To the Editor,
According to the ECDC, since December 31, 2019, and as of May 18, 2020, 4 679 511 cases of SARS‐CoV‐2 have been reported, including 246 979 deaths. 1 Between March 12, 2020, and May 18, 2020, in the Republic of Cyprus, a total of 917 confirmed cases have been reported. Only a few reports have been recently published regarding the course of SARS‐CoV‐2 in patients with human immunodeficiency virus (HIV). 2 ‐ 4
In our case study, we are presenting our experience from a 58‐year‐old Caucasian male patient with HIV who developed a severe SARS‐CoV‐2 infection. He was one of the first COVID‐19 cases in the Republic of Cyprus.
The patient had malaise, fever, and dry cough on illness day 1 (March 20, 2020). Breathing difficulty developed on day 4, which led him to seek medical attention. The patient was transferred to Limassol General Hospital according to the Republic of Cyprus regional plan for COVID‐19 suspected cases and was admitted to a dedicated ward.
On admission to our facility, the patient had a fever (38°C). The oxygen saturation was 92% while the patient was breathing ambient air, the respiratory rate 22 per minute, the blood pressure 117/72 mm Hg, and the heart rate 105 beats per minute. The patient was awake, alert, and fully oriented.
The patient's medical history is notable for HIV infection since 1995, followed in an outpatient HIV clinic. His most recent (August 2019) CD4 cell count was 1640 per μL, and viral load was undetectable under Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir Alafenamide Fumarate. He had no comorbidities.
The results of laboratory tests upon admission were unremarkable except for a mildly elevated CRP (52 mg per litter). Specimens were collected in accordance with ECDC guidance 5 and included nasopharyngeal and oropharyngeal swab specimens for influenza A and B and SARS‐CoV‐2 (Table 1). Chest radiography was performed, which showed bilateral air space pacifications (Figure 1).
Table 1.
Patient demographics and clinical characteristics
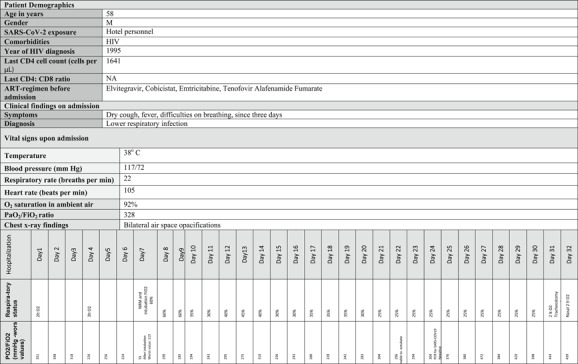

|
Figure 1.
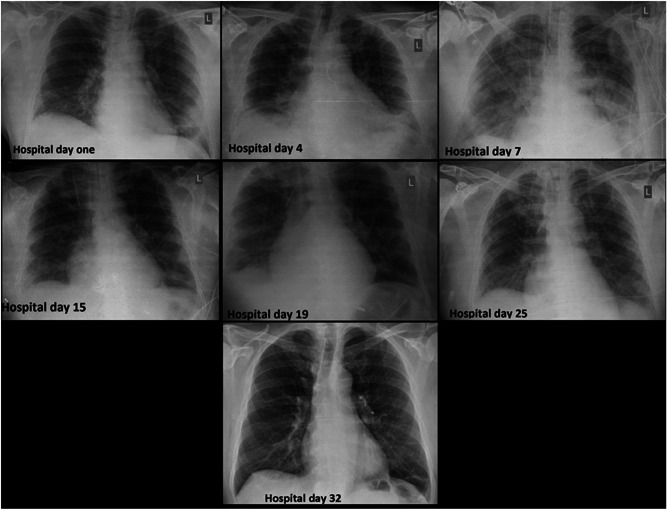
Chest X‐rays of the patient
The patient was started on levofloxacin (750 mg once daily) and oseltamivir (standard dose, 75 mg twice a day), pending results from polymerase chain reaction (PCR) analyses. The first (hospital day 1) and a repeat (hospital day 3) upper respiratory specimen tested with reverse transcriptase real‐time PCR (RT‐PCR) for SARS‐CoV‐2 returned a negative result. COVID‐19 was finally confirmed from a third nasopharyngeal/oropharyngeal sample on hospital day 6. Azithromycin (500 mg once daily) and Chloroquine (500 mg twice a day) was administered (Table 1). Influenza came out negative, and Oseltamivir was subsequently stopped.
The patient progressively developed severe acute respiratory distress syndrome (ARDS) (Figure 1) with a PO2/FiO2 ratio of 55 mm Hg on hospital day 7; he was electively intubated and admitted to the ICU (Table 1). Given the changing clinical presentation and concern about hospital‐acquired pneumonia, piperacillin‐tazobactam (4.5 g four times a day), and vancomycin (1750 mg loading dose followed by 1000 mg three times a day) were initiated. Nasal PCR testing for methicillin‐resistant Staphylococcus aureus was negative, as were all other obtained cultures. Serial procalcitonin was tested negative. Due to persistent fever, the antimicrobial treatment was upgraded to meropenem (2 g three times a day) and gentamicin (400 mg once daily), and upon failure to respond, empirical antifungal treatment with caspofungin (70 mg daily) was administered. The patient responded and remained afebrile after hospital day 24.
The mechanical ventilation aimed at minimizing ventilator‐induced lung injury (VILI). 6 Initially, we targeted a tidal volume of 6 mL/kg (predicted body weight), a plateau pressure lower than 30 cm H2O, PaO2 55 to 80 mm Hg, or SpO2 88% to 95% and pH ≥ 7.25. 7 His oxygenation did not respond to recruitment manoeuvres. His lung mechanics displayed a near‐normal static compliance of 40 L cm·H2O−1, which allowed the use of an individualized, higher tidal volume ventilation strategy, based on observations that COVID‐19 presents itself with impressive nonuniformity. 8 The oxygenation ratio was the worst on hospital day 9 (PO2/FiO2 185) (Figure 1) and gradually improved from that day forward. The patient did not need prone positioning.
On hospital day 14, the patient demonstrated a marked elevation of D‐dimer to 70 386 ng/mL (from 8854 ng/mL on day 6), accompanied by a rise in pCO2 and demand for ventilation. Despite a negative venous duplex ultrasound that excluded acute deep venous thrombosis, enoxaparin dose was increased to therapeutic, based on the procoagulant pattern of patients with COVID‐19. Central Venous Catheters were replaced with peripheral, in line with our thrombosis prevention and infection control bundles. 9 D‐dimers and gas exchange gradually normalized over the next days. A thrombus in the previously cannulated internal jugular vein was documented on hospital day 30.
Upon initiation to wean the patient from the mechanical ventilation, he developed severe hyperventilation, with high respiratory drive, large tidal volumes, and potentially injurious transpulmonary pressure swing, increasing the risk of patient self‐inflicted lung injury (P‐SILI). Sedation and controlled mechanical ventilation were reinitiated, allowing the lung more time to recover. In that perspective, percutaneous dilatational tracheostomy was performed on hospital day 24 after bronchial secretions resulted in negative for SARS‐CoV‐2. Despite the prolonged sedation and mechanical ventilation, the patient regained consciousness relatively quickly and remained oriented and cooperative during the entire stay. He was weaned off the ventilator on hospital day 29, and decannulation was performed on hospital day 31. The patient was discharged from the ICU the following day and transferred to a clinic for rehabilitation. So far, he makes a quick and uneventful recovery.
Standard antimicrobial treatment was used in combination with Chloroquine and azithromycin, based on studies that showed promising results. 10 The patient remained on his previous ART (on tenofovir‐containing regimen), 3 , 4 given his excellent virologic and immunologic condition. Remdesivir, Lopinavir/Ritonavir, Tocilizumab, and corticosteroids were not used due to inconclusive data about their efficacy and safety. 11 COVID‐19 characteristics such as serial false‐negative upper respiratory specimens, deterioration between days 7 and 10, near‐normal lung compliance, hyperventilation, prolonged need for mechanical ventilation, and increased risk for thrombotic complications, were observed in our patient, in line with findings from other case studies. The absence of comorbidities, the virologic and immunologic condition, as well as the use of standard antimicrobial treatment in combination with chloroquine and azithromycin and the maintenance of previous ART instead of adaptation ART seem to have an impact on patient's recovery despite his age. The early initiation of antimicrobial treatment may be translated into a lower risk for opportunistic infections.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
S.I., D.K., and V.R. have been involved in the writing of the manuscript. C.T.M. and D.M. are members of the ICU team and read the manuscript.
ETHICS STATEMENT
A single case report. The patient has given his informed consent.
REFERENCES
- 1. ECDC . Situation update worldwide, as of 3 May 2020. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases. Accessed May 3, 2020.
- 2. Zhu F, Cao Y, Xu S, Zhou M. Co‐infection of SARS‐CoV‐2 and HIV in a patient in Wuhan city, China. J Med Virol. 2020;92:529‐530. 10.1002/jmv.25732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blanco JL, Ambrosioni J, Garcia F, et al. COVID‐19 in patients with HIV: clinical case series. Lancet HIV. 2020;7(5):e314‐e316. 10.1016/s2352-3018(20)30111-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Härter G, Spinner CD, Roider J, et al. COVID‐19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection. 2020;14:15. 10.1007/s15010-020-01438-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European . Centre for Disease Prevention and Control. Laboratory support for COVID‐19 in the EU/EEA. https://www.ecdc.europa.eu/en/novel-coronavirus/laboratory-support. Accessed May 3, 2020.
- 6. Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome advances in diagnosis and treatment. J Am Med Assoc. 2018;319(7):698‐710. 10.1001/jama.2017.21907 [DOI] [PubMed] [Google Scholar]
- 7. Fan E, Del Sorbo L, Goligher EC, et al. An official American Thoracic Society/European Society of intensive care medicine/society of critical care medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253‐1263. 10.1164/rccm.201703-0548ST [DOI] [PubMed] [Google Scholar]
- 8. Gattinoni L, Chiumello D, Caironi P, et al. COVID‐19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020:1‐4. 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iordanou S, Middleton N, Papathanassoglou E, Palazis L, Raftopoulos V. Should the CDC's recommendations for promptly removing unnecessary centrally inserted central catheters be enhanced? Ultrasound‐guided peripheral venous cannulation to fully comply. J Vasc Access. 2019:112972981986355. 10.1177/1129729819863556 [DOI] [PubMed] [Google Scholar]
- 10. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1). 10.5582/BST.2020.01047 [DOI] [PubMed] [Google Scholar]
- 11. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nat Commun. 2020;11(1). 10.1038/s41467-019-13940-6 [DOI] [PMC free article] [PubMed] [Google Scholar]


