Abstract
Background
Current evidence suggests an important role of the interleukin‐6 (IL‐6) pathway in severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐related cytokine release storm in severely ill coronavirus disease 2019 (COVID‐19) patients. Inhibition of the IL‐6 pathway with tocilizumab has been employed successfully in some of these patients but the data is mostly consistent of case reports and series.
Methods
We performed a systematic search of PubMed, Embase, and Medline from 22nd April 2020 and again on 27th April 2020 using the following search terms alone or in combination: “COVID‐19,” “coronavirus,” “SARS‐CoV‐2,” “COVID,” “anti‐interleukin‐6 receptor antibodies,” “anti‐IL‐6,” “tocilizumab,” “sarilumab,” “siltuximab.” We included studies that reported individual patient data. We extracted and analyzed individual level data on baseline characteristics, laboratory findings, and clinical outcomes. The primary endpoint was in‐hospital mortality. Secondary endpoints included in‐hospital complications, recovery rates, effect of patient characteristics on the primary outcome and changes in levels of inflammatory markers.
Results
Three hundred fifty‐two records were identified through a systematic search, of which 10 studies met the inclusion criteria. A single study currently under review was also added. Eleven observational studies encompassing 29 patients were included in the present review. There were more males (24 [82.8%]), and hypertension was the most common comorbidity (16 [48.3%]). Over an average of 5.4 hospital days, the primary endpoint occurred in 6 (20.7%) patients. Among surviving patients, about 10% had worsened disease and 17% recovered. The most common complication was acute respiratory distress syndrome (8 [27.6%]). The IL‐6 level was significantly higher after the initiation of tocilizumab with median (interquartile range) of 376.6 (148‐900.6) pg/mL compared to the baseline of 71.1 (31.9‐122.8) pg/mL (P = .002). Mean (standard deviation) levels of C‐reactive protein (CRP) were significantly decreased following treatment 24.6 (26.9) mg/L compared to baseline 140.4 (77) mg/L (P < .0001). Baseline demographics were not significantly different among survivors and nonsurvivors by Fisher's exact test.
Conclusion
In COVID‐19 patients treated with tocilizumab, IL‐6 levels are significantly elevated, which are supportive of cytokine storm. Following initiation of tocilizumab, there is elevation in the IL‐6 levels and CRP levels dramatically decrease, suggesting an improvement in this hyperinflammatory state. Ongoing randomized control trials will allow for further evaluation of this promising therapy.
Importance
Recent data indicate that severe COVID‐19 causes a cytokine release storm and is associated with worse clinical outcomes and IL‐6 plays an important role. It is suggestive that anti‐IL‐6 results in the improvement of this hyperinflammatory state. However, to our knowledge, there is no individual patient data systematic review performed to summarize baseline characteristics and clinical outcomes of COVID‐19 patients who received tocilizumab.
Keywords: anti‐interleukin‐6 receptor antibody, COVID‐19, individual patient data, SARS‐CoV‐2, tocilizumab
Highlights
Interleukin‐6 (IL‐6) may play an important role in the pathogenesis of COVID‐19.
Data show that tocilizumab, an IL‐6 receptor antagonist, reduces COVID‐19 complications.
Our systematic review suggests that tocilizumab may improve survival in COVID‐19.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was first observed in Wuhan, China, in December 2019 and was later declared a pandemic by World Health Organization on 11th March 2020. 1 Severe COVID‐19 is characterized as a syndrome of hypoxemic pneumonitis with a profound inflammatory response. This inflammatory response is associated with elevated levels of inflammatory cytokines including interleukin‐6 (IL‐6) and leads to diffuse alveolar damage and acute respiratory distress syndrome (ARDS). 2‐4 Patients with severe disease often have high IL‐6 levels. 5 Therefore, the treatment of cytokine storm with IL‐6 blocking agents for severely ill COVID‐19 patients seems logical. Trials are ongoing to assess the effectiveness of three IL‐6 blocking agents: tocilizumab, siltuximab, and sarilumab in COVID‐19 patients. 6 , 7 , 8 Tocilizumab is FDA (Food and Drug Administration) approved for chimeric antigen receptor (CAR) T cell‐induced severe or life‐threatening cytokine release syndrome. 9 Currently, there are no published randomized trials on the use of tocilizumab in COVID‐19 patients. The best evidence thus far is from observational studies, which often report aggregate data. We performed a systematic review of individual patient data to summarize the baseline characteristics of COVID‐19 patients who received tocilizumab to identify nuanced predictors of response to therapy and other clinical outcomes.
2. METHODS
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses of the individual participant data (the PRISMA‐IPD) statement was followed for the conduct and reporting of this systematic review. 10
2.1. Data sources, searches and outcome definitions
DA and ZK performed a systematic search of PubMed, Embase, and Medline from 22nd April 2020 and again on 27th April 2020. The following search terms or keywords were used alone or in combination: “COVID‐19,” “coronavirus,” “SARS‐CoV‐2,” “COVID,” “anti‐interleukin‐6 receptor antibodies,” “anti‐IL‐6,” “tocilizumab,” “sarilumab,” “siltuximab.” We did not apply age, geographic, language, publication type, or date restrictions. Following the search and duplicate record removal, two independent reviewers (DA and ZK) screened titles and abstracts for inclusion in the review. The primary outcome of interest was in‐hospital mortality. Secondary outcomes included in‐hospital complications, recovery rates, effect of patient characteristics on the primary outcome, and changes in levels of inflammatory markers.
2.2. Study selection
Included studies reported a diagnosis of reverse‐transcriptase polymerase chain reaction‐confirmed COVID‐19 in patients who received either tocilizumab, sarilumab, or siltuximab alone or in addition to regular care for COVID‐19. A single study currently under review was also added. 11 All included studies reported individual patient data. We excluded reviews, letters, comments, editorials, society guidelines, nonhuman studies, and all studies which did not report individual patient data.
2.3. Study quality assessment and data extraction
Eligible studies were critically appraised by the two independent reviewers at the study level for methodological and reporting quality by adapting the ROBIN‐I tool for assessment of risk of bias to the selected observational studies. 12 Where the two independent reviewers disagreed on whether a study met the inclusion criteria or not, we resolved the disagreement through discussion, which involved a third author when necessary. We assessed inter‐reviewer reliability in selecting studies with Fleiss' kappa. Where required, authors of papers were contacted to request missing or additional data for clarification.
Two reviewers (DA and ZK) independently extracted relevant data using a data extraction form designed for this systematic review. Extracted data included study characteristics such as the name of the first author, study type, country of origin of the study, and the clinical setting of the patient encounter. Patient characteristics included age, sex, pre‐existing medical conditions, day of tocilizumab administration from the day of hospitalization, dose of tocilizumab, and administration of hydroxychloroquine, corticosteroids, antivirals, or antibiotics during index hospitalization. When available, we also extracted information on in‐hospital complications, levels of C‐reactive protein (CRP) and IL‐6 before and after the administration of tocilizumab, adverse effects of tocilizumab when reported, as well as, clinical outcomes.
2.4. Data synthesis and analysis
Continuous variables were summarized as mean (standard deviations [SD]) or median (interquartile range [IQR]), where applicable, and categorical variables were presented as number (percentage). We compared baseline demographics and month survival in patients using Fisher's exact test. Differences in the serum levels of IL‐6 and CRP before and after administration of tocilizumab were assessed with paired Student's t test or Wilcoxon‐signed rank test depending upon whether the normality assumptions were met. All tests were performed as two‐tailed and statistical significance levels set at a P < .05 with Stata (StataCorp version 14, College Station, TX) and XLSTAT (Addinsoft, New York, NY).
3. RESULTS
3.1. Search results
A total of 351 records were identified through the systematic search. A single study currently under review was also added. 11 Thus, 352 records were screened for eligibility for further full‐text review. Of these, 30 studies underwent full‐text review for inclusion in the analysis. Among these, 11 studies were identified for inclusion that encompassed 29 patients and were available for further analysis (Figure 1). The Fleiss' kappa for inter‐reviewer reliability in selecting studies was 0.85, with a sensitivity of 100% and specificity of 89%. Disagreement was on the inclusion of two studies. This was resolved after extensive discussion among the three reviewers.
Figure 1.
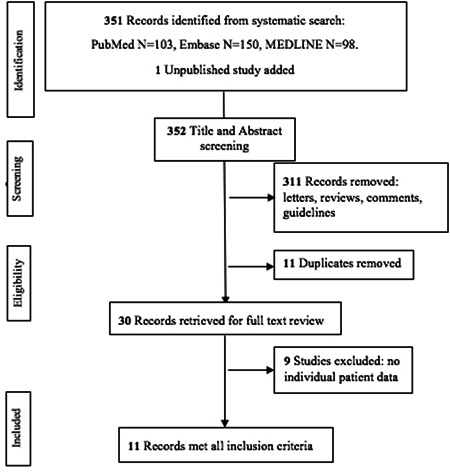
Flow diagram of the study selection process
3.2. Study characteristics and quality assessment
The majority of the patients came from the case series, of which 55% came from a single Chinese report. 13 Table 1 illustrates the characteristics of each included study. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 These studies were of high quality except for the lack of reports on tocilizumab adverse effects. There were inconsistencies in the definition of the severity of illness, comorbid conditions, and level of care received. The corresponding authors of the included studies provided data and the clarification on this upon request. Most patients (n = 24 [83%]) received intensive care unit (ICU) level of care.
Table 1.
Study characteristics and assessment of study quality
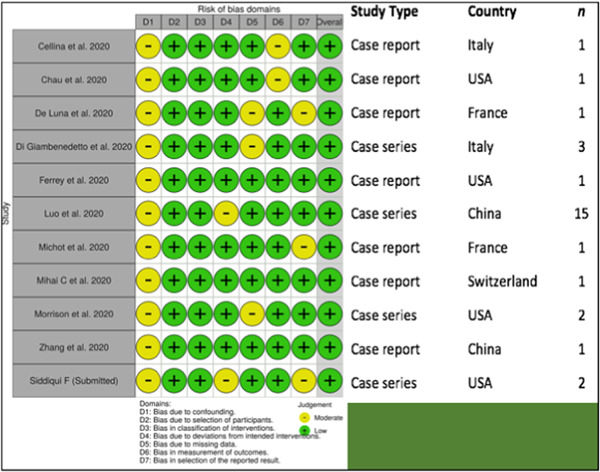
|
3.3. Patient characteristics, hospital course, and outcomes
The baseline patient characteristics are outlined in Table 2. There were more males (24 [82.8%]) compared to females. Hypertension was the most common comorbidity (16 [48.3%]). About 20% of patients did not have any reported comorbidities. Three patients were taking immunosuppressive medications before the diagnosis of COVID‐19. One was taking tocilizumab for systemic sclerosis. 20 Another was taking hydroxychloroquine for mixed connective tissue disease. 11 The other was taking thalidomide for multiple myeloma. 22 There was a considerable heterogeneity in the dosing and duration of tocilizumab therapy. Weight‐based dosing ranged from 6 to 8 mg/kg per dose, and frequency ranged from one to four times during the hospitalization. There was no uniform indication for the initiation of tocilizumab. However, most patients received the medication when there was an increased oxygen requirement along with a rise in inflammatory markers such as CRP. Of these 29 patients, 52% received only tocilizumab and the rest of the patients received corticosteroid, hydroxychloroquine, lopinavir‐ritonavir and/or other antiviral therapy in addition to tocilizumab (Table 2).
Table 2.
Baseline characteristics and clinical outcomes in COVID‐19 patients treated with tocilizumab
| Demographics | Number of patients (N = 29) |
|---|---|
| Male sex, n (%) | 24 (82.8) |
| Age in years, mean (SD) | 63 (±12) |
| Comorbidities, n (%) | |
| Hypertension | 16 (48.3) |
| Diabetes | 7 (24.1) |
| Obesity | 5 (17.2) |
| Cerebrovascular accident | 3 (10.3) |
| Heart failure | 2 (6.9) |
| Heart disease | 2 (6.9) |
| Lung disease | 1 (3.4) |
| Renal disease | 3 (10.3) |
| Rheumatologic disease | 2 (6.9) |
| Medications reported before diagnosis of COVID‐19, n (%) | |
| Antivirals | 1 (3.4) |
| Immunosuppressive agents | 2 (6.9) |
| Other medications used for COVID‐19, n (%) | |
| Corticosteroids | 10 (34.5) |
| Hydroxychloroquine | 7 (24.1) |
| Lopinavir/ritonavir | 6 (20.7) |
| Ribavarin | 1 (3.4) |
| Umifovir | 1 (3.4) |
| In‐hospital complications, n (%) | |
| Acute respiratory distress syndrome | 8 (27.6) |
| Mechanical ventilation | 6 (20.7) |
| Acute kidney injury | 1 (3.4) |
| Liver injury | 1 (3.4) |
| Myocardial injury | 3 (10.3) |
| Arrhythmia | 1 (3.4) |
| Shock | 2 (2.9) |
| Outcome, n (%) | |
| Death | 6 (20.7) |
| Alive | 23 (79.3) |
Abbreviations: COVID‐19, coronavirus disease 2019; SD, standard deviation.
Serum IL‐6 levels before and after initiation of tocilizumab were available for 17 patients. There were inconsistencies in reporting on the time when the first IL‐6 levels were drawn relative to the index dose of tocilizumab. However, follow‐up IL‐6 levels were obtained about 2 days after the first dose of tocilizumab. The IL‐6 level was significantly higher after the initiation of tocilizumab with median (IQR) of 376.6 (148‐900.6) pg/mL compared to the baseline of 71.1 (31.9‐122.8) pg/mL (P = .002) as seen in Figure 2A. CRP levels before and after tocilizumab initiation were available for 22 patients. The CRP levels were drawn 2.4 (±1.6) days before the initiation of tocilizumab, and repeat measures were drawn 3.4 (±2.5) days after the index dose of tocilizumab. The mean (SD) CRP levels before and after tocilizumab was 140.4 (77) mg/L and 24.6 (26.9) mg/L, respectively (Figure 2B). CRP level was significantly lower after the initiation of tocilizumab (P < .0001).
Figure 2.
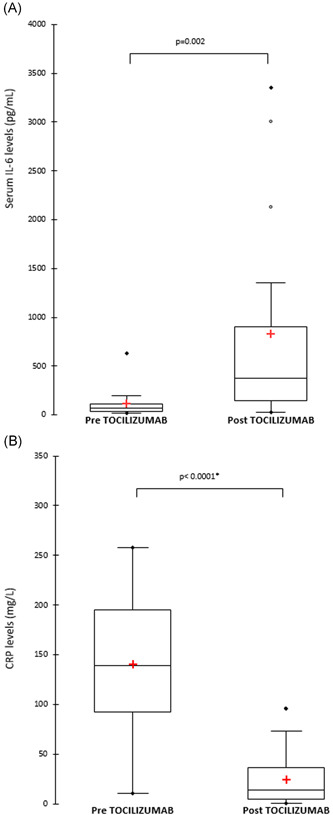
Plot of serum levels of inflammatory markers before and after initiation of tocilizumab. (A) IL‐6 (Wilcoxon‐signed rank test), (B) CRP (Student's paired t test). CRP, C‐reactive protein; IL‐6, interleukin‐6
Over an average of 5.4 hospital days, 6 (20.7%) of the 29 patients had died. Two‐way comparisons of the baseline demographics were not significantly different amongst survivors and nonsurvivors by Fisher's exact test. Among the survivors, about 10% had worsened disease, 17% recovered and 4 (17.3%) were discharged home. One patient was not hospitalized due to mild symptoms. That patient was, however, taking tocilizumab before COVID‐19 diagnosis. Disposition information on 18 patients was not available, although most appear to remain hospitalized at the end of the reports. The most common in‐hospital complication was ARDS (27.6%) requiring mechanical ventilation (Table 2), which is certainly an indication of disease progression rather than a consequence of treatment. There were three reports of medication‐related complications including hypertriglyceridemia in two patients and an incident of QT interval prolongation believed to be due to an adverse effect of hydroxychloroquine. 15 , 21
4. DISCUSSION
This systematic review was based on 11 studies that encompassed 29 patients who received tocilizumab during their admission. The results demonstrated that most patients were treated in the ICU, which is in line with most of the institutional guidelines where tocilizumab is usually administered at later or advanced stages of illnesses. The sex distribution was heavily skewed toward males, which is a common observation in this disease. 2 , 23 , 24 Other demographic features of the patients involved in the present study included the presence of comorbidities including hypertension, diabetes, obesity, cerebrovascular accident, cardiac, and lung diseases. The presence of these illnesses has important prognostic value, and as such, these patients were noted to have rapidly deteriorated and required admission to ICU and administration of anti‐IL‐6 agents. 25 A considerable proportion of these patients further developed ARDS and required mechanical ventilation. Review of clinical outcomes data was limited because of limitations encountered secondary to event reporting limitations and limited timeframe.
An interesting observation was an improvement in serum CRP levels, which could serve as a surrogate for dampening inflammation and clinical improvement. An initial rise in IL‐6 levels after initiation of tocilizumab is temporarily expected as its receptors have been blocked by tocilizumab. 26 There may exist an upper threshold beyond which this temporary feedback mechanism fails and elevated IL‐6 levels persist, as seen in two patients in this study. 13 Therefore it seems likely that the clinical improvement noted in some of these patients is the result of rapid control of hyper inflammation resulting from tocilizumab treatment.
The use of tocilizumab in COVID‐19 patients who develop respiratory failure and other complications is currently off the label, and hence its safety profile needs to be evaluated with further randomized control trials. Currently, the FDA has approved phase III clinical trial of tocilizumab for COVID‐19 pneumonia. There were several instances where successful treatment of patients with this drug was carried out as illustrated in this study. The small sample size from these observational studies and the heterogeneity of the results including the scarcity of the clinical outcomes data limit the generalizability of this study.
5. CONCLUSION
In conclusion, severe COVID‐19 patients may develop the dreaded hyperinflammatory cytokine release syndrome, which involves a complex interplay of IL‐6 and other chemokines. IL‐6 seems to play a pivotal role in this, and the use of anti‐IL‐6 treatment seems beneficial. Data from randomized control trials are needed for the appropriate evaluation of clinical outcomes using this promising therapy.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
FS conceived the study hypothesis. MSR and DA designed the study. DA and ZK performed systematic searches, study selection, and data extraction. MSR and DA analyzed the data. All authors contributed to the interpretation of the data, writing, and critical editing of the manuscript.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Veterans Affairs leadership for their continued help and support during this project: Sara Ashraf, MD; Amy Sanguinetti, MD, PhD; and Robert Kimmel, MD.
Antwi‐Amoabeng D, Kanji Z, Ford B, Beutler BD, Riddle MS, Siddiqui F. Clinical outcomes in COVID‐19 patients treated with tocilizumab: An individual patient data systematic review. J Med Virol. 2020;92:2516–2522. 10.1002/jmv.26038
REFERENCES
- 1. WHO. WHO Director‐General's opening remarks at the media briefing on COVID‐19—11 March 2020. https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19‐‐‐11‐march‐2020
- 2. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID‐19 and interleukin‐6 receptor (IL‐6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;29:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coomes EA, Haghbayan H. Interleukin‐6 in COVID‐19: a systematic review and meta‐analysis. medRxiv. 2020. 10.1101/2020.03.30.20048058 [DOI] [PMC free article] [PubMed]
- 6.Efficacy of Subcutaneous Sarilumab in Hospitalised Patients With Moderate‐severe COVID‐19 Infection (SARCOVID). https://ClinicalTrials.gov/show/NCT04357808. Accessed May 2, 2020. [DOI] [PMC free article] [PubMed]
- 7. Tocilizumab in COVID‐19 Pneumonia (TOCIVID‐19). https://ClinicalTrials.gov/show/NCT04317092. Accessed May 2, 2020.
- 8. Efficacy and Safety of Siltuximab vs. Corticosteroids in Hospitalized Patients With COVID‐19 Pneumonia. https://ClinicalTrials.gov/show/NCT04329650. Accessed May 2, 2020.
- 9. Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell‐induced severe or life‐threatening cytokine release syndrome. Oncologist. 2018;23(8):943‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for systematic review and meta‐analyses of individual participant data: the PRISMA‐IPD statement. JAMA. 2015;313(16):1657‐1665. [DOI] [PubMed] [Google Scholar]
- 11. Siddiqui F, Rai A, Riddle MS. Use of tocilizumab for the treatment of severe COVID‐19: experience from a Veterans Affairs hospital in Northern Nevada. Submitted to Case Rep Med. 2020. [Google Scholar]
- 12. McGuinness LA, Higgins JPT. Risk‐of‐bias VISualization (robvis): an R package and Shiny web app for visualising risk‐of‐bias assessments. Res Syn Meth. 2020:1‐7. 10.1002/jsrm.1411 [DOI] [PubMed] [Google Scholar]
- 13. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cellina M, Orsi M, Bombaci F, Sala M, Marino P, Oliva G. Favorable changes of CT findings in a patient with COVID‐19 pneumonia after treatment with tocilizumab. Diagn Interv Imaging. 2020;101(5):323‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chau VQ, Oliveros E, Mahmood K, et al. The imperfect cytokine storm: severe COVID‐19 with ARDS in patient on durable LVAD support [published online ahead of print April 8, 2020]. JACC Case Rep. 10.1016/j.jaccas.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Luna G, Habibi A, Deux JF, et al. Rapid and severe Covid‐19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. Am J Hematol. 2020. [published online ahead of print April 13, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Giambenedetto S, Ciccullo A, Borghetti A, et al. Off‐label use of tocilizumab in patients with SARS‐CoV‐2 infection [published online ahead of print April 16, 2020]. J Med Virol. 10.1002/jmv.25897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferrey AJ, Choi G, Hanna RM, et al. A case of novel coronavirus disease 19 in a chronic hemodialysis patient presenting with gastroenteritis and developing severe pulmonary disease. Am J Nephrol. 2020;51(5):337‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michot JM, Albiges L, Chaput N, et al. Tocilizumab, an anti‐IL6 receptor antibody, to treat Covid‐19‐related respiratory failure: a case report [published online ahead of print April 2, 2020]. Ann Oncol. 10.1016/j.annonc.2020.03.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mihai C, Dobrota R, Schröder M, et al. COVID‐19 in a patient with systemic sclerosis treated with tocilizumab for SSc‐ILD. Ann Rheum Dis. 2020;79(5):668‐669. [DOI] [PubMed] [Google Scholar]
- 21. Morrison AR, Johnson JM, Ramesh M, Bradley P, Jennings J, Smith ZR. Letter to the Editor: acute hypertriglyceridemia in patients with COVID‐19 receiving tocilizumab. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang X, Song K, Tong F, et al. First case of COVID‐19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4(7):1307‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li LQ, Huang T, Wang YQ, et al. COVID‐19 patients' clinical characteristics, discharge rate, and fatality rate of meta‐analysis [published online ahead of print March 12, 2020]. J Med Virol. 10.1002/jmv.25757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alattar R, Ibrahim TBH, Shaar SH, et al. Tocilizumab for the treatment of severe COVID‐19. J Med Virol. 2020:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid‐19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID‐19? J Transl Med. 2020;18(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]


