Long‐term durable mechanical circulatory support and particularly continuous‐flow left ventricular assist devices (LVAD) have become a standard of care in the treatment of end‐stage heart failure (HF). Complications related to this life‐saving treatment modality are not uncommon and include an elevated risk for infection. 1
COVID‐19
Coronavirus disease 2019 (COVID‐19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) binding to angiotensin‐converting enzyme 2 (ACE2). COVID‐19 is usually mild, but it may occasionally be severe with patients presenting with pneumonia, acute respiratory distress syndrome (ARDS), and circulatory shock. 2 Predictors of fatality include elevated ferritin and interleukin‐6 suggesting that the adverse outcome in COVID‐19 might be due to virally driven haemophagocytic lymphohistiocytosis. 3
Risk from COVID‐19 in left ventricular assist device patients
Left ventricular assist device patients are at increased risk of COVID‐19 infection for several reasons: (i) the demographics of most LVAD patients overlap with risk factors for COVID‐19 infection representing a very vulnerable population, (ii) LVAD patients may manifest hypo‐gammaglobulinaemia and abnormalities of T‐cell function with increased risk for opportunistic infections, 4 (iii) although abnormal values of cardiovascular biomarkers of myocardial stretch, fibrosis, fluid homeostasis, and inflammation may improve with long‐term LVAD use, they remain higher than in chronic HF, 5 (iv) activation or enhanced release of the inflammatory cytokines in COVID‐19 may augment the pre‐existing myocardial injury (acute coronary syndrome, myocarditis, left ventricular dysfunction, arrhythmias, or thromboembolic events) and increase in‐hospital mortality, and (v) sustained haemodynamic instability after LVAD implantation.
Serum lactate dehydrogenase is a recognized biomarker for early recognition of lung injury and assessment of severity in COVID‐19. 6 The relative change in these biomarkers may be more pertinent in grading COVID‐19 severity in LVAD patients. 3 However, the increase in lactate dehydrogenase in LVAD patients may raise specific concerns since it is also a marker of haemolysis and a harbinger of LVAD thrombosis and concomitant stroke. 7 Infection, itself, acts as a trigger for inflammatory response predisposing to pump thrombosis, ischaemic or haemorrhagic stroke in LVAD patients. 8 Of concern, abnormal coagulation parameters have been reported in hospitalized patients with severe COVID‐19 (D‐dimer, fibrin degradation product levels, prothrombin time and activated partial thromboplastin time). 9 The differential diagnosis between worsening HF and/or LVAD thrombosis may be challenging when dyspnoea and fatigue are reported in the COVID‐19 infected LVAD patient along with the aforementioned laboratory abnormalities.
Evaluation and management of the left ventricular assist device patient with suspected COVID‐19
Timely and accurate COVID‐19 laboratory testing is an essential part of the management of COVID‐19. Given the presence of a number of circulating respiratory viruses, differentiating COVID‐19 from other pathogens (e.g. influenza) is important and done using upper or lower respiratory tract samples for reverse transcriptase–polymerase chain reaction and bacterial cultures. Ground‐glass opacities on computed tomography are suggestive but not specific. Low‐risk LVAD patients with COVID‐19 can be treated at home, whereas those at high risk should be hospitalized, ideally at an experienced LVAD centre (Figure 1 ).
Figure 1.
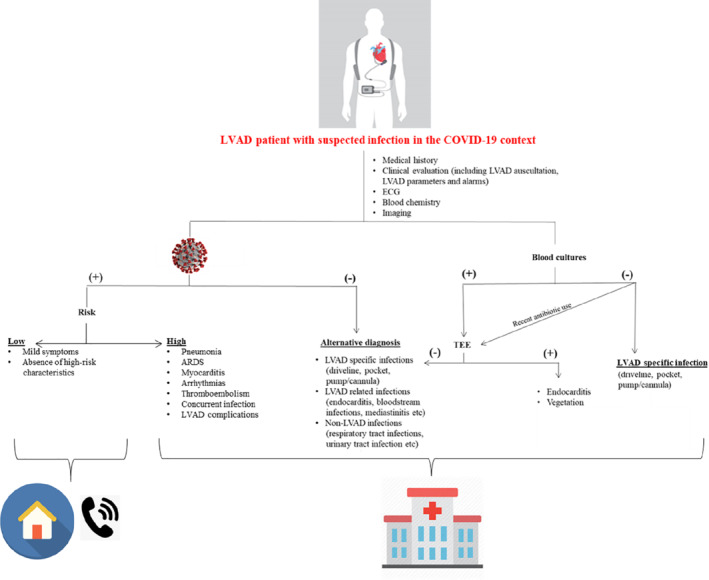
Flow diagram showing the management of left ventricular assist device (LVAD) patients with suspected coronavirus disease 2019 (COVID‐19). (+), present; (−), absent; ARDS, acute respiratory distress syndrome; ECG, electrocardiogram; TEE, transoesophageal echocardiography.
Home management requires that healthcare professionals innovate ways to follow LVAD patients virtually and advise them with instructions to self‐quarantine (‘stay at home’), take hygiene actions and social distancing measures for prevention of disease and transmission, since prevention is currently the best strategy for COVID‐19. Healthcare professionals should limit all elective medical visits and testing, arrange for in‐home blood draws and home international normalized ratio monitoring as well as emphasize the importance of nutrition, sleep and exercise for immune system health. Regarding medical treatment, many LVAD patients are treated with renin–angiotensin–aldosterone (RAAS) inhibitors, which may increase the expression of ACE2, and this has raised concerns regarding their safety. However, RAAS inhibitors are indispensable for HF management, and the scientific societies strongly recommended to physicians and patients not to discontinue treatment. Finally, the patients' families and caregivers must also be protected and practice self‐care measures for safety. Unfortunately, providers currently rely upon face to face visits to follow LVAD patients and there is limited embedded technology within current LVADs to provide remote monitoring capabilities. Most LVAD centres have adapted their face to face contacts to virtual or telephone contacts. Assessment provided with a virtual visit can include evaluation of LVAD patient's clinical status and screening for adverse events, medication review and management, review of LVAD controller parameters, as well as counselling. 10
Hospital management requires that healthcare organizations prepare to address the high demand for care of increased numbers of patients with moderate or severe respiratory illnesses, focusing on the availability of ventilation equipment. Patients with acute hypoxaemic respiratory failure due to COVID‐19 may have a poor tolerance to high positive end‐expiratory pressure, resulting from lung damage. 11 Although prone ventilation may be effective in COVID‐19‐related severe ARDS (improves lung mechanics and gas exchange), it may be problematic in HF patients on LVAD support as prone positioning could result in complications such as compression of outflow graft and driveline, impaired venous return from increased thoracic pressure, hardware malpositioning, and worsening right ventricular (RV) haemodynamics. A case report of an LVAD patient undergoing spine surgery documents the rapid physiologic changes with prone positioning and the need for an experienced clinician to make rapid clinical management decisions. 12 However, the probability of impaired functioning of the LVAD by rotation or mechanical compression seems to be very low and overcome with proper positioning and use of pillows.
A severe complication commonly encountered in ARDS is RV failure, which is associated with high mortality rate despite best standards of care. 13 RV failure in this setting is due to a combination of factors including depressed RV contractility, elevated pulmonary vascular tone, hypercapnia, sepsis, and positive pressure ventilation. Standard haemodynamic monitoring including right heart catheterization can provide evidence indicative of acute RV dysfunction. Point‐of‐care bedside echo may be informative and provide an assessment of RV structure and function (RV dilatation, hypocontractility, and septal shifting toward the left ventricle). Nevertheless, echocardiography and right heart catheterization should be deferred in mild cases or when the information derived from those tests is expected to have no impact on clinical decision making, in order to limit healthcare professionals' exposure to COVID‐19. 3
Medical management is the mainstay of therapy to manage RV failure in the LVAD patient with COVID‐19 and should be focused on four major areas 14 : (i) volume management and optimization of RV preload, (ii) improvement in the contractile state of the right ventricle, (iii) reduction in RV afterload, and (iv) optimization of cardiac rhythm. Haemodynamic goals should optimally include a central venous pressure target of 8 to 12 mmHg, with a cardiac index of 2.2 L/kg/m2 or greater. According to the most recent International Society for Heart and Lung Transplantation guidelines, no evidence base exists for blood pressure targets with continuous‐flow LVADs, but a mean arterial blood pressure goal of ≤80 mmHg seems reasonable. 15 Inotropes of choice are dobutamine and milrinone; renal failure and the need for vasopressor support will guide optimal pharmacologic choices. Vasopressor therapy with vasopressin or angiotensin II in conjunction with inotropes can be used whenever needed to counteract systemic vasodilatation.
Regulation of the LVAD parameters is important in the management of RV failure in the COVID‐19 patient. Although LVAD unloading of the left ventricle may decrease RV afterload and improve RV contractility, LVAD support has important haemodynamic effects that may adversely impact RV function. 16 Volume resuscitation during COVID‐19 infection may exacerbate RV dilatation and tricuspid regurgitation. Device management should focus on maintaining device speeds sufficient to obtain satisfactory haemodynamic goals without inappropriate left ventricular unloading. LVAD speed control should focus on maintaining a rightward or neutral position of the interventricular septum and limiting cardiac output while maintaining an adequate mean arterial pressure. Maintaining appropriate systemic perfusion pressure in the setting of LVAD support is another important aspect to facilitate device management. Vasodilatation or low systemic perfusion pressures result in inappropriate unloading of the left ventricle by the LVAD that can contribute to leftward septal shift and suction events. Suction events prevent adequate LVAD output, may further impair RV function, and may additionally trigger ventricular arrhythmias.
Although LVAD is an effective treatment for cardiogenic shock, it cannot protect for hypotension and vasoplegia with cytokine storm due to COVID‐19 and complicating infections. Septic shock and acute kidney injury may occur in a significant proportion of patients with COVID‐19‐related critical illness and are associated with increasing mortality.
COVID‐19 specific treatment
There is no definitive therapy for COVID‐19. Hydroxychloroquine is frequently chosen as initial treatment, as it presumably reduces in vitro SAR‐CoV‐2 cell entry COVID‐19 and preliminary data are encouraging. 3 , 17 Timely control of the cytokine storm in its early stage with immunomodulators, cytokine antagonists (tocilizumab), and reduction of lung inflammatory cell infiltration may be beneficial in COVID‐19. Remdesivir appears to be effective and may become the standard of care. 18 A small percentage (1–3%) of LVAD patients with COVID‐19 will require extracorporeal membrane oxygenation support, which is feasible only in very specialized centres. Finally, it is important to caution that new data emerge daily regarding clinical characteristics, treatment options, and outcomes for COVID‐19 and that currently optimized supportive care remains the mainstay of therapy.
Conclusion
Left ventricular assist device patients are a very high‐risk population that is vulnerable to COVID‐19. Plans for care of COVID‐19 positive LVAD patients for ambulatory, in‐hospital, and emergency care should be developed until specific measures for COVID‐19 prevention and/or treatment become available. Infected LVAD patients requiring hospitalization should be transported to centres experienced in the care of LVAD patients, which must prepare infrastructure and algorithms to care and innovate strategies for this unique patient population.
Conflict of interest: A.X. has received honoraria from Novartis. F.T. has received research support and honoraria from Amgen, Bayer, Boehringer Ingelheim, Elpen, Lilly, Menarini, Merck, Novartis, Sanofi, Servier, Vianex and WinMedica. R.C.S. reports research funding from Covia, Amgen, advisory board Medtronic, advisor and steering committee Cardiac Dimensions, steering committee Novartis.
References
- 1. Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: patient selection and outcomes. Eur J Heart Fail 2017;19:595–602. [DOI] [PubMed] [Google Scholar]
- 2. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A, Uriel N. COVID‐19 and cardiovascular disease. Circulation 2020;141:1648–1655. [DOI] [PubMed] [Google Scholar]
- 3. Chau VQ, Oliveros E, Mahmood K, Singhvi A, Lala A, Moss N, Gidwani U, Mancini DM, Pinney SP, Parikh A. The imperfect cytokine storm: severe COVID‐19 with ARDS in patient on durable LVAD support. JACC Case Rep 2020. Apr 8. 10.1016/j.jaccas.2020.04.001 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamani MH, Chuang HH, Ozduran V, Avery RK, Mawhorter SD, Cook DJ, Zilka C, Zeroske K, Buda T, Hobbs RE, Taylor DO, Smedira NG, Starling RC. The impact of hypogammaglobulinemia on infection outcome in patients undergoing ventricular assist device implantation. J Heart Lung Transplant 2006;25:820–824. [DOI] [PubMed] [Google Scholar]
- 5. Ahmad T, Wang T, O'Brien EC, Samsky MD, Pura JA, Lokhnygina Y, Rogers JG, Hernandez AF, Craig D, Bowles DE, Milano CA, Shah SH, Januzzi JL, Felker GM, Patel CB. Effects of left ventricular assist device support on biomarkers of cardiovascular stress, fibrosis, fluid homeostasis, inflammation, and renal injury. JACC Heart Fail 2015;3:30–39. [DOI] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, Rame JE, Acker MA, Blackstone EH, Ehrlinger J, Thuita L, Mountis MM, Soltesz EG, Lytle BW, Smedira NG. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33–40. [DOI] [PubMed] [Google Scholar]
- 8. Cho SM, Hassett C, Rice CJ, Starling R, Katzan I, Uchino K. What causes LVAD‐associated ischemic stroke? Surgery, pump thrombosis, antithrombotics, and infection. ASAIO J 2019;65:775–780. [DOI] [PubMed] [Google Scholar]
- 9. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gorodeski EZ, Goyal P, Cox ZL, Thibodeau JT, Reay R, Rasmusson K, Rogers JG, Starling RC. Virtual visits for care of patients with heart failure in the era of COVID‐19: a statement from the Heart Failure Society of America. J Card Fail 2020;26:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meng L, Qiu H, Wan L, Ai Y, Xue Z, Guo Q, Deshpande R, Zhang L, Meng J, Tong C, Liu H, Xiong L. Intubation and ventilation amid the COVID‐19 outbreak: Wuhan's experience. Anesthesiology 2020;132:1317–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kollmar JP, Colquhoun DA, Huffmyer JL. Anesthetic challenges for posterior spine surgery in a patient with left ventricular assist device: a case report. A A Case Rep 2017;9:77–80. [DOI] [PubMed] [Google Scholar]
- 13. Zochios V, Parhar K, Tunnicliffe W, Roscoe A, Gao F. The right ventricle in ARDS. Chest 2017;152:181–193. [DOI] [PubMed] [Google Scholar]
- 14. Pagani FD. Right heart failure after left ventricular assist device placement: medical and surgical management considerations. Cardiol Clin 2020;38:227–238. [DOI] [PubMed] [Google Scholar]
- 15. Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, Deng M, Dickstein ML, El‐Banayosy A, Elliot T, Goldstein DJ, Grady KL, Jones K, Hryniewicz K, John R, Kaan A, Kusne S, Loebe M, Massicotte MP, Moazami N, Mohacsi P, Mooney M, Nelson T, Pagani F, Perry W, Potapov EV, Eduardo Rame J, Russell SD, Sorensen EN, Sun B, Strueber M, Mangi AA, Petty MG, Rogers J; International Society for Heart and Lung Transplantation . The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157–187. [DOI] [PubMed] [Google Scholar]
- 16. Houston BA, Kalathiya RJ, Hsu S, Loungani R, Davis ME, Coffin ST, Haglund N, Maltais S, Keebler ME, Leary PJ, Judge DP, Stevens GR, Rickard J, Sciortino CM, Whitman GJ, Shah AS, Russell SD, Tedford RJ. Right ventricular afterload sensitivity dramatically increases after left ventricular assist device implantation: a multi‐center hemodynamic analysis. J Heart Lung Transplant 2016;35:868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID‐19. J Infect 2020;80:607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros‐Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora‐Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, De Zure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate use of remdesivir for patients with severe Covid‐19. N Engl J Med 2020;382:2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]


