Abstract
Since the outbreak of coronavirus disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was first reported in Wuhan, a series of confirmed cases of COVID‐19 were found on the Qinghai‐Tibet plateau. We aimed to describe the epidemiological, clinical characteristics, and outcomes of all confirmed cases in Qinghai, a province at high altitude. The region had no sustained local transmission. Of all 18 patients with confirmed SARS‐CoV‐2 infection, 15 patients comprising four transmission clusters were identified. Three patients were infected by direct contact without travel history to Wuhan. Of 18 patients, 10 patients showed bilateral pneumonia and two patients showed no abnormalities. Three patients with comorbidities such as hypertension, liver diseases, or diabetes developed severe illness. High C‐reactive protein levels and elevations of both alanine aminotransferase and aspartate aminotransferase were observed in three severely ill patients on admission. All 18 patients were eventually discharged, including the three severe patients who recovered after treatment with noninvasive mechanical ventilation, convalescent plasma, and other therapies. Our findings confirmed human‐to‐human transmission of SARS‐CoV‐2 in clusters. Patients with comorbidities are more likely to develop severe illness.
Keywords: clinical characteristics, COVID‐19, epidemiological, plateau, SARS‐CoV‐2
Highlights
All 18 patients with COVID‐19 including severe ones in Qinghai at high altitude were eventually discharged.
High of C‐reactive protein levels and elevations of both ALT and AST at early stage may predict the progression of severe patients with COVID‐19.
Second‐generation cases confirmed human‐to‐human transmission of SARS‐CoV‐2 in clusters.
Patients with comorbidities are more likely to develop severe illness.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), emerged in Wuhan, Hubei, China in December 2019 1 , 2 , 3 , 4 and rapidly spread worldwide. 5 The outbreak spread to 209 countries and the global number of reported cases surpassed 1 210 000 as of 6 April 2020. 6 With evidence that SARS‐CoV‐2 is spread by human‐to‐human transmission, 7 , 8 , 9 the increasing number of cases and widening geographical spread of the disease raise a global health concern. 10
So far, several studies have described the epidemiological and clinical features of COVID‐19, but the data mainly came from Wuhan. 4 , 11 Qinghai province, located on the Qinghai‐Tibet Plateau with an average altitude of more than 3000 m above sea level and a population of 6.03 million, reported a total of 18 confirmed cases by 6 April. During the outbreak, Qinghai rapidly instituted a number of strict control measures to lower transmission, including the enforcement of quarantine measures, early detection, reducing passenger flow, and strong social messaging. By 6 April 2020, no new confirmed cases had been found in Qinghai Province for 60 consecutive days since 6 February 2020. More importantly, all 18 patients including three severely ill cases had been discharged after treatment by 21 February 2020 (Figure 1).
Figure 1.
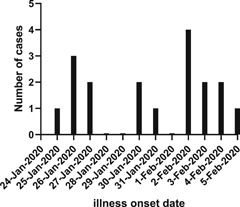
Date of illness onset of patients with laboratory‐confirmed 2019‐nCoV infection in Qinghai, China
In this study, we report the epidemiological and clinical characteristics, and outcomes of all 18 confirmed COVID‐19 patients in Qinghai including family clusters who returned to Qinghai from Wuhan, and family members who did not travel to Wuhan.
2. METHODS
2.1. Study design and patients
For this retrospective study, we enrolled all 18 patients infected with SARS‐CoV‐2 from the hospitals designated for treatment by the Health Commission of Qinghai Province from 21 January to 6 April 2020. A total of 15 patients were from the Fourth People's Hospital of Qinghai Province and three patients were from the Third People's Hospital of Xining. All confirmed patients enrolled in this study were diagnosed with COVID‐19 according to World Health Organization interim guidance. 12 A confirmed case of COVID‐19 was defined as a positive result on real‐time reverse‐transcriptase polymerase chain reaction (RT‐PCR) assay from nasopharyngeal swab specimens. Under the condition of tertiary protection, nasopharyngeal swabs of patients were collected in the isolation ward for SARS‐CoV‐2 nucleic acid detection. All biological samples were sealed and transferred to the laboratory of Qinghai Center for Diseases Prevention and Control in strict accordance with the standard process. Total RNA was extracted using RNA isolation kit (Tianlong Science Technology, Xi'an, China). To target the nucleocapsid (N) gene and open reading frame lab (ORF1ab) gene, a SARS‐Cov‐2 real‐time PCR kit (Bio‐germ, Shanghai, China) was used in fluorescent PCR method. Both internal controls and negative controls were routinely performed with each batch of tests. Incubation period was defined from the first point of contact with a symptomatic case to illness onset. This study was approved by the ethics commissions of the two hospitals. Oral consent was obtained from patients.
2.2. Data collection
The epidemiological, demographic, clinical, laboratory and radiological characteristics, and treatment and outcomes data were obtained from patients’ medical records. Information recorded included demographic data, exposure history, comorbidities, symptoms, laboratory findings, and computed tomographic (CT) scans. Laboratory confirmation of SARS‐CoV‐2 by real‐time PCR was done in Qinghai Center for Diseases Prevention and Control. The severity of COVID‐19 was defined based on COVID‐19 Guidelines (5th version) made by the National Health Commission of the People's Republic of China (NHCC). Qinghai is located on a plateau, with an average altitude of 2261 m above sea level in Xining area. The atmospheric pressure and air oxygen content are low. Therefore, oxygenation index should be calculated as follows: PaO2/(FIO2 × [barometric pressure/760]) QJ;according to the Berlin Definition of Acute Respiratory Distress Syndrome (ARDS). 13
Mild cases: clinical symptoms were mild without pneumonia manifestation through image results. Moderate cases: having fever and other respiratory symptoms with pneumonia manifestation through image results. Severe cases: meeting any one of the following: respiratory distress, RR more than 30/minutes; SpO2 ≤90% at rest in Xining adjusted according to altitude; PaO2/FiO2 ≤300mm Hg needed to be corrected according to altitude as mentioned above.
All treatment measures were collected during the hospitalization, such as antiviral therapy, antibacterial therapy, corticosteroid therapy, traditional Chinese medicine therapy, immune support therapy, convalescent plasma therapy, and respiratory support. Discharge criteria were based on COVID‐19 Guidelines (5th version) by NHCC as follows: body temperature normal for more than 3 days, respiratory symptoms and pulmonary imaging improved significantly, and respiratory tract specimen nucleic acid amplification test negative on two consecutive occasions at least 24 hours apart.
2.3. Statistical analysis
Categorical variables were described as proportions and percentages, and continuous variables were described using median and range (min‐max) values. Statistical analyses were done using the Graphpad Prism software, version 8.02, unless otherwise indicated. For unadjusted comparisons, a two‐sided α of less than .05 was considered statistically significant. Median values were compared by Mann‐Whitney test.
3. RESULTS
3.1. Epidemiological analysis
All 18 patients identified as confirmed SARS‐CoV‐2 cases were included in this study from 25 January to 5 February 2020 (Figure 1). Among them, 3 (17%), 13 (72%), and 2 (11%) patients were categorized into severe, moderate, and mild groups, respectively, during hospitalization. Of three severe patients, two were initially classified as moderate and then changed to severe as disease progressed. In total, 15 patients returned from Wuhan, Hubei Province of China. Of the three remaining cases, two patients had contact with a confirmed case and one patient had contact with a family member who returned from Wuhan with a negative result on nucleic acid amplification test. In total, four clusters of SARS‐CoV‐2 infection were identified, involving 15 cases. The median age was 32 years (range: 7‐47 years), and 12 (67%) were men. A total of 5 (28%) patients, including three severe patients, had chronic diseases, including hypertension, hyperlipidemia, diabetes, liver injury, and polymyositis (Tables 1 and 2). On admission, the most common symptoms were cough (9 [50%]), sputum production (6 [33%]), chest tightness (6 [33%]), fever (3 [17%]), and fatigue (3 [17%]). All three severe patients showed cough, sputum production, and chest tightness but only two showed fever (Table 2). Less common symptoms were sore throat, and diarrhoea (Table 1). Three second‐generation patients did not travel to Wuhan. The two source patients came from Wuhan and showed fever, cough, and fatigue when they contacted. Two second‐generation patients contacted with one source patient for 2 days and the incubation periods were 14 and 15 days separately. The third second‐generation patient contacted with the other source patient for 5 days and the incubation periods was 5 days. Therefore, three second‐generation cases had incubation periods of 5, 14, and 15 days, respectively. The median time from departure from Wuhan to admission was 8 days (range: 1‐16).
Table 1.
Demographics, comorbidities, clinical symptoms, and radiology findings on admission
| All patients (n = 18) | |
|---|---|
| Age, median (range), y | 32 (7‐47) |
| Gender | |
| Male | 12 (67%) |
| Female | 6 (33%) |
| Travel to Wuhan | 15 (83%) |
| Interval between admission to hospital and departure from Wuhan, median (min, max), d | 8 (1,16) |
| Second‐generation cases | 3 (17%) |
| Incubation period, d | 5, 14, 15 |
| Cluster (cases) | 4 (15) |
| Interval from nucleic acid amplification test positive to negative, median (min, max), d | 9 (5, 18) |
| Length of hospital stay, median (min, max), d | 13.5 (8,20) |
| Comorbidities | 5 (28%) |
| Hypertension | 3 (17%) |
| Hyperlipidemia | 1 (6%) |
| Diabetes | 1 (6%) |
| Liver injury | 3 (17%) |
| Polymyositis | 1 (6%) |
| Signs and symptoms | |
| Fever | 3 (17%) |
| Cough | 9 (50%) |
| Expectoration | 6 (33%) |
| Chest tightness | 6 (33%) |
| Fatigue | 3 (17%) |
| Sore throat | 2 (11%) |
| Diarrhoea | 1 (6%) |
| More than one sign or symptom | 10 (56%) |
| Radiology manifestation | |
| Normal | 2 (11%) |
| Unilateral involvement | 6 (33%) |
| Bilateral involvement | 10 (56%) |
| Multiple mottling and ground‐glass opacity | 12 (67%) |
Table 2.
Clinical characteristics of three severe patients with COVID‐19
| On admission | Severe | Discharged | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Comorbidities | symptoms | Radiology | ALT, U/L | AST, U/L | C‐reactive protein, mg/L | Interval from onset to severe illness | Corrected PaO2/FiO2 | Length of hospital stay | |
| Case 1 | Hypertension, hyperlipidemia, diabetes, liver injury | Cough, sputum production, chest tightness, fatigue | Bilateral pneumonia | 151 | 76 | >20 | 13 | 217 | 16 |
| Case 2 | Hypertension, liver injury | Fever, cough, sputum production, chest tightness, fatigue | Bilateral pneumonia | 78 | 64 | 53.9 | 8 | 174 | 18 |
| Case 3 | Hepatitis B | Fever, cough, sputum production, chest tightness | Bilateral pneumonia | 82 | 101 | 17.9 | 8 | 163 | 18 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID‐19, coronavirus disease 2019; FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen.
3.2. Imaging features
Chest X‐ray or CT examination was performed on all patients on admission. Of 18 patients, 10 (56%) patients showed bilateral pneumonia while 6 (33%) patients showed unilateral pneumonia, and 2 (11%) patients showed no abnormalities (Table 1). The most common abnormalities were ground‐glass opacities (12 [67%]) and patchy shadows (Figure 2).
Figure 2.
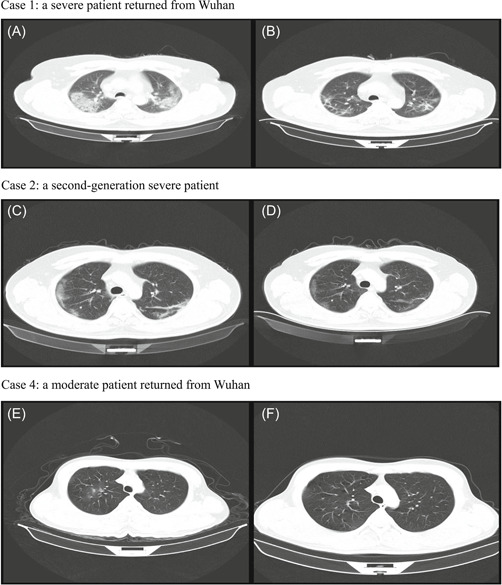
Chest computed tomographic (CT) images of patients infected with SARS‐CoV‐2. Case 1: a severe patient returned from Wuhan. Case 2: a second‐generation severe patient. Case 4: a moderate patient returned from Wuhan. Case 1: A, CT scan showing mass shadows of consolidation and bilateral ground‐glass opacities on day 13 after symptom onset; B, image showing the resolution of bilateral ground‐glass opacities and a decrease of consolidation after treatment. Case 2: C, CT scan showing bilateral ground‐glass opacities on day 6 after symptom onset; D, image showing the resolution of bilateral ground‐glass opacities and low‐density shadow after treatment. Case 4: E, CT scan showing ground‐glass opacities on day 8 after symptom onset; F, image showing the resolution of the lesions after treatment
3.3. Laboratory findings
On admission, 3 (17%), 3 (17%), 4 (22%), and 5 (28%) patients had leucopenia, lymphopenia, neutropenia, and eosinophilia, respectively (Table 3). Hemoglobin was above the normal range in 6 (33%) patients, which may be attributed to high altitude. Both alanine aminotransferase (ALT) and aspartate aminotransferase (AST) concentrations were elevated in four patients including three severe patients (Table 2). Elevated levels of lactate dehydrogenase (LDH) and creatine kinase were found in 6 (40%) and 2 (13%) cases, respectively. One patient with polymyositis showed abnormal creatine kinase (3510 U/L), LDH (527 U/L), ALT (82 U/L), and AST (101 U/L). Elevations of glucose and lactic acid were found in about 44%. Similarly, 7/16 and 5/16 patients demonstrated elevated C‐reactive protein levels and erythrocyte sedimentation rates, respectively. Notably, the levels of C‐reactive protein in three severe patients were higher than those of mild and moderate patients (P < .01; Tables 2 and 3). Levels of D‐dimer and procalcitonin were in the normal range in 12 detected patients.
Table 3.
Laboratory examination of patients infected with SARS‐CoV‐2 on admission
| Normal range | Median (min, max) | Proportion of cases with higher than normal value | Proportion of cases with lower than normal value | |
|---|---|---|---|---|
| Blood routine and lymphocyte classification | ||||
| White blood cells, ×109/L | 4.00‐10.00 | 4.8 (2.4, 9.5) | 0/18 | 3/18 |
| Lymphocytes, ×109/L | 0.8‐4 | 1.4 (0.6, 3.2) | 0/18 | 3/18 |
| Neutrophils, ×109/L | 2‐7.7 | 2.8 (1.0, 8.2) | 1/18 | 4/18 |
| Eosinophils, ×109/L | 0.02‐0.5 | 0.04 (0, 0.13) | 0/18 | 5/18 |
| Hemoglobin, g/L | 110‐160 | 156 (133, 229) | 6/18 | 0 |
| Platelets, ×109/L | 100‐300 | 171 (91, 281) | 0/18 | 2/18 |
| Blood biochemistry | ||||
| Alanine aminotransferase, U/L | 0‐50 | 45 (15, 151) | 6/15 | 0 |
| Aspartate aminotransferase, U/L | 0‐50 | 28 (15, 101) | 4/15 | 0 |
| Total bilirubin, µmol/L | 0‐22 | 10.5 (5.2, 16.4) | 0/15 | 0 |
| Albumin, g/L | 35‐55 | 43 (35, 51) | 0/15 | 0 |
| Lactate dehydrogenase, U/L | 110‐245 | 215 (142, 599) | 6/15 | 0 |
| Creatine kinase, U/L | 25‐200 | 65 (37, 3510) | 2/15 | 0 |
| Blood urea nitrogen, mmol/L | 1.7‐8.6 | 5.0 (2.9, 7.7) | 0/15 | 0 |
| Glucose, mmol/L | 3.8‐6.2 | 6.1 (4.7, 15.6) | 8/18 | 0 |
| Lactic acid, mmol/L | 0.5‐1.6 | 1.6 (0.7, 2.7) | 7/16 | 0 |
| Potassium, mmol/L | 3.5‐5.5 | 3.8 (3.0, 5.8) | 2/18 | 6/18 |
| Sodium, mmol/L | 135‐145 | 138 (131, 143) | 0/18 | 3/18 |
| Coagulation function | ||||
| Prothrombin time, s | 10‐15 | 12.9 (11.4, 13.8) | 0/15 | 0 |
| Activated partial thromboplastin time, s | 22‐40 | 33 (23, 40.3) | 1/15 | 0 |
| Fibrinogen degradation products, µg/mL | 0‐5 | 1.2 (0.2, 3.3) | 0/13 | 0 |
| D‐dimer, μg/mL | 0‐1 | 0.24 (0.14, 0.8) | 0/12 | 0 |
| Infection‐related parameters | ||||
| Procalcitonin, ng/mL | 0‐0.15 | Normal | 0/12 | 0 |
| C‐reactive protein, mg/L | 0‐3 | 2.2 (0.3, 53.9) | 7/16 | 0 |
| Erythrocyte sedimentation rate, mm/h | 0‐20 | 13.5 (2, 72) | 5/16 | 0 |
Abbreviation: SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
3.4. Treatment
All 18 patients received antiviral treatment, including lopinavir/ritonavir (18 [100%]), interferon‐α2b (18 [100%]), oseltamivir (5 [28%]), and ribavirin (6 [33%]; Table 4). A total of 17 patients initiated lopinavir/ritonavir and interferon‐α2b treatment within 1 day after admission. The median duration of lopinavir/ritonavir and interferon‐α2b treatment was 8 days (range: 2‐13). A total of 11 (61%) patients were given antibacterial treatment (moxifloxacin) and all patients were treated with various traditional Chinese medicines according to different symptoms and signs of each person. One patient with moderate COVID‐19 showed cardiotoxicity with high level of creatine kinase (2756 U/L) after treatment with moxifloxacin and lopinavir/ritonavir.
Table 4.
Complications and treatments of all 18 patients infected with SARS‐CoV‐2
| No. (%) | |
|---|---|
| Complications | |
| ARDSa | 3 (17%) |
| Liver injurya | 3 (17%) |
| Treatment | |
| Oxygen therapy | |
| Nasal cannula | 10 (56%) |
| Noninvasive ventilation or high‐flow nasal cannulab | 3 (17%) |
| Mechanical ventilation (noninvasive)b | 3 (17%) |
| Antiviral treatment | |
| Lopinavir/ritonavir, interferon‐α2b | 18 (100%) |
| Oseltamivir | 5 (28%) |
| Ribavirin | 6 (33%) |
| Antibacterial treatment, moxifloxacin | 11 (61%) |
| Methylprednisoloneb | 3 (17%) |
| Immunoregulation therapy, thymalfasin, immunoglobulinb | 3 (17%) |
| Convalescent plasma | 4 (22%) |
| Traditional Chinese medicine | 18 (100%) |
Abbreviations: ARDS, acute respiratory distress syndrome; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Complications occurred in three severe patients.
These therapies were only used for three severe patients.
A total of 10 (56%) patients received oxygen therapy by nasal cannula. The corrected PaO2/FiO2 values of three severe patients were 217, 174, and 163 mm Hg, respectively, which were below 300 mm Hg. During hospitalization, all three severe patients had complications including ARDS, acute respiratory injury, and liver injury. These three severe cases were given noninvasive mechanical ventilation for respiratory support. Besides antiviral therapy, antibacterial therapy, and traditional Chinese medicine, all three severe patients received methylprednisolone (40 mg, twice a day or bid, 3 days), immunoglobulin (20‐25 g, every day or qd, 3 days), thymalfasin (0.4 mg, every other day or qod, three times), and convalescent plasma (50 mL, every other day or qod, twice; Table 4).
3.5. Outcomes
With supportive care, the condition of all 18 patients improved significantly and CT images showed obvious regression of ground‐glass opacities (Figure 2). All patients including three severe cases were discharged. The median time for conversion of nucleic acid amplification test from positive to negative was 9 days (range: 5‐18) and the median length of hospital stay was 13.5 days (range: 8‐20).
4. DISCUSSION
The first laboratory‐confirmed case of COVID‐19 in Qinghai was reported on 25 January 2020 and the province leadership initiated first‐level response on the same day. A total of 15 cases were imported from Wuhan before the city was sealed off on 23 January. Overall, four transmission clusters involving 15 cases (2‐5 cases/clusters) were identified. Three patients without history of travel to Wuhan were infected by direct contact with cases returned from Wuhan. These data clearly confirmed human‐to‐human transmission of COVID‐19 7 , 8 , 14 and the effectiveness of isolation to control the epidemic. Chinese and Qinghai governments issued very strict and efficient measures to stop the spread of SARS‐CoV‐2 such as joint prevention and control, mobility reduction, cancellation of gathering activities, enforcement of quarantine measures, and social messaging about personal protection. With the substantial public health prevention measures and huge efforts from medical professionals to treat patients, 18 patients were confirmed infected with SARS‐CoV‐2 from 25 January to 5 February 2020 and all of them were discharged between 5 February and 21 February 2020. A total of 437 close contacts of the confirmed cases were released after medical observation. Those close contacts that had any relevant symptoms during 14‐day quarantine underwent RT‐PCR testing for SARS‐CoV‐2. By 16 April, no new cases were found in Qinghai Province for 60 consecutive days since 6 February 2020. Personal protective equipments including N95 respirator, isolation gown, protective eyewear, and gloves were used to treat patients for protecting healthcare personnel. Due to high‐level protection and infection control measures, our medical workers had zero infection during this outbreak.
Due to the early detection and early diagnosis strategies, most patients were confirmed at early stage. Five symptomatic patients infected with SARS‐CoV‐2 were admitted within 3 days of returning from Wuhan. Seven patients had no self‐perceived relevant clinical symptoms on admission. However, they had clear epidemiological history and positive nucleic acid test. Six of them had pneumonia symptoms on CT imaging and showed headache and fatigue, ocular discomfort, sore throat, dry cough, fatigue, diarrhea on days 2, 2, 4, 5, 8, 8 after admission, respectively. The remaining one had fever several hours before admission but no pneumonia symptoms on CT imaging. According to COVID‐19 Guidelines made by the NHCC at that time, they were admitted before 5 February. It is worth noting that one second‐generation patient (47‐year‐old male) had contact with, and presumably contracted COVID‐19 from, his son who returned from Wuhan with negative nucleic acid test. His son had mild symptoms including fever, cough, and fatigue at the next day after returning from Wuhan. However, the nucleic acid tests for SARS‐CoV‐2 were negative at four different time points and CT imaging showed normal. So his son was quarantined at home and only contacted with him for 5 days. Then the second‐generation patient showed cough, fatigue, and sore throat and was confirmed of COVID‐19 by nucleic acid test.
We observed a greater number of men than women among the 18 cases of SARS‐CoV‐2 infection, consistent with a previous study. 15 Additionally, three children were infected with SARS‐CoV‐2 and showed mild or moderate symptoms. All three severe cases had comorbidities such as hypertension, liver disease, or diabetes. SARS‐CoV‐2 infects host cells through angiotensin‐converting enzyme 2 (ACE2) receptors. 2 ACE2 is highly expressed in the heart and lungs, which is involved in heart function and the development of hypertension and diabetes. 16 Liver injury in patients with SARS‐CoV‐2 infections might be also directly caused by the viral infection of liver cells. 17 Elevation of both ALT and AST was observed in four patients including three severe cases and one case with polymyositis on admission. Therefore, liver damage is more prevalent in severe cases than in mild and moderate cases of COVID‐19 consistent with previous reports. 4 , 14 As the elevated amount of C‐reactive protein may be associated with the inflammatory response and cytokine storms caused by the virus in the blood vessels, 18 a previous study showed that the C‐reactive protein level was positively correlated with the severity of the pneumonia. 19 Similarly, we found that the amount of C‐reactive protein was higher in three severe patients than the other 15 mild and moderate patients.
Qinghai is located on an elevated plateau with lower ambient oxygen levels. Compared to those living at lower altitudes, patients at high altitude are less tolerant to hypoxia and lung diseases are more likely to cause respiratory failure. 20 Therefore, oxygen supply is important for patients with COVID‐19, especially severe patients. Our three severe patients had ARDS with low PaO2/FiO2 prompting noninvasive mechanical ventilation. In addition to antiviral and antibiotic therapy and traditional Chinese medicine, all three severe cases received immunoglobulin and low dose of methylprednisolone for a short duration. Although there are different opinions on using corticosteroids, A team of front‐line physicians from China found that short courses of corticosteroids at low‐to‐moderate dose were beneficial for critically ill patients. 21 In our study, all three severe patients received methylprednisolone 40 mg bid for 3 days, which were in line with the recommendations on the use of corticosteroids.
Convalescent plasma has been used to improve the survival rate of patients with SARS‐CoV infection. 22 Thus, our three severe patients were given convalescent plasma (50 mL, qod, on 10 and 12 February) collected from two patients who had recovered from COVID‐19. We detected SARS‐CoV‐2 antibodies (immunoglobulin G [IgG] and immunoglobulin M [IgM]) from the convalescent plasma of one donated patient using chemiluminescent immunoassay. SARS‐CoV‐2 antibodies IgM and IgG CLIA kits were from Shenzhen Snibe Co, Ltd (China), with two antigens of SARS‐CoV‐2 coated on the magnetic beads of the CLIA assay (Nucleocapsid protein and Spike Protein). The amount of anti‐SARS‐CoV‐2 antibodies IgM and IgG is positively correlated with the relative light units measured by the chemiluminescence analyzer. CLIA analyzer automatically calculates the concentration (AU/mL) based on the calibration curve. Cut off value proposed by manufacturer is 1 AU/mL both for IgM and IgG antibodies: hence samples with IgM and IgG concentration ≥1 AU/mL are considered positive. The level of IgG was very high (>30 AU/mL) and IgG (1:80) was 3.464 AU/mL. As expected, the level of IgM was very low (0.093 AU/mL). The CT images, blood gas analysis, and symptoms improved after convalescent plasma transfusion. No adverse events were observed.
Our study has several limitations. First, there was no detailed collection of symptom data at different phases of illness. There is no detailed serial RT‐PCR sampling depicted to show the viral dynamics in this small cohort. Second, with the limited number of cases in Qinghai, the results should be interpreted with caution. Third, there was no control group for any of the treatments given, so no conclusion can be drawn about their role in recovery. At the time of convalescent plasma transfusion, the antibody level test (IgG, IgM et al) had not yet been routinely introduced at the hospital and no treatment guideline for using convalescent plasma was released. We did not measure the antibody concentrations in severe patients before and after convalescent plasma transfusion, so it is difficult to accurately evaluate the efficacy related to convalescent plasma.
In summary, all 18 patients including three severely ill patients with COVID‐19 were discharged after treatment on Qinghai plateau. Patients with comorbidities are more likely to develop severe illness. High C‐reactive protein levels and elevations of both ALT and AST were observed in three severely ill patients on admission. The strategies of early detection, early diagnosis, early isolation, and early treatment of COVID‐19 in Qinghai are useful to prevent the transmission and improve the cure rate.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
This study was funded by the Science and Technology Department of Qinghai Province (number: 2020‐SF‐158). We acknowledge all healthcare workers involved in the diagnosis and treatment of patients in Qinghai. We thank all patients involved in the study. We thank Qinghai Center for Diseases Prevention and Control for SARS‐CoV‐2 nucleic acid test and Xiangren A. from Qinghai Provincial People's Hospital for detection of coronavirus antibody.
Xi A, Zhuo M, Dai J, et al. Epidemiological and clinical characteristics of discharged patients infected with SARS‐CoV‐2 on the Qinghai Plateau. J Med Virol. 2020;92:2528–2535. 10.1002/jmv.26032
Aiqi Xi, Ma Zhuo, Jingtao Dai, Yuehe Ding and Xiuzhen Ma equally contributed to this work.
REFERENCES
- 1. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 6. WHO . Coronavirus disease 2019 (COVID‐19) Situation Report – 77. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. accessed April 7, 2020.
- 7. Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382:1199‐1207. 10.1056/NEJMoa2001316 published online Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease‐2019 (COVID‐19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. March 13, 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected accessed April 7, 2020.
- 13. ARDS Definition Task F , Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526‐2533. [DOI] [PubMed] [Google Scholar]
- 14. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259‐260. 10.1038/s41569-020-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428‐430. 10.1016/S2468-1253(20)30057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, YZ, Yang Z, Xia D, Geng S. Clinical characteristics of patients with severe pneumonia caused by the 2019 novel coronavirus in Wuhan, China. medRxiv. 2020;1–15. 10.1101/2020.03.02.20029306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China: Life Sci. 2020;63:364‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang S, Lin S, Gao W, et al. Report of the consensus conference on diagnostic criteria of ALI/ARDS at high altitudes in Western China. Intensive Care Med. 2001;27:1539‐1546. [DOI] [PubMed] [Google Scholar]
- 21. Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019‐nCoV pneumonia. Lancet. 2020;395(10225):683‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng Y, Wong R, Soo YOY, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]


