Abstract
Acute lung injury (ALI) represents the most severe form of the viral infection sustained by coronavirus disease 2019 (COVID‐19). Today, it is a pandemic infection, and even if several compounds are used as curative or supportive treatment, there is not a definitive treatment. In particular, antiviral treatment used for the treatment of several viral infections (eg, hepatitis C, HIV, Ebola, severe acute respiratory syndrome–coronavirus) are today used with a mild or moderate effect on the lung injury. In fact, ALI seems to be related to the inflammatory burst and release of proinflammatory mediators that induce intra‐alveolar fibrin accumulation that reduces the gas exchange. Therefore, an add‐on therapy with drugs able to reduce inflammation, edema, and cell activation has been proposed as well as a treatment with interferon, corticosteroids or monoclonal antibodies (eg, tocilizumab). In this article reviewing literature data related to the use of escin, an agent having potent anti‐inflammatory and anti‐viral effects in lung injury, we suggest that it could represent a therapeutic opportunity as add‐on therapy in ALI related to COVID‐19 infection.
Keywords: acute lung injury, coronavirus‐2, COVID‐19, escin, pneumonia
The current pandemic infection of coronavirus disease 2019 (COVID‐2019) is caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), a novel RNA β‐coronavirus, that shares 79% sequence identity with SARS‐CoV, the virus that caused a major outbreak in 2002‐2003. Usually, COVID‐19 is transmitted by inhalation or contact with infected droplets or touching surfaces contaminated by them and then touching the nose, mouth, or eyes, even if a transmission via the aerosolization/fecal‐oral route is also hypothesized. 1 The most common symptoms of patients with COVID‐19 are fever, cough, and fatigue, while other patients also have or manifest only headache, diarrhea, and lymphopenia. Chest computed tomography scans generally present as pneumonia, often with RNAemia and acute respiratory distress syndrome (ARDS) in severely ill patients. There are similarities in the symptoms between COVID‐19 and coronavirus infections, such as SARS. 2 However, COVID‐19 showed some unique clinical features, especially mainly targeting the lower airway. As of April 19, 2020, there have been more than 2.3 million reported cases and 160 000 deaths in >200 countries. Currently, there is no evidence from randomized clinical trials to show any potential therapy improving outcomes in patients with COVID‐19. 3
SARS‐CoV‐2 invading results in immune and inflammatory responses, even cytokine storm. Pathological observation showed that exudative inflammation occurred in the early to late phase of COVID‐19 pneumonia. Therefore, effective antiviral and potent anti‐inflammatory drugs should be administered to patients with COVID‐19 pneumonia.
In this article, we review the pathophysiology and the therapeutic agents of COVID‐19 up to now, then discuss the possible role of escin, which has shown potent anti‐inflammatory and some antiviral effects in vitro and the effects on acute lung injury in vivo, including animals and humans, in the management of acute lung injury (ALI) during COVID‐19 infection.
Pathophysiology of COVID‐19 and Therapeutic Agents
COVID‐19 is characterized by pneumonia, lymphopenia, exhausted lymphocytes, and a cytokine storm (CS). Defining the immunopathological changes in patients with COVID‐19 provides potential targets for drug discovery and is important for clinical management. 4
Pathophysiology of COVID‐19 and the Immune Response, CS
During the viral infection, host factors trigger an immune response to be against the virus that could result in pulmonary tissue damage, functional impairment, and reduced lung capacity if the immune pathogenesis associated with an immune response is out of control. 5 In the immune response macrophages present SARS‐CoV‐2 antigens to T cells, that activate, differentiate, and release chemokines and cytokines, such as interleukin (IL)‐1, IL‐6, IL‐8, IL‐21, tumor necrosis factor (TNF)‐β, and monocyte chemotactic protein (MCP)‐1, causing the CS that recruits lymphocytes and leukocytes to the site of infection. Whether it is in an infected cell or in an immune cell, nuclear factor kappa B (NF‐κB) activation itself may play an important role in immune response and acute inflammatory lung injury (Figure 1). 5 , 6
Figure 1.
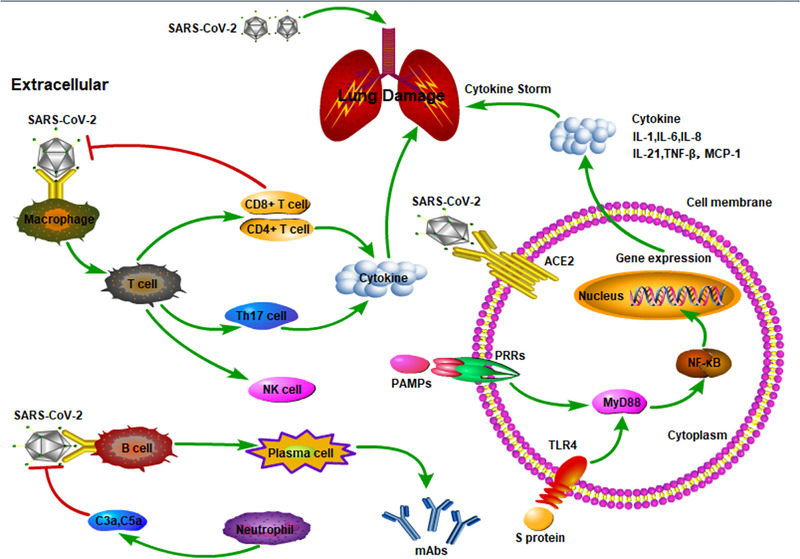
The immune and inflammatory responses in coronavirus infections. In the immune response, macrophages present SARS‐CoV‐2 antigens to T cells that activate, differentiate, and release chemokines and cytokines such as interleukin (IL)‐1, IL‐6, IL‐8, IL‐21, tumor necrosis factor‐β (TNF‐β), and monocyte chemotactic protein‐1 (MCP‐1), causing the cytokine storm that recruits lymphocytes and leukocytes to the site of infection. Whether it is in an infected cell or in an immune cell, nuclear factor kappa B (NF‐κB) activation itself may play an important role in immune response and acute inflammatory lung injury. ACE2, angiotensin‐converting enzyme 2; PAMPs, pathogen‐associated molecular patterns; PRRs, pattern recognition receptors; TLR4, Toll‐like receptor 4.
Growing evidence suggests that a subgroup of patients with severe COVID‐19 might have a CS syndrome with systemic inflammation, multiple organ failure, and high inflammatory parameters. 7 CS has been previously documented in patients with coronavirus pneumonia infection, that is, SARS and Middle East respiratory syndrome (MERS) accompanied by rapid viral replication and inflammatory cell infiltration leading to ALI and death. 8 , 9 In a recent clinical trial in 41 COVID‐19 infected patients in Wuhan, Huang et al 10 documented high amounts of IL‐1β, interferon (IFN)‐γ, IFN‐γ–inducible protein, and MCP‐1, probably leading to activated T‐helper‐1 cell responses. Moreover, the same authors documented higher concentrations of granulocyte colony‐stimulating factor, IFN‐γ–inducible protein, MCP‐1, macrophage inflammatory protein 1α, and TNF‐α in intensive care unit hospitalized SARS‐CoV‐2–infected patients than did those not requiring intensive care unit admission, suggesting that CS was associated with disease severity. 10 Finally, several studies documented a significant increase in IL‐6 plasma levels in critically ill patients with COVID‐19. 11 , 12 , 13 , 14 This increase in CS is associated with ALI during COVID‐19 infection, and it is characterized by an in intra‐alveolar fibrin accumulation that stimulates the production of both profibrotic growth factors 15 and profibrotic cytokine transcripts, such as transforming growth factor‐β, connective tissue growth factor, and platelet‐derived growth factor, with a decrease in pulmonary surfactant protein, leading to collapse or closure of alveoli and loss of lung compliance (changes in lung volumes). 16 In combination, a high‐fibrin/low‐surfactant intra‐alveolar environment provides an ideal environment for fibroblast adherence and growth, resulting in collagen deposition and development of lung fibrosis. 17 Finally, fibrin and fibrin breakdown products increase vascular permeability, stimulate migration and proliferation of inflammatory cells, and promote recruitment of neutrophils to the lung. 18 , 19 Taken together, these factors decrease the capacity to cleave and remove fibrin deposits corresponding with a poor clinical patient outcome. 20 Moreover, the increased release of proinflammatory factors (IL‐1, IL‐6, IL‐8, IL‐21, TNF‐β, and MCP‐1, etc) is associated with hypercoagulation and disseminated intravascular coagulation, that could induce an impairment in clinical conditions. Additionally, the subgroup of patients having immunosuppression could improve mortality; therefore, it is of great significance to pay attention to sepsis immunosuppression caused by COVID‐19. 21 , 22 , 23
Pathological Changes of Lungs in Patients With COVID‐19
Tian et al 24 described 2 patients who underwent lung lobectomies for adenocarcinoma (pT1bN0) and were later found to have been infected with SARS‐CoV‐2. Pathological findings from these 2 patients are edema and prominent proteinaceous exudates, vascular congestion, and inflammatory clusters with fibrinoid material and multinucleated giant cells. 24 Liu et al 25 and Xu et al 26 found that the pathological features of COVID‐19 resemble those exhibited in MERS and SARS. In particular, during the autopsy of a COVID‐19 cadaver, Liu et al 25 documented in right pleura the presence of an intense inflammation, with edema and fiber bands. Similarly, histologic examination performed by Xu et al 26 showed bilateral diffuse alveolar damage with cellular fibromyxoid exudates and interstitial mononuclear inflammatory infiltrates. Using minimally invasive autopsies in 3 patients died from COVID‐19 pneumonia, Yao et al 27 described alveolar exudative inflammation and interstitial inflammation, alveolar epithelium proliferation and hyaline membrane formation. All findings above suggest that exudative inflammation occurred in the early to late phase of COVID‐19 pneumonia. Therefore, potent anti‐inflammatory drugs should be administered for COVID‐19 pneumonia besides antiviral drugs.
Based on the pathophysiology and pathological changes of lungs in patients with COVID‐19, it may be better for them if antiviral and anti‐inflammatory drugs are administered early. In the late stage, uncontrolled pulmonary inflammation caused by SARS‐CoV‐2 infection will lead to CS, immunosuppression, and ARDS, and it may be difficult to attenuate the severe ALI with any management.
Drug Treatment for COVID‐19
Antiviral Treatment of COVID‐19
To date, there is not a specific treatment for COVID‐19, 28 , 29 even if some clinical trials are ongoing to evaluate their effects as add‐on therapy in COVID‐19 patients. Drugs possibly effective for COVID‐19 are reported in Table 1. Lopinavir‐ritonavir and add‐on IFNα‐1b has been studied for MERS treatment, 30 , 31 and in recent clinical trials it has been also proposed for COVID‐19 treatment. 14 , 32 However, Cao et al, 33 in a randomized, controlled, open‐label trial in 199 hospitalized adult patients with COVID‐19, documented that the lopinavir‐ritonavir combination (400 mg and 100 mg, respectively, twice daily for 14 days) was not associated with clinical improvement compared with standard care procedures. In a recent retrospective cohort study in COVID‐19 patients, Deng et al 34 documented that a 14‐day treatment with lopinavir‐umifenovir (also called arbidol) induced a significant improvement (P < .05) of clinical symptoms and laboratory finding (virus detection), compared to lopinavir alone. Favipiravir, a purine nucleic acid analog (Table 1) approved for use in influenza, is studied in the management of COVID‐19. 35 In particular, to date (April 14, 2020), 9 clinical trials have been recorded in the World Health Organization database: 7 favipiravir alone (JPRN‐jRCTs041190120, JPRN‐jRCTs031190226, ChiCTR2000030254, ChiCTR2000030113, ChiCTR2000029600, ChiCTR2000029548, ChiCTR2000029544), 1 plus chloroquine phosphate (ChiCTR2000030987), and 1 plus tocilizumab (ChiCTR2000030894).
Table 1.
Antiviral Drugs Used to Treat COVID‐19–Infected Patients
| Drug | Anti‐infective Mechanism | Dosage/Duration | Route of Administration | NCT in WHO |
|---|---|---|---|---|
| Lopinavir/ritonavir | Inhibition of HIV‐1 protease for protein cleavage | 200 mg/100 mg, 2 tablets per day, every 12 hours for 7‐10 days | Oral | NCT01700790 |
| Not indicated | Oral | ChiCTR2000029603 | ||
| Favipiravir | Acting on viral genetic copying to prevent its reproduction, without affecting host cellular RNA or DNA synthesis | Not indicated | Oral |
ChiCTR2000030254 ChiCTR2000029600 |
| On the first day, 1600 mg each time, twice a day; from the second to the seventh day, 600 mg each time, twice a day; the maximum number of days taken is not more than 7 days | Oral | NCT04310228 | ||
| Day 1: 1800 mg twice daily; Day 2 and thereafter: 600 mg 3 times daily, for a maximum of 14 days | Oral | NCT04336904 | ||
| Chloroquine and hydroxychloroquine | Increase of endosomal pH, immunomodulation, autophagy inhibitors | 600 mg daily for 7 days | Oral | NCT04340544 |
| First dose: 800 mg; from second day on, each patient will get 600 mg once a day until day 7 | Oral | NCT04342221 | ||
| Not indicated | Oral |
ChiCTR2000031782 ChiCTR2000030054 |
||
| Umifenovir (Arbidol) | Inhibitor of viral fusion with the targeted membrane | 200 mg, 3 times a day for 14 days | Oral | ChiCTR2000029621 ChiCTR2000029759 ChiCTR2000029592 ChiCTR1900028586 NCT04252885 NCT04246242 NCT04260594 |
| Remdesivir | RNA polymerase inhibitor | Day 1: 200 mg; Day 2 and thereafter: 100 mg every 24 h. Available as 5‐mg/mL vial (reconstituted) | Intravenous | |
| Lianhuaqingwen Capsule | Inhibitor of viral replication, | Routine treatment + Lianhuaqingwen Capsules; 4 capsules/time or 1 bag/time, 3 times a day | Oral | ChiCTR2000029434 ChiCTR2000029433 |
NCT, national clinical trial number; WHO, World Health Organization.
Wang et al 36 suggest that chloroquine and hydroxychloroquine, used in the prevention and treatment of malaria, 37 could play a role in the management of COVID‐19, due to their mechanism of action (Table 1). 38 , 39 , 40 However, the problem of chloroquine is its safety related to the dosage; in fact, as suggested by Duan et al, 41 the dosage for COVID‐19 treatment is 500 mg twice daily for no more than 10 days, which is larger than of the dosage for previous treatment of malaria, so it is very important to evaluate the development of adverse reactions. In a small group of patients with COVID‐19 (n = 20), Guatret et al 42 demonstrated a significant viral load decrease, 3 days after the beginning of hydroxychloroquine plus azithromycin treatment respect to the control group (57.1% and 12.5%, respectively). However, the use of either chloroquine or hydroxychloroquine plus azithromycin for COVID‐19 infection is only supported by in vitro data and weak studies, and physicians should consider the development of adverse effects to determine their benefit, if any, in treating or preventing COVID‐19. 43
Remdesivir is a monophosphate prodrug that undergoes metabolism to an active C‐adenosine nucleoside triphosphate analogue. Currently, remdesivir is a promising potential therapy for COVID‐19 due to its broad‐spectrum, potent in vitro activity against several novel coronaviruses, including SARS‐CoV‐2 with half maximal effective concentration and concentration for 90% of maximal effect (EC90) values of 0.77 µM and 1.76 µM, respectively. Clinical trials are ongoing to evaluate the safety and antiviral activity of remdesivir in patients with mild to moderate or severe COVID‐19. 3 Notably, Williamson et al 44 recently used the Rhesus macaque model of SARS‐CoV‐2 infection to investigate the antiviral activity of remdesivir. Therapeutic remdesivir treatment initiated early during infection has a clear clinical benefit in SARS‐CoV‐2–infected Rhesus macaques. These data support early remdesivir treatment initiation in COVID‐19 patients to prevent progression to severe pneumonia. 44
Moreover, recently, Chen et al 45 reported that the viral genome of COVID‐19 encodes >20 proteins; among these, two proteases (PLpro and 3CLpro) are vital to virus replication, and they suggested that sofosbuvir in the fixed combination with velpatasvir or ledipasvir could be effective in the treatment of COVID‐19 owing to their dual inhibitory actions on 2 viral enzymes. Other antiviral drugs, umifenovir, oseltamivir, and ribavirin currently are also being tested in ongoing randomized trials. 3
Drugs Treatment for ALI of COVID‐19 With Anti‐inflammatory Mechanisms
In this concern, several drugs could be used as add‐on therapy to inhibit lung inflammation and reduce CS. Azithromycin, commonly used to treat bacterial infections, could be used as add‐on antiviral therapy in patients with SARS‐CoV‐2 infection, 28 due to its immunomodulant effect. 46 In fact, to date (April 14, 2020), there are 21 clinical trials recorded on clinicaltrial.gov that evaluate the effect of azithromycin add‐on to hydroxychloroquine sulfate or tocilizumab in the treatment of hospitalized patients with moderate or severe COVID‐19. One prospective trial that is in recruiting in Utah (NCT04334382) evaluates the effect of oral azithromycin (500 mg on day 1, then 250 mg on days 2‐5) vs oral hydroxychloroquine (400 mg twice daily on day 1, then 200 mg twice daily on days 2‐4) in outpatients >45 years old with COVID‐19. Recently, it has been reported that tocilizumab (recombinant human IL‐6 monoclonal antibody used for rheumatic diseases), to block IL‐6 signaling and its mediated inflammatory response, is approved to treat the cytokine release syndrome caused by chimeric antigen receptor T‐cell immunotherapy. 47 , 48 Until now (April 14, 2020), several clinical trials have been registered on safety and efficacy of tocilizumab (8 mg/kg intravenously, single infusion up to a maximum of 800 mg/dose) alone or with other drugs in the treatment of severe COVID‐19 pneumonia in both China registry (ChiCTR2000029765, ChiCTR2000030796, ChiCTR2000030442, and ChiCTR2000030894) and clinicaltrial.gov registry (16 trials up to April 14, 2020). Concerning the similar mechanism of action, other monoclonal antibodies or small‐molecule inhibitors are under investigation to evaluate their efficacy and safety in COVID‐19 (Table 2).
Table 2.
Immunomodulatory Drugs Studied for COVID‐19 and Recorded in Identified at Clinicaltrials.gov up to April 14, 2020
| Drug | Mechanism of Action | Dosage/Duration | Route of Administration | Comparator | NCT in WHO |
|---|---|---|---|---|---|
| Bevacizumab | Anti‐VEGF | 7.5 mg/kg + 0.9% NaCl 100 mL | Intravenous | None | NCT04305106 |
| 500 mg + saline 100 mL, intravenous drip ≥90 min | Intravenous | None | NCT04275414 | ||
| Anakinra | Anti IL‐1 | 200 mg 3 times daily for 7 days | Intravenous | Tocilizumab | NCT04339712 |
| Situximab | 11 mg/kg, single infusion | Intravenous |
Placebo Anakinra 100 mg for 28 days Anakinra + Siltuximab Tocilizumab Anakinra + Tocilizumab |
NCT04330638 | |
|
Sarilumab |
Anti IL‐6 | High dose | Intravenous |
Placebo Sarilumab low dose i.v. |
NCT04315298; NCT04327388 |
| 400 mg | Intravenous | None | |||
|
Baricitinib |
JAK inhibitors |
2 mg for 14 days | Oral | None | NCT04340232 |
| 2 mg for 10 days | Oral |
Lopinavir/ritonavir 200 mg/50 mg twice daily for 10 days Hydroxychloroquine 200 mg twice daily for 10 days Sarilumab 200 mg subcutaneously single infusion |
NCT04321993 | ||
| 4 mg/day for 14 days | Oral | Add‐on ritonavir 600 mg twice daily | NCT04320277 | ||
| Pembrolizumab | Anti PD‐1 | 200 mg, single dose. | Intravenous | Tocilizumab | NCT04335305 |
| Nivolumab | Anti PD‐1 | 0.3 mg/kg, single infusion | Intravenous | Tocilizumab | NCT04333914 |
| RH‐ACE2 | Anti ACE2 | Not indicated, bid | Intravenous | Placebo | NCT04335136 |
ACE2, angiotensin‐converting enzyme 2; IL, interleukin; NCT, national clinical trial number; RhACE2, recombinant human angiotensin‐converting enzyme 2; VEGF, vascular endothelial growth factor; WHO, World Health Organization.
SARS‐CoV‐2 is able to invade and enter cells using angiotensin‐converting enzyme 2 receptor through endocytosis, which is regulated by adaptor‐associated protein kinase 1. Angiotensin‐converting enzyme 2 receptor is a cell‐surface protein widely existed on cells in the heart, kidney, blood vessels, especially lung alveolar epithelium type 2 cells. 49 , 50 , 51 Considering this mechanism, drugs able to block adaptor‐associated protein kinase 1 (eg, sarilumab and baricitinib) can interrupt both the passage of the virus into cells and the cytokine storm and could be used in COVID‐19 treatment. 52
Metronidazole, decreasing the levels of proinflammatory cytokines, (ie, IL‐1α and β, IL‐6, IL‐8, IL‐12, IFN‐γ, TNF‐α) as well as the levels of C‐reactive protein and neutrophil count, could be efficacious in the management of immunopathological manifestations of the COVID‐19 53 ; however, clinical studies must be performed to further validate this experimental observation.
Concerning the role of corticosteroids as add‐on therapy in COVID‐19 patients, even if they have anti‐inflammatory and immunomodulatory activity, 54 , 55 , 56 to date, there is no evidence concerning their efficacy to improve the clinical outcomes in these patients. 57 , 58 Moreover, corticosteroids can delay the viral clearance 59 , 60 , 61 ; therefore, Arabi et al 61 advise against their continuous use in SARS‐CoV‐2–infected patients. In addition, in the late stage of COVID‐19, immunosuppression can often be seen in patients; therefore, the use of corticosteroids may aggravate this condition in patients with COVID‐19. 4 In agreement with these data, the guideline of the World Health Organization 62 does not suggest the use of systemic corticosteroids for the treatment of viral pneumonia and ARDS for suspected COVID‐19 cases.
Finally, some natural products and traditional medicines are investigated to treat patients with COVID‐19. Lianhuaqingwen Capsule, a traditional Chinese medicine formula, has been used to treat influenza and exerted broad‐spectrum antiviral effects on a series of influenza viruses and immune regulatory effects. 63 Recently, Lianhuaqingwen Capsule has been registered in clinical center of China (ChiCTR2000029434, ChiCTR2000029433) to treat patients with COVID‐19 pneumonia (Table 1) and was finally approved (Approval No. 2020B02813) by the National Medical Products Administration of China for the treatment of mild and moderate coronavirus pneumonia on April 12, 2020.
Escin represents some isomers of escin saponins or the natural mixture of triterpene saponins in different production extracted from seeds of Aesculus hippocastanum L., Aesculus wilsonii Rehd, and others. In China, sodium aescinate injection consists of A, B, C, and D escin, of which A and B are the main components that belong to β‐escin (Figure 2). Accumulating evidence suggests that escin exerts potent anti‐inflammatory, anti‐edematous effects and has been used to treat acute edema in clinic. Recently, the sodium of escin, sodium aescinate injection, was registered in the clinical center of China (ChiCTR2000029742) and Italy (NCT04322344) to treat patients with COVID‐19 pneumonia.
Figure 2.
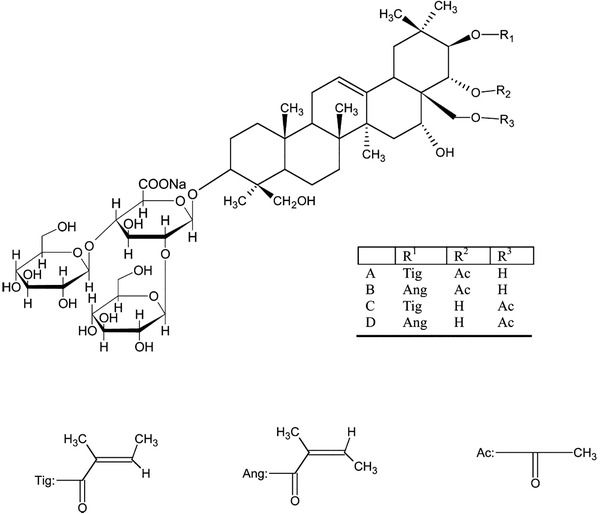
Chemical structure of escin. Escin is a natural mixture of triterpene saponins extracted from seeds of Aesculus hippocastanum. It mainly consists of A, B, C, and D escin, of which A and B are the main components that belong to β‐escin.
Anti‐inflammatory, Antiviral Effects of Escin In Vitro and Its Effects on ALI
Anti‐inflammatory Effects of Escin In Vitro
Xin et al 64 reported that 10 µg/mL of escin significantly inhibits the secretion of inflammatory factors including nitric oxide (NO), TNF‐α, and IL‐1β in lipopolysaccharide (LPS)‐stimulated RAW264.7 macrophage cells. Furthermore, the combination of suboptimal concentrations of 0.1 µg/mL of escin with 1 ng/mL of corticosterone, which alone could not depress the release of inflammatory factors, inhibited the secretion of NO, TNF‐α, and IL‐1β in vitro. These findings suggest that escin can synergize with glucocorticoids to enhance their anti‐inflammatory effect. In human endothelial cells under inflammatory conditions, β‐escin (3 µM) inhibits the inflammation blocking nuclear translocation of p50 and p65. 65 Montopoli et al 66 reported that escin shows dose‐dependent (0.1‐1 µM) effects in LPS‐activated endothelial cells, reducing IL‐6 release and vascular cell adhesion molecule‐1 activity. In another experimental study in human endothelial cells, Chen et al67 documented that escin (10 µg/mL) inhibits the overexpression of the aquaporin‐1 and alleviates barrier dysfunction induced by proinflammatory high mobility group protein 1.
Antiviral Effects of Escin In Vitro
Escin shows antiviral activities against SARS‐CoV, respiratory syncytial virus (RSV), and some other viruses (Table 3). In SARS‐CoV‐infected Vero E6 cells, the half maximal effective concentration for escin against SARS‐CoV was 6 µM. 68 RSV infection is the leading cause of acute respiratory tract infections in childhood. In RSV‐infected epithelial and macrophage cell lines, 5 µg/mL of escin and 25 µg/mL of Aesculus hippocastanum seed extract show significant virucidal and antiviral activities against RSV by modulating activities of NF‐κB, activator protein‐1, and cytokine. 69 In addition, herpes simplex virus‐1, vesicular stomatitis virus, and dengue virus are also inhibited by escin and seed extract in vitro. In agreement, Michelini et al 70 reported that both escin and seed extract decrease levels of TNF‐α and IL‐6 in J774A.1 cells infected with HSV‐1 or stimulated with Toll‐like receptor ligands, probably through the inhibition of NF‐κB and activator protein‐1 signaling pathways. These results support the use of escin in COVID‐19 pneumonia because escin has potent anti‐inflammatory activities by inhibiting inflammatory cytokines such as IL‐6, TNF‐α, and IL‐1β, and shows antiviral activity on SARS‐CoV, RSV, dengue virus, and others.
Table 3.
Antiviral Effect of Escin
| Escin | Target Virus | Virucidal/Antiviral | EC50 | Cell Model | Ref. |
|---|---|---|---|---|---|
| β‐escin | SARS‐CoV | Antiviral | 6 µM | Vero E6 | 68 |
| β‐escin | RSV | Antiviral |
1.6 µg/mL 2.4 µg/mL 1.5 µg/mL |
HEp‐2 A549 Vero |
69 |
| Virucidal | 14.5 µg/mL | … | |||
| RSV A2 | Antiviral |
1.4 µg/mL 1.8 µg/mL 2.6 µg/mL |
HEp‐2 A549 Vero |
||
| Virucidal | 15.1 µg/mL | … | |||
| β‐escin | HSV‐1 | Antiviral |
1.5 µg/ml 2.4 µg/mL 1.9 µg/mL |
HCLE NHC Vero |
70 |
| Virucidal | 15.9 µg/mL | … | |||
| VSV | Antiviral | 10 µg/mL | Vero | ||
| Dengue type 2 | (dose) |
A549, human lung carcinoma cell line; EC50, half maximal effective concentration; HCLE, human corneal cells; HEp‐2, human epidermoid cancer cell line; HSV, herpes simplex virus; NHC, human conjunctival cells; RSV, respiratory syncytial virus; VSV, vesicular stomatitis virus.
Effects of Escin on ALI In Vivo
Several authors described the effects of escin on ALI in animal models. In particular, Wang et al 71 reported that β‐escin reduces the degree of lung injury and improves function of gas exchange in LPS‐induced lung inflammation in mice by inhibiting both the lipid peroxidation and the expression of proinflammatory factors, for example, NO, TNF‐α, IL‐1β, and IL‐6. Further, Xin et al 72 described that escin could increase glucocorticoid receptor in the lung of animals, especially reversing the decrease of glucocorticoid receptor resulting from LPS, supporting the idea that the anti‐inflammatory effect of escin could involve the upregulation of glucocorticoid receptor with an increase of endogenous antioxidant activity. In a mouse model of pneumococcal pneumonia, a 4‐day administration of escin (1.8 mg/kg intravenously), significantly reduced lung inflammation, suggesting a role for escin as add‐on treatment. 73 , 74
Finally, several authors reported that escin is able to attenuate nonpathogenic lung injury. In rats with ALI induced by phosgene (Psg), which is used extensively in industry, escin injection decreased the mortality of animals. 74 In an experimental model of ALI induced by oleic acid injection (0.1 mL/kg intravenously), Wei et al 75 documented that both pretreatment (1 mg/kg) and treatment (1‐6 mg/kg) with sodium aescinate reduces oleic acid–induced ALI by modulating the levels of superoxide dismutase, malondialdehyde and matrix metallopeptidase 9 in plasma and lung tissue. These results have been confirmed by Huang et al 76 ; experimental models of oleic acid–induced pulmonary fibrosis showed that sodium aescinate improves lung fibrosis by inhibiting the levels of proinflammatory factors, such as IL‐1β, TNF‐α, and transforming growth factor‐β1.
Clinical Studies on Escin
The clinical efficacy of escin, administered for 6 to 20 days, on respiratory diseases has been reported (Table 4). Xiao 77 documented that the injection of sodium aescinate in SARS‐infected patients has anti‐inflammatory and antiexudative effects and reduces the adverse drug reaction related to glucocorticoid administration. Other authors reported the effects of escin on acute pulmonary edema in patients with thoracic trauma, 78 , 79 , 80 radiation pneumonitis, 81 thoracotomy, 82 and pneumonia. 83 Finally, escin improves pulmonary function in patients with acute exacerbation of chronic obstructive pulmonary disease and chronic pulmonary heart disease through the inhibition of cytokine release. 84 , 85 , 86 , 87
Table 4.
Roles of Escin Administered Intravenously in Human Lung Injury
| Escin | Dosage/Duration | Diseases | Biomarkers Used for Assessment of Efficacy | Ref. |
|---|---|---|---|---|
| Sodium aescinate | 20 mg/d; 10 d | SARS | Reducing inflammatory exudation in the lung | 77 |
| Aescine | 20 mg/d; 6 d | Traumatic acute lung injury | Inhibiting TNF‐α, IL‐1, IL‐6, IL‐8 in serum; improving pulmonary function including PaO2, PaCO2, PaO2/FiO2 | 77 |
| β‐sodium aescinate | 0.4 mg/kg; 7 d | Traumatic acute lung injury | Improving pulmonary function including SpO2, PaO2, PaCO2, PaO2/FiO2, FEV1, FVC, PEF, MMEF | 79 |
| β‐sodium aescinate | 20 mg/d; 14 d | Pulmonary contusion | Reducing the incidence of pulmonary infection, ARDS, and mortality | 80 |
| β‐sodium aescinate | 20 mg/d; 20 d | Acute radiation pneumonitis | Reducing the incidence of radiation‐induced lung injury; improving pulmonary function including PaO2, MVV, FEV/FVC | 81 |
| Sodium aescinate | 20 mg/d; 12 d | Thoracotomy | Reducing pleural effusion | 82 |
| Sodium aescinate | 10 mg/d; 10 d | Pneumonia‐like pleural effusion | Reducing pleural effusion; improving pulmonary function including FEV1, FVC | 83 |
| Sodium aescinate | 10 mg/d; 14 d | COPD | Inhibiting TNF‐α and IL‐6 in serum; improving pulmonary function including PaO2, PaCO2, FEV/FVC | 84 , 85 |
| β‐sodium aescinate | 20 mg/d; 10 d | COPD | Improving pulmonary function including FEV/FVC | 86 |
| Sodium aescinate | 20 mg/d; 10 d | CPHD | Reducing peripheral edema; improving pulmonary function including PaO2, PaCO2 | 87 |
ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; CPHD, chronic pulmonary heart disease; FEV1, forced expiratory volume in 1 second; FiO2, fraction of inspiration O2, FVC, forced vital capacity; MMEF, maximum midexpiratory flow; MVV, maximum ventilatory volume PaCO2, arterial partial pressure of CO2; PaO2, arterial partial pressure of O2; PEF, peak expiratory flow; SARS, severe acute respiratory syndrome; SpO2, pulse oxygen saturation.
Potential Role and Clinical Trials of Escin in the Management of COVID‐19
Potential Role of Escin in COVID‐19
It is well known that SARS‐CoV and SARS‐CoV‐2 share very similar gene structure and clinical symptoms. RSV‐induced pneumonia and COVID‐19 pneumonia have some similar pathological changes. For example, they all have alveolar inflammatory exudation, inflammatory cell infiltration, and the formation of hyaline membrane. Dengue virus also shares some characteristics with COVID‐19, such as sudden onset of high fever and lymphocytopenia. The dosage of escin in clinic, that is, sodium aescinate injection, is until 20 mg/day, the blood concentration in vivo is estimated to reach the similar level as that in vitro. The characteristic of escin to inhibit the acute inflammation is similar to dexamethasone or methylprednisolone. 74 , 88 Psg has been a chemical warfare agent and can result in severe acute lung injury. In ALI induced by Psg, after latent stage of exposure, the burst inflammatory response is very similar to the cytokine storm in the late stage of COVID‐19. Proinflammatory cytokines TNF‐a, IL‐6, and IL‐8 are critical factors, and their concentrations are known to be altered in the process of ALI. TNF‐a, IL‐6, IL‐8, IL‐4, and IL‐10 levels were significantly increased in the serum and bronchoalveolar lavage fluid of Psg‐exposed rats. However, escin injection still could significantly decrease the mortality of rats resulting from phosgene. 89 , 90 Escin has shown that it could both attenuate ALI and inhibit the secretion of cytokines in animals and humans.
Escin is a traditional medicine that displays anti‐inflammatory, antiedematous and antioxidant properties, and has been widely used in the clinical treatment of traumatic edema, hemorrhoids, and chronic venous insufficiency, 91 without showing severe adverse reactions besides a few gastroenterological reactions for oral tablets and local reaction with injection.
Taking the effects of escin in vitro and in vivo, the pathophysiology and the inflammatory response in COVID‐19 into account, it can be deduced that escin may have a potential role to treat patients with COVID‐19 effectively, and it may be predicted that effective antiviral drugs, such as remdesivir, in combination with potent anti‐inflammatory drugs, such as escin, could have a possible therapeutic effects on the severe ALI of patients with COVID‐19.
Clinical Trials of Escin in COVID‐19
In China, a randomized, parallel, controlled trial for the efficacy and safety of sodium aescinate injection in the treatment of patients with COVID‐19 pneumonia is being conducted. The aim is to evaluate the efficacy and safety of sodium aescinate for injection compared with conventional treatment in patients with COVID‐19 and to compare the efficacy of conventional therapy plus sodium aescinate for injection and conventional therapy plus glucocorticoids. Chest imaging (computed tomography), C‐reactive protein, and IL‐6 plasma levels are used to evaluate the efficacy for patients with COVID‐19. In an Italian clinical trial, escin is administered to COVID‐19 patients as an oral formulation (40 mg 3 times daily) or injection (20 mg intravenously once daily) for 12 days, compared with standard treatment. Generally, a randomized, double‐blind, and parallel controlled trial is requested to evaluate the efficacy of the treatment and should be performed to reach scientific conclusion even if the treatment of patients with COVID‐19 pneumonia is urgent. Thus, the clinical trials in the future for COVID‐19 pneumonia need to be intensively designed.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
The authors thank Dr. Ge Baoming for his efforts and support in preparing this article.
Author Contributions
G.L. was responsible for review design, literature search, writing, and editing. L.Z. and T.W. were responsible for literature search, writing, and editing. F.F. was responsible for review design, literature search, writing, editing, and supervision.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review [published online ahead of print April 13, 2020]. JAMA. 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 4. Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fu Y, Cheng Y, Wu Y. Understanding SARS‐CoV‐2‐mediated inflammatory responses: from mechanisms to potential therapeutic tools [published online ahead of print March 3, 2020]. Virol Sin. 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID‐19. J Infect. 2020;pii: S0163‐4453(20)30165‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39(5):517‐528. [DOI] [PubMed] [Google Scholar]
- 10. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [published online ahead of print March 13, 2020]. JAMA. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wygrecka M, Jablonska E, Guenther A, Preissner KT, Markart P. Current view on alveolar coagulation and fibrinolysis in acute inflammatory and chronic interstitial lung diseases. Thromb Haemostasis. 2008;99(3):494‐501. [DOI] [PubMed] [Google Scholar]
- 16. Stinson SF, Ryan DP, Hertweck S, Hardy JD, Hwang‐Kow SY, Loosli CG. Epithelial and surfactant changes in influenzal pulmonary lesions. Arch Pathol Lab Med. 1976;100(3):147‐153. [PubMed] [Google Scholar]
- 17. Burkhardt A. Alveolitis and collapse in the pathogenesis of pulmonary fibrosis. Am Rev Resp Dis. 1989;140(2):513‐524. [DOI] [PubMed] [Google Scholar]
- 18. Dang CV, Bell WR, Kaiser D, Wong A. Disorganization of cultured vascular endothelial cell monolayers by fibrinogen fragment D. Science. 1985;227(4693):1487‐1490. [DOI] [PubMed] [Google Scholar]
- 19. Leavell KJ, Peterson MW, Gross TJ. The role of fibrin degradation products in neutrophil recruitment to the lung. Am J Resp Cell Mol Biol. 1996;14(1):53‐60. [DOI] [PubMed] [Google Scholar]
- 20. Idell S, James KK, Levin EG, et al. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest. 1989;84(2):695‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sarzi‐Puttini P, Giorgi V, Sirotti S, et al. COVID‐19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38(2):337‐342. [PubMed] [Google Scholar]
- 23. Zhou H, Li XL, Li B. Pay attention to 2019 coronavirus disease (COVID‐19) induced sepsis immunosuppression. J Third Milit Med Univ. 2020;42(6):539‐544. [Google Scholar]
- 24. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary Pathology of Early‐Phase 2019 Novel Coronavirus (COVID‐19) Pneumonia in Two Patients With Lung Cancer. J Thorac Oncol. 2020;15(5):700‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Q, Wang RS, Qu GQ, et al. General view of the systemic anatomy of a dead cadaver resulted from COVID‐19. J Foren Med. 2020;36(1):21‐23. [Google Scholar]
- 26. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. J Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies. Chinese J Pathol. 2020;49:E009. [DOI] [PubMed] [Google Scholar]
- 28. Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019‐nCoV) infected pneumonia (standard version). Milit Med Res. 2020;7(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li H, Wang YM, Xu JY, Cao B. Potential antiviral therapeutics for 2019 novel coronavirus. Chinese J Tubercu Resp Dis. 2020;43(3):170‐172. [DOI] [PubMed] [Google Scholar]
- 30. Arabi YM, Asiri AY, Assiri AM, et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon‐beta1b (MIRACLE trial): statistical analysis plan for a recursive two‐stage group sequential randomized controlled trial. Trials. 2020;21(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arabi YM, Alothman A, Balkhy HH, et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir‐ritonavir and interferon‐beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Habibzadeh P, Stoneman EK. The novel coronavirus: a bird's eye view. Int J Occup Environ Med. 2020;11(2):65‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cao B, Wang Y, Wen D, et al. A Trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19 [published online ahead of print March 18, 2020]. N Engl J Med. 2020;382(19):1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020;pii: S0163‐4453(20)30113‐30114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Du YX, Chen XP. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019‐nCoV infection [published online ahead of print April 4, 2020]. Clin Pharmacol Ther. 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- 36. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30(3):269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6(2):67‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID‐19? [published online ahead of print March 12, 2020] Int J Antimicrob Agents. 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3(11):722‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou D, Dai SM, Tong Q. COVID‐19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression [published online ahead of print March 20, 2020]. J Antimicrob Chemother. https://doi.org/1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duan YJ, Liu Q, Zhao SQ, et al. The trial of chloroquine in the treatment of corona virus disease 2019 (COVID‐19) and its research progress in forensic toxicology. J Foren Med. 2020;36(2):1‐7. [DOI] [PubMed] [Google Scholar]
- 42. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. [published online ahead of print March 20, 2020] 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Juurlink DN. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS‐CoV‐2 infection. CMAJ. 2020;192(17):E450‐E453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Williamson BR, Feldmann F, Schwarz B, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS‐CoV‐2 [published online ahead of print April 15, 2020]. BioRxiv. 10.1101/2020.04.15.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen YW, Yiu CB, Wong KY. Prediction of the SARS‐CoV‐2 (2019‐nCoV) 3C‐like protease (3CL pro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. Version 2. F1000Res. 2020;9:129. 10.12688/f1000research.22457.2. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hui DS, Lee N, Chan PK, Beigel JH. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antiviral Res. 2018;150:202‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell‐induced severe or life‐threatening cytokine release syndrome. Oncologist. 2018;23(8):943‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell‐induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15(8):813‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li W, Zhang C, Sui J, et al. Receptor and viral determinants of SARS‐coronavirus adaptation to human ACE2. EMBO J. 2005;24(8):1634‐1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor‐binding domain complexed with receptor. Science. 2005;309(5742):1864‐1868. [DOI] [PubMed] [Google Scholar]
- 52. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019‐nCoV acute respiratory disease. Lancet. 2020;395(10223):e30‐e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gharebaghi R, Heidary F, Moradi M, Parvizi M. Metronidazole: a potential novel addition to the COVID‐19 treatment regimen. Arch Acad Emerg Med. 2020;8(1):e40. [PMC free article] [PubMed] [Google Scholar]
- 54. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. He L, Ding Y, Zhang Q, et al. Expression of elevated levels of pro‐inflammatory cytokines in SARS‐CoV‐infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210(3):288‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pelaia G, Gallelli L, D'Agostino B, et al. Effects of TGF‐beta and glucocorticoids on map kinase phosphorylation, IL‐6/IL‐11 secretion and cell proliferation in primary cultures of human lung fibroblasts. J Cell Physiol. 2007;210(2):489‐497. [DOI] [PubMed] [Google Scholar]
- 57. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus‐Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395(10223):473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lansbury LE, Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated cochrane systematic review and meta‐analysis [published online ahead of print November 15, 2019]. Crit Care Med. 2020;48(2):e98‐e106. [DOI] [PubMed] [Google Scholar]
- 61. Arabi YM, Mandourah Y, Al‐Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757‐767. [DOI] [PubMed] [Google Scholar]
- 62. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (2019‐nCoV) infection is suspected: interim guidance. https://apps.who.int/iris/handle/10665/330893. Published 2020. Accessed January 28, 2020.
- 63. Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti‐viral and anti‐inflammatory activity against novel coronavirus (SARS‐CoV‐2). Pharm Res. 2020;56:104761. 10.1016/j.phrs.2020.104761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xin W, Zhang L, Sun F, et al. Escin exerts synergistic anti‐inflammatory effects with low doses of glucocorticoids in vivo and in vitro. Phytomedicine. 2011;18(4):272‐277. [DOI] [PubMed] [Google Scholar]
- 65. Domanski D, Zegrocka‐Stendel O, Perzanowska A, et al. Molecular mechanism for cellular response to beta‐escin and its therapeutic implications. PloS One. 2016;11(10):e0164365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Montopoli M, Froldi G, Comelli MC, Prosdocimi M, Caparrotta L. Aescin protection of human vascular endothelial cells exposed to cobalt chloride mimicked hypoxia and inflammatory stimuli. Planta Med. 2007;73(3):285‐288. [DOI] [PubMed] [Google Scholar]
- 67. Chen C, Wang S, Chen J, et al. Escin suppresses HMGB1‐induced overexpression of aquaporin‐1 and increased permeability in endothelial cells. FEBS Open Bio. 2019;9(5):891‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu CY, Jan JT, Ma SH, et al. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc Natl Acad Sci U S A. 2004;101(27): 10012–10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Salinas FM, Vazquez L, Gentilini MV, et al. Aesculus hippocastanum L. seed extract shows virucidal and antiviral activities against respiratory syncytial virus (RSV) and reduces lung inflammation in vivo. Antiviral Res. 2019;164:1‐11. [DOI] [PubMed] [Google Scholar]
- 70. Michelini FM, Alche LE, Bueno CA. Virucidal, antiviral and immunomodulatory activities of beta‐escin and Aesculus hippocastanum extract. J Pharm Pharmacol. 2018;70(11):1561‐1571. [DOI] [PubMed] [Google Scholar]
- 71. Wang B, Mao X, Zhu J. beta‐aescin alleviates acute lung injury induced by lipopolysaccharide by inhibiting lipid peroxidation and inflammation in mice. Chinese J Cell Mol Immunol. 2018;34(7):600‐604. [PubMed] [Google Scholar]
- 72. Xin W, Zhang L, Fan H, Jiang N, Wang T, Fu F. Escin attenuates acute lung injury induced by endotoxin in mice. Eur J Pharm Sci 2011;42(1‐2):73‐80. [DOI] [PubMed] [Google Scholar]
- 73. Muller‐Redetzky H, Kellermann U, Wienhold SM, et al. Neutralizing Complement C5a Protects Mice with Pneumococcal Pulmonary Sepsis. Anesthesiology. 2020;132(4):795‐807. [DOI] [PubMed] [Google Scholar]
- 74. Fu F, Wang Z, Tian J, Jiang W, Wang C. Application of aescinate and its salts in the preparation of drugs for treating acute inflammation of lung. Patent of China. 2009;ZL03136127.7:2009‐2006‐2024. [Google Scholar]
- 75. Wei T, Tong W, Wen‐ping S, et al. The impact of sodium aescinate on acute lung injury induced by oleic acid in rats. Exp Lung Res. 2011;37(10):585‐599. [DOI] [PubMed] [Google Scholar]
- 76. Huang S, Meng L, Wang Z, Ling T, Zeng Z. The intervening effect of sodium aescinate on pulmonary fibrosis in ALI rats. J Guangdong Pharm Univ. 2018;34(1):59‐63. [Google Scholar]
- 77. Xiao X. Fighting against SARS by traditional Chinese medicine integrated efficiently with Chinese materia medica. Chinese Tradi Herbal Drug. 2003;34(7):669‐671. [Google Scholar]
- 78. Liu C, Hu P, Li C, Wang X, Xiao XT. Therapeutic value of aescine in traumatic acute lung injury. J Trauma Surg. 2013;15(6):497‐499. [Google Scholar]
- 79. Zhang K, Lu Y, Li D, Liang S. The effect of Shunqi Huoxue decoction combined with β‐sodium aescinate in the treatment of acute lung injury caused by thoracic trauma and influences on serum ferritin superoxide dismutase and lung function. Shaanxi J Tradi Chinese Med. 2018;39(12):1798‐1801. [Google Scholar]
- 80. Liu W, Liang Y, Wang L, Hu J, Li Z. Effect of β‐sodium aescinate on 35 cases of pulmonary contusion. Hebei Med J. 2009;31(22):3074‐3075. [Google Scholar]
- 81. Wang H, Fu L, Wang H, Li Q. Clinical study on the prevention of acute radiation lung injury by β‐aescin sodium. Jiangsu Tradi Chinese Med. 2008;40(10):39‐40. [Google Scholar]
- 82. Wang Z, Gao Q. Clinical analysis on the treatment of 60 cases of increased thoracic drainage after thoracotomy with sodium aescinate. Shandong Med J. 2012;52(13):9. [Google Scholar]
- 83. Wang Z, Zhao F, Jiang G, et al. Clinical study of hyperthermia combined with Aescinate Sodium for Injection in treating pneumonia‐like pleural effusion. China Med Herald. 2019;16(8):113‐116. [Google Scholar]
- 84. Wang Y, Liu Z, Zhang F, Wu X, Guo W, Liu M. Effects of sodium aescinate on cytokine during acute exacerbation in patients with chronic obstructive pulmonary disease. China J Mode Med. 2012;22(28):51‐54. [Google Scholar]
- 85. Wang Y, Liu Z, Zhang F, et al. Effects of sodium aescinate combined with tiotropium bromide on pulmonary function and inflammatory mediators in patients with acute exacerbation of COPD. Chinese J Gerontol. 2013;33(19):4742‐4743. [Google Scholar]
- 86. Hao G, Qin J. A randomized controlled study of sodium aescinate on chronic pulmonary heart with peripheral edema. Chinese J Coal Ind Med. 2008;2008(11):8. [Google Scholar]
- 87. Tang S, Gong F, Wu Z. Clinical trial of β‐sodium aescinate injection in the treatment of chronic obstructive pulmonary disease complicated with pneumocardial disease and heart failure. Chin J Clin Pharmacol. 2019;35(21):2651‐2654. [Google Scholar]
- 88. Pan L, Niu J, Wang Z, Zhang L, Fu F. Effect of escin on acute inflammation of lung. China Pharmacist. 2010;13(3):321‐323. [Google Scholar]
- 89. Russell D, Blain PG, Rice P. Clinical management of casualties exposed to lung damaging agents: a critical review. Emergency medicine journal: EMJ. 2006;23(6):421‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. He DK, Shao YR, Zhang L, et al. Adenovirus‐delivered angiopoietin‐1 suppresses NF‐kappaB and p38 MAPK and attenuates inflammatory responses in phosgene‐induced acute lung injury. Inhal toxicol. 2014;26(3):185‐192. [DOI] [PubMed] [Google Scholar]
- 91. Gallelli L. Escin: a review of its anti‐edematous, anti‐inflammatory, and venotonic properties. Drug Des Devel Ther. 2019;13:3425‐3437. [DOI] [PMC free article] [PubMed] [Google Scholar]


