact of COVID‐19 on the course of liver disease. The SECURE‐Cirrhosis Registry (Americas, China, Japan, and Korea) and the COVID‐HEP registry (Europe) have been created to collect data on patients with preexisting liver disease and SARS‐CoV‐2 infection.
COVID‐19 in Patients With Alcohol Use Disorder
The psychosocial stressors and social distancing needed for initial control of the pandemic have resulted in significantly increased alcohol use and alcohol use disorders (AUDs) throughout the world, as well as limiting in‐person participation in substance use disorder support groups. 3 , 4 AUD is often associated with comorbidities such as diabetes mellitus and chronic kidney disease, which also increase the risk for complications in COVID‐19. 5 Furthermore, the immunosuppressive effects of corticosteroid therapy for alcoholic hepatitis, as well as AUD itself, are risk factors for severe disease. 5 Although there is no specific guidance on management of alcoholic hepatitis during the COVID‐19 pandemic, caution is advised regarding glucocorticoids therapy in SARS‐CoV‐2 infection. 5 , 6 The pandemic has also raised significant barriers for patients with AUD being considered for liver transplantation, resulting in both increased risk for relapse and difficulties in maintaining linkage to care. 5
Unfortunately, many have consumed alcohol in a misguided attempt to protect against coronavirus infection, resulting not only in the risk for alcoholic hepatitis but also in hundreds of deaths from acute methanol poisoning. 7
Observations that use of nonsteroidal anti‐inflammatory drugs (NSAIDS) were linked to more severe disease in COVID‐19 led health authorities to recommend avoiding use of NSAIDS. 8 This subsequently resulted in increased usage of acetaminophen (paracetamol), and although this guidance has since been rescinded, 9 the potential remains for increased utilization of acetaminophen and associated hepatotoxicity, especially when combined with alcohol use.
COVID‐19 in Patients With Autoimmune Liver Disease
Preliminary guidance on the management of autoimmune hepatitis (AIH) has been published by both the American Association for the Study of Liver Diseases (AASLD) 6 and the European Association for the Study of the Liver (EASL). 10 In addition, a detailed management protocol was developed for patients in three referral centers in Europe (Table 1). 11 This recommends stratification of existing patients by risk of complications, with all health care encounters performed via a “COVID‐free” pathway.
Table 1.
Expert Guidance for Management of Autoimmune Liver Disease During COVID‐19 Pandemic
| Risk for Complications from AIH | Suggested Management Pathway |
|---|---|
| Low (i.e., AIH stable on immunosuppressive therapy) |
|
| Moderate (i.e., acute symptoms in patients without cirrhosis, chronic management of decompensated cirrhosis) |
|
| High (i.e., flare of AIH, obstructive jaundice, variceal hemorrhage) |
|
| All |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The effect of immune‐modulating therapy on COVID‐19, as well as the effect of COVID‐19 on immunosuppressed patients with chronic liver disease, is incompletely understood; however, insight may be gained from transplant and other immunosuppressed patients. A preliminary evaluation of solid organ transplant (SOT) recipients hospitalized with COVID‐19 showed significantly higher rates of critical illness, mechanical ventilation, and death, although this likely undercounted mild illness. 12 Conversely, preliminary analysis of COVID‐19 in SOT recipients from Italy showed no increases in severe illness or hospitalization. 13 Finally, use of immune‐modulating agents for other conditions such as rheumatological disease 14 has not resulted in an increased risk for severe SARS‐CoV‐2 infection.
In an attempt to clarify these data, AASLD and EASL have provided guidance on the use of immune‐modulating therapy during the COVID‐19 pandemic, which is summarized in Table 2. These guidelines emphasize that reduction in immune modulation is not recommended in the absence of SARS‐CoV‐2 infection and may be considered in certain subsets of patients with COVID‐19 (i.e., severe disease, lymphopenia, bacterial or fungal superinfection). 6 , 10
Table 2.
Expert Guidance on Immunosuppression for Liver Disease in the Setting of COVID‐19
| Condition | Society Guidance | |
|---|---|---|
| AASLD 6 | EASL/ESCMID 10 | |
| Patients with immunosuppressed liver disease without COVID‐19 | Avoid anticipatory adjustment of immunosuppressive therapy | Avoid reducing immunosuppressive therapy |
| Patients with immunosuppressed liver disease with COVID‐19 |
|
Reduce only in special circumstances (drug‐induced lymphopenia, severe bacterial or fungal superinfection) after consultation with specialist |
| Patients with liver disease with strong indication for initiation of immunosuppressive therapy | Initiate treatment regardless of presence of SARS‐CoV‐2 infection | Not specifically addressed |
| All patients with liver disease and COVID‐19 | Carefully assess risk/benefit ratio when initiating glucocorticoids or other immunosuppressive therapy | Not specifically addressed |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
COVID‐19 in Patients With Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis
Given the association of COVID‐19 severity with metabolic disease, the association with nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) has also been investigated (Table 3). In a report of more than 5000 patients in New York with COVID‐19, hypertension, obesity, and diabetes were the most common comorbidities in patients with COVID‐19, and diabetes appeared to be associated with higher risk for requiring mechanical ventilation. 2 Although the presence of NAFLD was not directly evaluated, only 23% of patients were Hispanic, the group most prone to NASH, and prior reports actually suggest higher death rates among African Americans (who are less likely to have NASH) with COVID‐19. 15 In an early report of 324 patients with COVID‐19 in China, 70 (21.6%) were diagnosed with fatty liver on computed tomography (CT) scan and also made up a significantly higher percentage of the severe COVID‐19 cases than the general prevalence of NAFLD in the population. 16
Table 3.
Studies of MAFLD/NAFLD/NASH and COVID‐19
| Study | Site | Number of patients | Diagnostic Criteria | Outcomes Described | Conclusions |
|---|---|---|---|---|---|
| Qian et al. (2020) 16 | Shanghai, China | 70 | CT scan | Patients with NAFLD accounted for 34.6% of severe patients | High prevalence of NAFLD among patients with severe COVID‐19 |
| Ji et al. (2020) 17 | China | 76 | HSI +/− ultrasound | 34/39 (87.2%) progressive disease versus 42/163 (25.8%) stable disease | Higher risk for progression to severe COVID‐19; longer viral shedding time |
| Zheng et al. (2020) 18 | Wenzhou, China | 66 | CT scan + MAFLD consensus diagnostic criteria | 6‐Fold increased risk for severe COVID‐19 in patients with MAFLD | Risk of obesity to COVID‐19 severity is higher in those with MAFLD |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
In a study from China, conducted at two designated COVID‐19 hospitals, NAFLD was defined using the hepatic steatosis index (HSI) or by abdominal ultrasound examination. 17 Patients with COVID‐19 were divided into stable and progressive, and NAFLD was found to be associated with COVID‐19 progression (defined by worsening respiratory distress or lung CT findings during hospitalization) with an odds ratio of 6.4 (95% confidence interval: 1.5‐31.2). However, although higher body mass index was also associated with progression, diabetes and other metabolic factors were not considered as unique risk factors, so their individual contribution to increased disease severity could not be determined. Patients with NAFLD were also found to have higher risk for disease progression, higher rates of abnormal liver chemistries during admission and disease course, and longer duration of viral shedding (17 versus 12 days) when compared with patients without NAFLD.
Finally, in another study of 214 patients in China, the presence of obesity in metabolic‐associated fatty liver disease (MAFLD) was associated with a 6‐fold increased risk for severe COVID‐19. This risk persisted after adjusting for age, sex, and metabolic features, such as diabetes, hypertension, and dyslipidemia. 18 Because SARS‐CoV‐2 uses the angiotensin‐converting enzyme 2 (ACE2) receptor for cellular entry, increased ACE2 expression on hepatocytes of patients with NAFLD, 19 as well as impaired hepatic innate immune system in patients with NAFLD, are potential mechanisms for increased risk for disease, although further study is required. Given these data, patients with NAFLD, particularly those with diabetes and obesity, should be considered high risk for COVID‐19.
COVID‐19 in Patients With Compensated Cirrhosis
There are several key issues surrounding COVID‐19 infection in patients with cirrhosis, including potentially increased risk for SARS‐CoV‐2 infection, higher risk for severe disease, and increased risk for hepatic decompensation (Fig. 1). Furthermore, the pandemic has significantly affected the management of patients with cirrhosis, both with and without COVID‐19.
Fig 1.
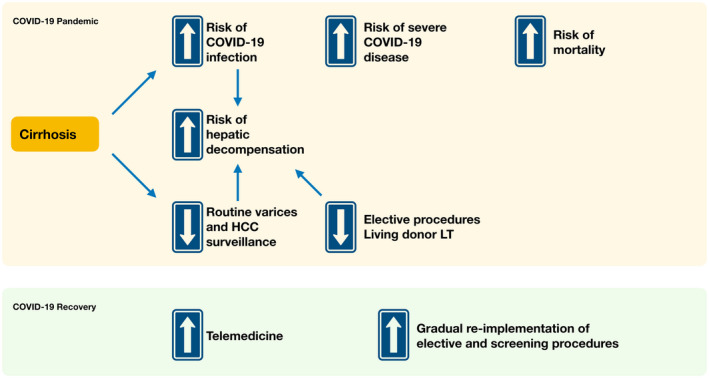
Implications of COVID‐19 on cirrhosis.
There is currently a global effort to determine severity of disease, mortality, and incidence of complications in patients with COVID‐19 with cirrhosis. In the combined COVID‐HEP and SECURE‐Cirrhosis registries, there have been 151 patients reported to date. Alcohol is the most common causative factor, followed by NASH and hepatitis B. 2 Of patients reported thus far, 24% required intensive care admission, 16% required mechanical ventilation, and 40% had reported deaths. Although this is a small sample size, this is significantly higher than the 6% overall mortality rate reported by the Centers for Disease Control and Prevention, 4 underscoring the increased severity of disease in patients with cirrhosis.
Of patients in the combined registries, 38% decompensated during their disease course (worsening ascites, encephalopathy, or acute kidney injury). It is well established that infection places patients at risk for decompensation, and in this particular setting of COVID‐19 characterized by significant cytokine activation, cytokine‐induced hepatocyte apoptosis and necrosis in the setting of diminished liver reserve may lead to hepatic decompensation. 20 Thus, patients with cirrhosis who present with decompensation should be tested for COVID‐19 as a potential cause.
Aside from complications of SARS‐CoV‐2 infection, cirrhosis care in general has significantly been impacted by the COVID‐19 pandemic, with significant disruptions in care flow. 21 Screening for esophageal varices and hepatocellular carcinoma (HCC) is now considered nonurgent and is delayed for all but the highest‐risk patients (i.e., endoscopy for variceal bleeding or follow‐up band ligation in those with recent variceal bleed). AASLD guidance suggests that it is appropriate to defer HCC surveillance for 2 months, after a discussion of the risks and benefits of doing so with the patient. 6 These changes in care pathways have the potential to increase the risk for variceal bleed and later‐stage HCC occurrence. In addition, elective procedures, such as living donor liver transplantation and locoregional therapy for HCC, have been deferred in many centers, potentially increasing both progression of disease and overall mortality. 21 Alternative management strategies, such as increased use of nonselective beta‐blockade, greater use of serum‐based markers, increased outpatient interventions (including albumin infusions), and emphasis on integrated telehealth, have been proposed. 21
Conclusions
Our knowledge of the impact of chronic liver disease on risk and severity of COVID‐19 infection, as well as understanding of the COVID‐19 pandemic and effect on hepatic disease, will continue to evolve. A paradoxical effect of immune suppression on disease severity should not be discounted and may play a significant role in future therapeutic strategies. Optimizing care pathways for those with these liver diseases, particularly if infected with SARS‐CoV‐2, will be critical to improve outcomes of both liver disease and COVID‐19.
Potential conflict of interest: J.C. advises and received grants from Gilead.
References
- 1. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol 2020;5:428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. Available at: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahmed MZ, Ahmed O, Aibao Z, et al. Epidemic of COVID‐19 in China and associated psychological problems. Asian J Psychiatr 2020;51:102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffman J. With meetings banned, millions struggle to stay sober on their own New York Times. Available at: https://www.nytimes.com/2020/03/26/health/coronavirus-alcoholics-drugs-online.html. Published March 26, 2020. [Google Scholar]
- 5. Da BL, Im GY, Schiano TD. COVID‐19 hangover: a rising tide of alcohol use disorder and alcohol‐associated liver disease. Hepatology. Available at: https://doi.org/10.1002/hep.31307 [DOI] [PubMed] [Google Scholar]
- 6. Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID‐19 pandemic: AASLD expert panel consensus statement. Hepatology. Available at: 10.1002/hep.31281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shalbafan M, Khademoreza N. What we can learn from COVID‐19 outbreak in Iran about the importance of alcohol use education. Am J Drug Alcohol Abuse. Available at: 10.1080/00952990.2020.1753759 [DOI] [PubMed] [Google Scholar]
- 8. Day M. Covid‐19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ 2020;368:m1086. [DOI] [PubMed] [Google Scholar]
- 9. Torjesen I. Covid‐19: ibuprofen can be used for symptoms, says UK agency, but reasons for change in advice are unclear. BMJ 2020;369:m1555. [DOI] [PubMed] [Google Scholar]
- 10. Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID‐19 pandemic: EASL‐ESCMID position paper. JHEP Rep 2020;2:100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lleo A, Invernizzi P, Lohse AW, et al. Highlights for management of patients with Autoimmune Liver Disease during COVID‐19 pandemia. J Hepatol. Available at: 10.1016/j.jhep.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. Available at: 10.1111/ajt.15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. Available at: 10.1002/lt.25756 [DOI] [PubMed] [Google Scholar]
- 14. Monti S, Balduzzi S, Delvino P, et al. Clinical course of COVID‐19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis 2020;79:667‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thebault R, Tran AB, Williams V. The coronavirus is infecting and killing black Americans at an alarmingly high rate. Washington Post. Available at: https://www.washingtonpost.com/nation/2020/04/07/coronavirus-is-infecting-killing-black-americans-an-alarmingly-high-rate-post-analysis-shows/?arc404=true. Published April 7, 2020. [Google Scholar]
- 16. Qian ZP, Mei X, Zhang YY, et al. Analysis of baseline liver biochemical parameters in 324 cases with novel coronavirus pneumonia in Shanghai area. Zhonghua Gan Zang Bing Za Zhi 2020;28:E005. [DOI] [PubMed] [Google Scholar]
- 17. Ji D, Qin E, Xu J, et al. Non‐alcoholic fatty liver diseases in patients with COVID‐19: a retrospective study. J Hepatol. Available at: 10.1016/j.jhep.2020.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng KI, Gao F, Wang XB, et al. Obesity as a risk factor for greater severity of COVID‐19 in patients with metabolic associated fatty liver disease. Metabolism 2020;20:154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paizis G, Tikellis C, Cooper ME, et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut 2005;54:1790‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9:727‐738. [DOI] [PubMed] [Google Scholar]
- 21. Tapper EB, Asrani SK. The COVID‐19 pandemic will have a long‐lasting impact on the quality of cirrhosis care. J Hepatol. Available at: 10.1016/j.jhep.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


