Cardiovascular (CV) disease is particularly prevalent among patients with severe coronavirus disease 2019 (COVID‐19) and confers a fivefold higher risk of mortality. 1 Cancer is further associated with a 3.5‐fold higher risk of major events including death or need for mechanical ventilation or intensive care unit admission in patients with COVID‐19 and this risk is higher in patients having undergone chemotherapy or surgery in the past month (75% vs. 43%). 2
Cardio‐oncology represents the intersection of the two entities, CV disease and cancer, and although epidemiological evidence is still missing, it is expected that it deals with patients with a high propensity and/or severity of COVID‐19. This is particularly true for patients receiving treatment for active cancer, those experiencing cardiotoxicity from anticancer therapy or those with immunosuppression due to haematological malignancies, specific anticancer therapies or stem cell transplantation. In addition to the risk of COVID‐19 infection, cardio‐oncology patients may also be deprived of medical services by not getting to the hospital or not receiving the indicated care once there, because of the quarantine measures and the inevitable reallocation of medical resources. As a result, there are several challenges that the cardio‐oncology service needs to address during the COVID‐19 pandemic in terms of required adaptations in clinical practice and management of patients with suspected or confirmed COVID‐19.
Exposure of cancer patients or survivors to potential COVID‐19 cases should be minimized, in accordance with the social distancing measures imposed in many countries, without compromising at the same time the role of cardio‐oncology consultation in patients' outcomes. 3 The risk of exposure to COVID‐19 is higher in medical facilities, particularly referral hospitals, so an important measure would be to secure a separate and protected access to oncology, haematology and cardio‐oncology departments and clinics. For the public spaces and other departments that may be used by cancer patients like the radiology department, all the general prophylactic measures should be applied.
Cardiotoxicity risk stratification before the initiation of anticancer therapy is now more crucial than ever. To minimize the risk of exposure, baseline cardiological evaluation can be omitted in patients with low or very low cardiotoxicity risk, including those with no history of CV disease, CV risk factors, previous cardiotoxicity or previous cardiotoxic therapies and those not being scheduled for anticancer regimens with established cardiotoxicity profile (Table 1 ). In contrast, medium and high‐risk patients should be assessed according to current guidelines and local practices. During cancer therapy, the regular CV follow‐up of patients should be modified in a way that mitigates the risk of not identifying and treating cardiotoxicity on time while attenuating the potential exposure to COVID‐19. Biomarkers, including cardiac troponins (cTn) and natriuretic peptides (NP), have proven their role in early identification of cardiotoxicity in the form of myocardial dysfunction and can replace follow‐up echocardiographic studies in asymptomatic patients during this period. These biomarkers can be assessed during scheduled cancer therapy, without the need for additional hospital visits as in the case of imaging. It should be stressed though that cardiotoxicity is not only about myocardial dysfunction and biomarkers are not always available and cannot identify other toxicities.
Table 1.
Proposed adaptations in cardio‐oncology services during the COVID‐19 pandemic
| Baseline assessment before cancer therapy |
|
| Follow‐up during cancer therapy |
|
| Routine follow‐up of cancer survivors |
|
| Diagnostic modalities |
|
| Tele‐health and information |
|
Imaging and mainly echocardiography is a pillar of cardio‐oncology, but its reasonable and modified use will minimize the exposure of patients and physicians. Apart from the careful selection of patients who need imaging on the basis of a result that will impact on therapy or outcome, point of care or focused echocardiographic protocols for cardio‐oncology may be used to address specific clinical questions while minimizing the examination time 4 , 5 , 6 ; further precautions are needed concerning the disinfection of probes and devices. 6 Alternative imaging modalities (e.g. multi‐gated acquisition scans) can be suggested to minimize patient and physician or sonographer exposure. 4 Specific instructions and measures have been published recently for the application of all imaging modalities, including echocardiography, 6 cardiac computed tomography, magnetic resonance and nuclear imaging techniques, during this outbreak. 7 , 8 , 9
Follow‐up appointments for cancer survivors should be deferred, prioritizing patients' safety and allocating appropriately the existing health care resources. Cancer patients under treatment and cancer survivors should be well informed and updated through all available resources and maintain contact with the cardio‐oncology team through telephone calls, mobile applications and smart digital web‐based applications. 10 Telecommunication‐based consultation between physicians and patients and among physicians can help to continue many of the cardio‐oncology service functions to promote safe delivery of cancer care without disruption while limiting the patients' exposure.
Cardiovascular complications of COVID‐19 include myocardial injury, acute myocardial infarction, heart failure, fulminant myocarditis, takotsubo syndrome, arrhythmias, conduction disturbances, cardiogenic shock and venous thromboembolism. In a cancer patient, these events can also represent CV toxicity of anticancer treatments, thus creating important diagnostic and therapeutic dilemmas. 11 Acute cardiac injury, presenting as cTn or NP elevation, is the most commonly reported COVID‐19‐related CV complication, with an incidence of 8–12% in the general population. 12 Although an advisory by the American College of Cardiology discourages random measurement of cardiac biomarkers, 13 this cannot be applied in many cancer patients, in whom these biomarkers can be used for cardiotoxicity surveillance, attenuating the need for echocardiography or other imaging modalities. cTn elevation, electrocardiographic and echocardiographic abnormalities in the setting of COVID‐19 are further indicative of disease severity and prognostic markers of adverse outcomes. In addition, a cytokine release syndrome represents a manifestation of advanced COVID‐19, while it may also be a complication of anticancer therapy and particularly of chimeric antigen receptor T‐cell agents used in chemotherapy‐refractory haematological and other malignancies. A comprehensive assessment of clinical and laboratory findings placed in the context of CV and oncological history is the safest way to patients' evaluation. For example, the differential diagnosis in the case of cTn elevation between cardiac injury caused by COVID‐19 and myocarditis due to concurrent immune checkpoint inhibitor (ICI) therapy, besides further clinical and laboratory findings, should take under consideration that COVID‐19‐related troponin elevation is frequent, particularly in the presence of CV comorbidities, while ICI‐related myocarditis is quite rare (<1%), being more possible during the first 4–6 weeks of ICI therapy and in the presence of combined ICI.
Angiotensin‐converting enzyme (ACE) inhibitors and angiotensin II receptor blockers represent the cornerstone therapy for myocardial dysfunction caused by anticancer therapy and are also widely used for the treatment of arterial hypertension among cancer patients and survivors. The knowledge that the SARS‐CoV‐2 uses ACE II (ACE2) receptor for its cell entry, gave rise to multiple pathophysiological hypotheses of potential harm or benefit. Given the complexity of the issue and the lack of solid evidence on either harm or benefit in the context of COVID‐19, CV societies across the world has recently released statements recommending the continuation of these drugs. Recently published data support these recommendations as inhibitors of the renin–angiotensin system are not associated with higher plasma ACE2 concentrations 14 or increased risk for COVID‐19.
In the field of research, recruitment of patients has been at least temporarily stopped for all non COVID‐19 trials. Ongoing trials have modified follow‐up to balance compliance with minimal exposure of participants to COVID‐19. Running cardio‐oncology registries in parallel to the numerous currently ongoing COVID‐19 studies and surveys would record clinical experience and generate valuable evidence for this vulnerable population.
The current COVID‐19 pandemic and previous coronavirus outbreaks show that probability of a new epidemic caused by the same or similar airborne viruses in the near future is quite high. Therefore, the proposed adaptations of cardio‐oncology services (Table 1 ) may constitute the core of a pre‐defined plan of care ready to become effective immediately in the case of a new epidemic. The three pillars of this plan should consist of the exploitation of the current telecommunication technologies, including the recent smartphone/watch or other wearable device applications, that allow replacement of conventional clinic visits with remote consultation and telemonitoring of patients at home, the selective use of diagnostic modalities such as imaging, and the constant provision of updated information to patients.
The COVID‐19 pandemic imposes the implementation of strategies that will limit exposure of the vulnerable cardio‐oncology patient population without compromising the essentials of their healthcare. Cardio‐oncology services need to adapt efficiently to deal with the unprecedented challenges in everyday clinical practice during the present and future pandemics (Figure 1 ). At the same time, this outbreak can pave the road to shape specific disciplines and establish mechanisms that would be useful for every similar crisis in the future.
Figure 1.
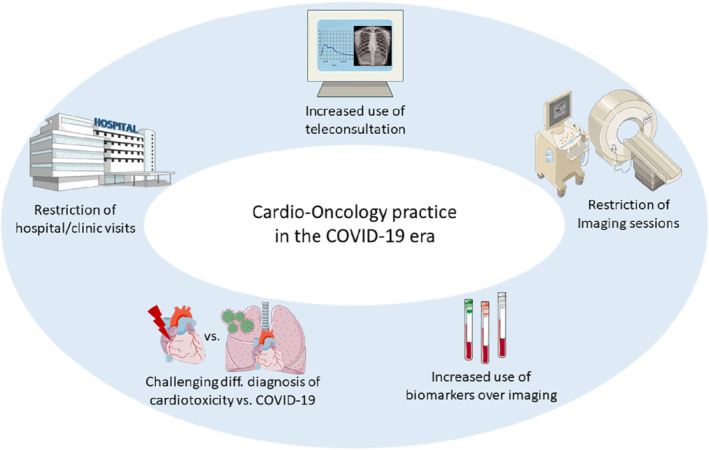
Main adaptations and challenges in cardio‐oncology services during the COVID‐19 pandemic.
Acknowledgements
Part of the figure was created using copyright‐free artwork provided online by Servier Medical Art.
Conflict of interest: D.F. has received modest consultation or lecture fees from Abbott Laboratories, Bayer, Boehringer Ingelheim, Menarini, Novartis, Orion Pharma and Roche Diagnostics. K.K. reports no conflict of interest. G.F. has served as member of committees in trials sponsored by Bayer, Boehringer Ingelheim, Medtronic, Novartis, Servier and Vifor.
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020. Feb 24. 10.1001/jama.2020.2648 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farmakis D, Keramida K, Filippatos G. How to build a cardio‐oncology service? Eur J Heart Fail 2018;20:1732–1734. [DOI] [PubMed] [Google Scholar]
- 4.Routine cardiotoxicity echo screening for chemotherapy patients during COVID‐19. The Council of Cardio‐Oncology of the European Society of Cardiology (ESC). 15 April 2020. https://www.escardio.org/Councils/council‐of‐cardio‐oncology/News/routine‐cardiotoxicity‐echo‐screening‐for‐chemotherapy‐patients‐during‐covid‐19 (24 April 2020).
- 5. American Society of Echocardiography (ASE) . ASE statement on point‐of‐care ultrasound (POCUS) during the 2019 novel coronavirus pandemic https://www.asecho.org/wp‐content/uploads/2020/04/POCUS‐COVID_FINAL2_web.pdf (24 April 2020). [DOI] [PMC free article] [PubMed]
- 6. Skulstad H, Cosyns B, Popescu BA, Galderisi M, Salvo GD, Donal E, Petersen S, Gimelli A, Haugaa KH, Muraru D, Almeida AG, Schulz‐Menger J, Dweck MR, Pontone G, Sade LE, Gerber B, Maurovich‐Horvat P, Bharucha T, Cameli M, Magne J, Westwood M, Maurer G, Edvardsen T. COVID‐19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging 2020;21:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi AD, Abbara S, Branch KR, Feuchtner GM, Ghoshhajra B, Nieman K, Pontone G, Villines TC, Williams MC, Blankstein R. Society of Cardiovascular Computed Tomography guidance for use of cardiac computed tomography amidst the COVID‐19 pandemic endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 2020;14:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American College of Radiology (ACR) . ACR guidance on COVID‐19 and MR use. https://www.acr.org/Clinical‐Resources/Radiology‐Safety/MR‐Safety/COVID‐19‐and‐MR‐Use (24 April 2020).
- 9. Paez D, Gnanasegaran G, Fanti S, Bomanji J, Hacker M, Sathekge M, Bom HS, Cerci JJ, Chiti A, Herrmann K, Scott AM, Czernin J, El‐Haj N, Estrada E, Pellet O, Orellana P, Giammarile F, Abdel‐Wahab M. COVID‐19 pandemic: guidance for nuclear medicine departments. Eur J Nucl Med Mol Imaging 2020;47:1615–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. European Society of Medical Oncology (ESMO) . Cancer care during the COVID‐19 pandemic: an ESMO guide for patients. 8 April 2020. https://www.esmo.org/for‐patients/patient‐guides/cancer‐care‐during‐the‐covid‐19‐pandemic (24 April 2020).
- 11. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, Zamorano JL, Aboyans V, Achenbach S, Agewall S, Badimon L, Barón‐Esquivias G, Baumgartner H, Bax JJ, Bueno H, Carerj S, Dean V, Erol Ç, Fitzsimons D, Gaemperli O, Kirchhof P, Kolh P, Lancellotti P, Lip GY, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Roffi M, Torbicki A, Vaz Carneiro A, Windecker S. ESC Position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail 2017;19:9–42. [DOI] [PubMed] [Google Scholar]
- 12. Bansal M. Cardiovascular disease and COVID‐19. Diabetes Metab Syndr 2020;14:247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American College of Cardiology (ACC) . ACC clinical bulletin focuses on cardiac implications of coronavirus (COVID‐19). 13 February 2020. https://www.acc.org/latest‐in‐cardiology/articles/2020/02/13/12/42/acc‐clinical‐bulletin‐focuses‐on‐cardiac‐implications‐of‐coronavirus‐2019‐ncov (24 April 2020).
- 14. Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, Cleland JG, Rienstra M, Friedrich AW, Samani NJ, Ng LL, Dickstein K, Lang CC, Filippatos G, Anker SD, Ponikowski P, Metra M, van Veldhuisen DJ, Voors AA. Circulating plasma concentrations of angiotensin‐converting enzyme 2 in men and women with heart failure and effects of renin‐angiotensin‐aldosterone inhibitors. Eur Heart J 2020;41:1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]


