Abstract
Background
The pandemic of new severe acute respiratory syndrome (SARS) due to coronavirus (CoV) 2 (SARS‐CoV‐2) has stressed the importance of effective diagnostic and prognostic biomarkers of clinical worsening and mortality. Epidemiological data showing a differential impact of SARS‐CoV‐2 infection on women and men have suggested a potential role for testosterone (T) in determining gender disparity in the SARS‐CoV‐2 clinical outcomes.
Objectives
To estimate the association between T level and SARS‐CoV‐2 clinical outcomes (defined as conditions requiring transfer to higher or lower intensity of care or death) in a cohort of patients admitted in the respiratory intensive care unit (RICU).
Materials and methods
A consecutive series of 31 male patients affected by SARS‐CoV‐2 pneumonia and recovered in the respiratory intensive care unit (RICU) of the “Carlo Poma” Hospital in Mantua were analyzed. Several biochemical risk factors (ie, blood count and leukocyte formula, C‐reactive protein (CRP), procalcitonin (PCT), lactate dehydrogenase (LDH), ferritin, D‐dimer, fibrinogen, interleukin 6 (IL‐6)) as well as total testosterone (TT), calculated free T (cFT), sex hormone–binding globulin (SHBG), and luteinizing hormone (LH) were determined.
Results
Lower TT and cFT were found in the transferred to ICU/deceased in RICU group vs groups of patients transferred to IM or maintained in the RICU in stable condition. Both TT and cFT showed a negative significant correlation with biochemical risk factors (ie, the neutrophil count, LDH, and PCT) but a positive association with the lymphocyte count. Likewise, TT was also negatively associated with CRP and ferritin levels. A steep increase in both ICU transfer and mortality risk was observed in men with TT < 5 nmol/L or cFT < 100 pmol/L.
Discussion and conclusion
Our study demonstrates for the first time that lower baseline levels of TT and cFT levels predict poor prognosis and mortality in SARS‐CoV‐2‐infected men admitted to RICU.
Keywords: COVID‐19, inflammatory markers, mortality, prognosis, sex hormones
1. INTRODUCTION
The new severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has spread rapidly around the world in the last few months, thus resulting in more than 3.8 million persons globally infected and 265,961 deaths as of May 9, 2020 (https://covid19.who.int/). The SARS‐CoV‐2 infection presents with a broad range of clinical manifestations, encompassing asymptomatic infection, mild upper respiratory tract illness, and dyspnea, to a life‐threatening SARS, with a great number of patients being hospitalized with pneumonia (https://www.who.int/docs/default‐source/coronaviruse/situation‐reports). The sharp increase in SARS‐CoV‐2 infection and the dramatic strain on healthcare systems worldwide have pressured international researchers to urgently investigate SARS‐CoV‐2 disease pathogenesis and to explore factors, which could have an impact toward its prognosis. Earlier prediction models suggest that elderly patients or patients with comorbidities (eg, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, cardiovascular disease) run a greater risk for disease progression and even fatal outcomes. 1 , 2 , 3 However, delineating efficient prognostic factors for both worse clinical course and mortality is claimed as a major unmet need in order to tackle clinical management of the severe cases. 4
SARS‐CoV‐2 infection has been shown to have a differential impact on women and men. Sex disaggregated data indeed showed greater mortality rates in men as compared with women. In particular, men are up at threefold higher odds of lethality than women, 5 thus indicating a firm gender inequality in SARS‐CoV‐2‐associated sequelae. Several social factors, genetic, immunological, and hormonal differences, as well as lifestyle habits (ie, smoking and alcohol consumption), have been considered to play a role in this gender disparity. 6 Among sex hormones, a potential role for testosterone (T) in defining sex‐related differences in terms of SARS‐CoV‐2 infection clinical outcomes has been hypothesized. 7 , 8 Obviously, due to the known higher level of T in men than in women, the prevailing theory is that T might be a promoter of SARS‐CoV‐2 infection and progression. 7 , 8 This theory stems from the positive regulatory effect of androgen receptor (AR) and T on the transcription of a transmembrane protease, serine 2 (TMPRSS2), a critical factor enabling cellular infection by coronaviruses, including SARS‐CoV‐2. Conversely, others have hypothesized that low T levels could theoretically be detrimental, increasing the risk of severe disease among patients with SARS‐CoV‐2 infection, 8 as for other comorbidities. 9 , 10 Indeed, T has been also described to induce the angiotensin‐converting enzyme 2 (ACE2) expression, 11 an important lung protective enzyme. 12 This view would agree with the evidence reporting that in some countries (at least Italy and Spain), SARS‐CoV‐2 infection among female health workers is twice that of their male counterpart (https://data.unwomen.org/resources/Covid‐19‐emerging‐gender‐data‐and‐why‐it‐matters). Of relevance, it is well established that T level declines according to aging and in the presence of comorbidities like obesity, diabetes mellitus, and cardiovascular diseases, which have been found to be highly prevalent in SARS‐CoV‐2 patients. 13 Owing to the high global prevalence of hypogonadism (estimated to be 15%‐20% among middle‐aged/elderly men), 14 , 15 the relation between hypogonadism and SARS‐CoV‐2 infection outcomes deserves deep a thorough investigation. More importantly, the prevalence of hypogonadism in elderly male patients admitted to the hospital for acute illness raises up to 53%. 16 , 17
Thereof, we sought to estimate the association between the T levels and the SARS‐CoV‐2 infection clinical outcomes (defined as conditions requiring to transfer to a higher or lower intensity of care units or even death) as well as the biochemical prognostic predictors of severe and fatal SARS‐CoV‐2 infection in a cohort of patients admitted in the respiratory intensive care unit (RICU) of a single Hospital in Mantua, one of the epicenter of the global SARS‐CoV‐2 pandemic in Italy. This study reports findings from 31 patients with SARS‐CoV‐2 infection and follows 25 of them through to discharge or death. The follow‐up until discharge or death is a point of difference from other case series to date.
2. METHODS
Data from a consecutive series of 31 male patients with SARS‐CoV‐2 pneumonia and recovered in the respiratory intensive care unit (RICU) of the “Carlo Poma” Hospital in Mantua, Italy, were analyzed. A laboratory (pharyngeal‐nose swab positivity) of SARS‐CoV‐2 infection was confirmed by chest X‐ray. Comorbidity burden was assessed by the Charlson comorbidity index (CCI).
Acute respiratory distress syndrome (ARDS) was defined by “Berlin definition,” and patients were segregated into mild ARDS (PaO2/FiO2 ≤ 300 mm Hg and > 200 mm Hg), moderate ARDS (PaO2/FIO2 ≤ 200 mm Hg and > 100 mm Hg), and severe ARDS (PaO2/FiO2 ≤ 100 mm Hg) 18 ; therefore, for the specific purpose of this analysis, patients have been further subdivided into either men with severe ARDS or with mild‐moderate ARDS at the RICU admission.
According to the protocol (approved by Local Ethics Committee Val Padana, Mantua, Italy), each patient underwent a standardized diagnostic workup. Specifically, blood samples were drawn the first morning after the admission at the RICU within 8.00 AM, after an overnight fast, for determination of blood count and leukocyte formula, creatinine, uric acid, electrolytes, transaminases, creatine phosphokinase (CPK), C‐reactive protein (CRP), procalcitonin (PCT), lactate dehydrogenase (LDH), ferritin, D‐dimer, fibrinogen, interleukin 6 (IL‐6), total testosterone (TT), sex hormone–binding globulin (SHBG), and luteinizing hormone (LH). Blood sample analyses were performed in the central laboratory of the “Carlo Poma” Hospital (Mantua, Italy) with commercially available kits normally used for clinical practice of the hospital. TT was measured once by immunoassay (Electrochemiluminescence Immunoassay, ECLIA), and free T was calculated by the Vermeulen formula (http://www.issam.ch/freetesto.htm). 19
Hence, patients were divided according to the outcome throughout the hospitalization in RICU. For the first analyses, patients have been segregated in four groups: (a) The first group composed by patients with improved clinical conditions overtime who have been transferred to the internal medicine (IM) units; clinically, they no longer needed non‐invasive ventilation (NIV) but only low‐flow oxygen therapy; (b) the second group composed by patients still in RICU and under NIV at the time of the current analysis (data on the definitive outcomes are not available yet); (c) the third composed by patients transferred to intensive care unit (ICU) for intubation; and (d) the fourth group composed by patients who eventually died.
2.1. Statistical methods
Differences among groups with different prognoses were evaluated eventually merging the two groups with the worst outcomes (the aforementioned third and fourth groups). Thereafter, a sensitivity analysis was performed to compare the groups of which post‐RICU outcome was known at the time of the analysis (thus excluding the aforementioned second group). Either Mann‐Whitney U test or one‐way ANOVA on ranks (Kruskal‐Wallis test) was used to test the differences concerning continuous variables between two or more than two groups, respectively. Differences between continuous categorical variables were assessed by the likelihood‐ratio test. Data were expressed as medians [interquartile ranges] or percentage for continuous and categorical variables, respectively. Univariate relationships were firstly assessed by Spearman's rank correlation and afterward checked for non‐linearity by the locally weighted scatterplot smoothing (LOWESS) analysis. When linearity could not be assumed, threshold levels for TT or calculated free T (cFT) were identified by the LOWESS analysis and further confirmed by linear regression models with linear spline functions for TT and cFT levels. This analysis allowed identifying threshold levels at which a significant change in the slope of the association between T and other blood markers occurred. The relationship between the clinical outcomes (transferred to IM, in charge to RICU, transferred to ICU or deceased) and TT or cFT was assessed by ordinal logistic regressions. The probability of each outcome based on T according to this regression model was calculated and fitted in a LOWESS curve as a function of TT or cFT.
All the analyses were performed by Statistical Package for the Social Sciences Statistics 26 (IBM Corporation). Spline linear functions were carried out by Stata MP 13 (StataCorp).
3. RESULTS
Of 31 patients, 21 (67.7%) were transferred from RICU to IM after overall improvement of the respiratory conditions, six (19.4%) were stable at time of the present analysis and maintained in RICU, and four (12.9%) worsened their conditions and were either transferred to ICU (n = 2) or eventually died (n = 2).
Table 1 details descriptive statistics of the whole cohort of patients as segregated according to the different outcomes. Overall, significant difference among groups was found in terms of previous history of psychiatric diseases and of prevalence of severe ARDS at RICU admission. Moreover, lymphocyte count was the lowest in both RICU and ICU/deceased patients, whereas PCT and LDH had the highest values in the ICU/deceased group. Of note, no differences in D‐dimer serum levels were observed. LH, TT, and cFT were significantly different among groups, with higher LH and lower TT and cFT in the ICU/deceased group. These differences were confirmed when comparing the groups transferred from RICU because of either improved respiratory conditions or adverse outcomes, respectively (Table 1). In addition, the comparison of these two groups revealed that men in the adverse outcome group (ie, transferred to ICU or deceased) more frequently had a history of arrhythmia and were less frequently obese. Besides the aforementioned biochemical and hormonal parameters, men in the ICU/deceased group had significantly higher neutrophil count, potassium, and CRP levels, as compared with men transferred to IM.
Table 1.
Descriptive statistics of the whole cohort as stratified according to clinical outcomes
|
Reference range |
Transferred to IM (n = 21) |
In charge in RICU (n = 6) |
Transferred to ICU/deceased (n = 4) |
p for trend | p1 | |
|---|---|---|---|---|---|---|
| Demographics and previous medical history | ||||||
| Age (years) | ‐‐‐ |
63.0 [55.0‐66.5] |
72.0 [33.0‐83.5] |
74.5 [59.5‐85.0] |
0.162 | 0.068 |
| Smoking habits (%) | ||||||
| Former smoker | ‐‐‐ | 42.9 | 0.0 | 50.0 | 0.128 | 0.823 |
| Current smoker | ‐‐‐ | 4.8 | 0.0 | 0.0 | ||
| Obesity (%) | ‐‐‐ | 42.9 | 16.7 | 0.0 | 0.860 | 0.046 |
| Hypertension (%) | ‐‐‐ | 57.1 | 33.3 | 50.0 | 0.584 | 0.793 |
| Dyslipidemia (%) | ‐‐‐ | 23.8 | 33.3 | 25.0 | 0.899 | 0.959 |
| Diabetes (%) | ‐‐‐ | 28.6 | 33.3 | 0.0 | 0.267 | 0.119 |
| Hypothyroidism (%) | ‐‐‐ | 14.3 | 0.0 | 25.0 | 0.347 | 0.610 |
| Chronic renal failure (%) | ‐‐‐ | 4.8 | 0.0 | 0.0 | 0.672 | 0.550 |
| Arrhythmia (%) | ‐‐‐ | 0.0 | 0.0 | 25.0 | 0.114 | 0.048 |
| Psychiatric diseases (%) | ‐‐‐ | 0.0 | 33.3 | 25.0 | 0.023 | 0.048 |
| Hematologic diseases (%) | ‐‐‐ | 4.8 | 16.7 | 0.0 | 0.501 | 0.550 |
| CVD (%) | ‐‐‐ | 4.8 | 0.0 | 0.0 | 0.672 | 0.550 |
| Liver diseases (%) | ‐‐‐ | 4.8 | 0.0 | 0.0 | 0.672 | 0.550 |
| Parameters during hospitalization in RICU | ||||||
| Time in RICU (days) | ‐‐‐ |
7.0 [4.0‐9.0] |
10.0 [7.0‐14.3] |
5.0 [4.0‐12.0] |
0.338 | 0.456 |
| PaO2/FiO2 (mm Hg) | <300 for ARDS |
130.4 [104.8‐165.4] |
119.5 [99.5‐155.0] |
87.3 [80.8‐157.9] |
0.226 | 0.132 |
| Severe ARDS (%) |
PaO2/FiO2 ≤100 mm Hg |
14.3 | 16.7 | 75.0 | 0.050 | 0.016 |
| WBC (103/µL) | 4.4‐11 |
7.3 [4.3‐9.0] |
7.2 [5.3‐8.6] |
11.6 [7.7‐17.3] |
0.115 | 0.056 |
| Neutrophils (103/µL) | 2.0‐7.5 |
4.0 [2.6‐6.7] |
6.3 [4.2‐7.2] |
10.6 [6.4‐16.4] |
0.085 | 0.027 |
| Lymphocytes (103/µL) | 1.3‐4.8 |
1.2 [0.9‐1.6] |
0.7 [0.5‐0.9] |
0.7 [0.5‐0.9] |
0.017 | 0.035 |
| Hemoglobin (g/dL) | 13.5‐17‐5 |
11.8 [9.4‐14.0] |
12.4 [10.3‐14.0] |
11.6 [9.4‐13.0] |
0.849 | 0.794 |
| Hematocrit (%) | 40‐50 |
34.8 [28.4‐42.1] |
37.4 [33.1‐41.8] |
36.2 [30.2‐42.1] |
0.771 | 0.911 |
| Platelets (103/µL) | 150‐400 |
278.5 [173.5‐387.3] |
299.5 [229.0‐346.8] |
284.0 [197.3‐391.0] |
0.993 | 0.911 |
| Creatinine (mg/dL) | 0.6‐1.3 |
0.9 [0.8‐1.1] |
1.1 [0.8‐1.2] |
0.8 [0.7‐2.3] |
0.592 | 0.592 |
| Uric acid (mg/dL) | 2.5‐7.2 |
4.0 [2.9‐5.1] |
3.4 [2.7‐5.9] |
4.2 [2.7‐8.4] |
0.973 | 0.803 |
| Sodium (mEq/L) | 135‐145 |
137.0 [132.0‐138.5] |
137.5 [131.5‐139.5] |
137.5 [133.0‐154.8] |
0.726 | 0.452 |
| Potassium (mEq/L) | 3.4‐4.7 |
3.8 [3.6‐3.9] |
3.7 [3.4‐4.0] |
4.4 [3.8‐4.5] |
0.088 | 0.047 |
| AST (U/L) | 10‐33 |
35.0 [30.5‐63.0] |
31.0 [27.5‐45.0] |
46.5 [21.8‐90.8] |
0.530 | 0.748 |
| ALT (U/L) | 5‐37 |
47.0 [32.5‐65] |
28.5 [21.5‐51.8] |
79.0 [28.0‐114.3] |
0.108 | 0.231 |
| CPK (U/L) | 25‐200 |
56.0 [28.0‐95.3] |
39.5 [22.3‐143.8] |
94 [53.5‐582.3] |
0.284 | 0.183 |
| CRP (mg/L) | 0‐5 |
15.7 [3.4‐64.2] |
24.9 [10.5‐72.5] |
143.1 [46.1‐257.2] |
0.060 | 0.023 |
| Procalcitonin (ng/mL) | 0‐0.09 |
0.08 [0.05‐0.15] |
0.10 [0.05‐0.33] |
1.33 [0.46‐2.88] |
0.006 | <0.001 |
| LDH (U/L) | 150‐450 |
414.5 [347.0‐515.0] |
621.0 [563.3‐954.8] |
935.5 [623.8‐1070.3] |
0.002 | 0.003 |
| Fibrinogen (mg/dL) | 150‐450 |
426.0 [303.0‐535.0] |
455.5 [386.0‐617.5] |
471.0 [152.3‐756.0] |
0.811 | 0.969 |
| D‐dimer (ng/mL) | <500 |
1836.0 [515.0‐3697.0] |
1343.5 [631.0‐3197.3] |
2108.5 [858.0‐8171.0] |
0.867 | 0.667 |
| Ferritin (ng/mL) | 30‐400 |
993.0 [656.0‐1365.0] |
1679.5 [511.0‐3791.0] |
1809.0 [876.0‐2199.8] |
0.167 | 0.081 |
| IL‐6 (pg/mL) | <7 |
50.3 [13.1‐86.0] |
56.1 [30.1‐77.6] |
137.2 [50.3‐205.8] |
0.291 | 0.148 |
| Total T (nmol/L) | 8.6‐29 |
8.8 [4.1‐16.2] |
5.0 [1.8‐7.6] |
1.0 [0.2‐1.9] |
0.005 | 0.001 |
| Calculated free T (pmol/L) | <225 |
146.5 [93.8‐287.0] |
118.0 [40.8‐133.5] |
17.5 [5.8‐37.0] |
0.006 | 0.001 |
| SHBG (nmol/L) | 18.3‐54.1 |
35.6 [22.0‐59.0] |
24.0 [19.6‐37.4] |
21.3 [12.2‐39.6] |
0.159 | 0.157 |
| LH (U/L) | 1.7‐8.6 |
6.6 [4.6‐9.6] |
16.3 [7.9‐20.3] |
11.2 [9.0‐19.3] |
0.043 | 0.037 |
Data are reported as median and interquartile range for continuous variables and as percentage for categorical variables. Differences in continuous variables were assessed by one‐way ANOVA on ranks (Kruskal‐Wallis test) for comparison among the three groups or by Mann‐Whitney U test for comparison between the groups with better (transferred to IM) or adverse (transferred to ICU/deceased) outcomes. Differences in categorical variables were evaluated by the likelihood‐ratio test. p for trend refers to the comparisons between all the groups. p1 refers to comparison between men transferred to IM inpatient clinics and men transferred to ICU/deceased.
Abbreviations: ARDS, acute respiratory distress syndrome; AST, alanine transaminase; AST, aspartate transaminase; CPK, creatine phosphokinase; CRP, C‐reactive protein; CVD, cardiovascular disease; ICU, intensive care unit; IL‐6, interleukin 6; IM, internal medicine; LDH, lactate dehydrogenase; LH, luteinizing hormone.; RICU, respiratory intensive care unit; SHBG, sex hormone–binding globulin; T, testosterone; WBC, white blood cell.
Bold values denote statistically significant P‐values.
Italics values denote P‐values close to the statistically significance.
In order to further explore the trends of hormonal levels according to the severity of the outcome, hormones were analyzed in each category after splitting the adverse outcome group into men transferred to ICU and men eventually deceased. Figure 1 confirms the aforementioned differences and shows a stepwise decrease in both TT and cFT according to the severity of the outcome at the time of the final assessment. Despite not achieving the statistical significant, LH had higher values in men with stable or adverse outcome categories. SHBG had comparable values among the four groups. Besides the outcome classes, LH, TT, and cFT were significantly different in patients with or without severe ARDS (LH = 12.0 U/L [8.5‐21.7] vs 6.9 U/L [5.0‐10.4]; TT = 2.19 nmol/L [1.25‐4.58] vs 7.0 nmol/L [4.07‐13.8] and cFT = 50.5 pmol/L [18.6‐84.0] vs 138.0 pmol/L [102.0‐221.0], respectively; all P < .05). No differences in SHBG were found (data not shown). In this cohort, TT and cFT were not significantly associated with age (B= −0.93 [−0.287; 0.101], P = .337 and B= −2.84 [−5.96; 0.274], P = .072, respectively); similarly, TT and cFT did not differ in obese and non‐obese men (TT = 6.75 nmol/L [3.19‐14.86] vs 4.61 nmol/L [2.12‐11.65], P = .633 and cFT = 127.0 pmol/L [58.1‐167.0] vs 120.0 pmol/L [46.8‐204.5], respectively; P = .929).
Figure 1.
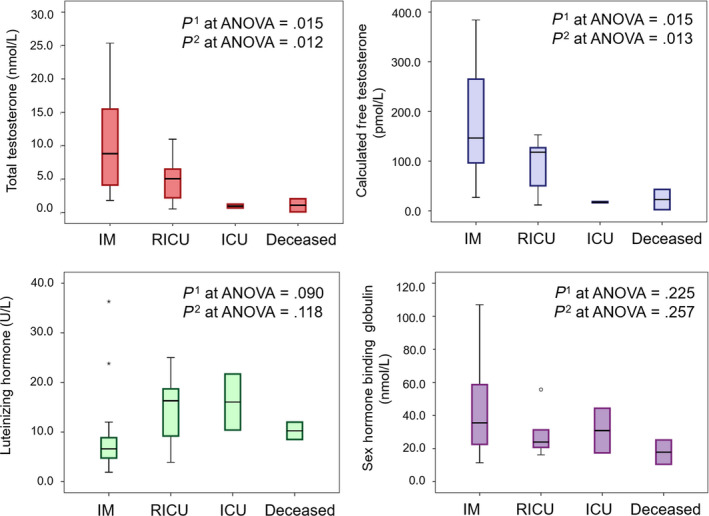
Hormone and sex hormone–binding globulin levels according to different clinical outcomes. Data are expressed as box plot, with median and interquartile range; whiskers represent minimum and maximum values; circles and asterisks represent the outliers and extreme outliers. p1 is the significance level for one‐way ANOVA on ranks (Kruskal‐Wallis test) for testing the differences among the four groups; p2 is the significance level for one‐way ANOVA on ranks (Kruskal‐Wallis test) after excluding the RICU group. Abbreviations: IM = internal medicine inpatient clinics; RICU = respiratory intensive care unit; ICU = intensive care unit
As both TT and cFT showed a significant and progressive decline according to worsening outcomes, we evaluated the relationship between T and markers of disease severity, as emerged by Table 1. Table 2 shows the correlation for TT and cFT with markers of disease severity. Both TT and cFT showed a negative significant correlation with the neutrophil count, LDH, and PCT levels and a positive one with the lymphocyte count. TT was also negatively correlated with CRP and ferritin; cFT showed a similar but not statistically significant trend. Neither TT nor cFT was correlated with potassium. In order to further explore these relationships, we conducted LOWESS analyses to check for their non‐linearity. Figures 2 and 3 show the results for TT and cFT, respectively. Several non‐linear relationships were observed with TT. The visual inspection of LOWESS curves allowed hypothesizing a possible threshold for TT levels around 5 nmol/L (Figure 2). For cFT, the linearity could be assumed for all of the variables but LDH, which showed a steeper increase for cFT levels below 100 pmol/L (Figure 3). In order to check formally these putative thresholds, linear regressions with linear spline functions were performed. Table 3 reports the results of these analyses: PCT, LDH, and ferritin levels depicted different trends above and below TT 5 nmol/L. In particular, for TT < 5 nmol/L, PCT, LDH, and ferritin increased on average of 0.18 ng/mL, 72.72 U/L, and 232.17 ng/mL, for each nmol/L decrease in TT, respectively; these values were higher than those predicted assuming linearity. This was not the case for the neutrophil and lymphocyte counts for which a linear association with TT may be assumed. The association between TT and CRP was not confirmed in this analysis. After adjustment for age and comorbidities, all the above‐mentioned linear relationships were confirmed (neutrophils: B= −0.19 [−0.36;‐0.03], P = .026; lymphocyte: B = 0.03 [0.00;0.06]; P = .043; PCT: B= −0.03 [−0.07; 0.01]; P = .137; LDH: B= −16.45 [−28.22;‐4.69], P = .008; CRP: B= −2.81 [−6.57;0.94], P = .135; ferritin: B= −21.76 [−80.36;36.83]; P = .451).
Table 2.
Correlation between total and free testosterone with parameters associated with different outcomes in SARS‐CoV‐2 pneumonia patients
| Total testosterone (nmol/L) | Calculated free testosterone (pmol/L) | |||
|---|---|---|---|---|
| Spearman's rho | P | Spearman's rho | P | |
| Neutrophils (103/µL) | −0.462 | .012 | −0.450 | .014 |
| Lymphocytes (103/µL) | 0.493 | .007 | 0.461 | .012 |
| CRP (mg/L) | −0.385 | .035 | −0.357 | .053 |
| Procalcitonin (ng/mL) | −0.448 | .015 | −0.454 | .013 |
| LDH (U/L) | −0.490 | .006 | −0.465 | .010 |
| Ferritin (ng/mL) | −0.401 | .031 | −0.320 | .091 |
| Potassium (mEq/L) | −0.134 | .471 | −0.202 | .285 |
Data derived from Spearman's rank correlation test.
Abbreviations: CRP, C‐reactive protein; LDH, lactate dehydrogenase; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Bold values denote statistically significant P‐values.
Figure 2.
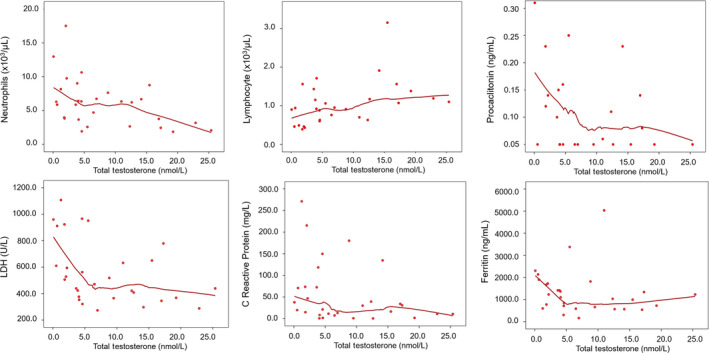
Relationship between total testosterone and blood inflammatory markers of severity of SARS‐CoV‐2 pneumonia. The smooth curves were carried out by locally weighted scatterplot smoothing (LOWESS) analysis. Abbreviations: LDH = lactate dehydrogenase
Figure 3.
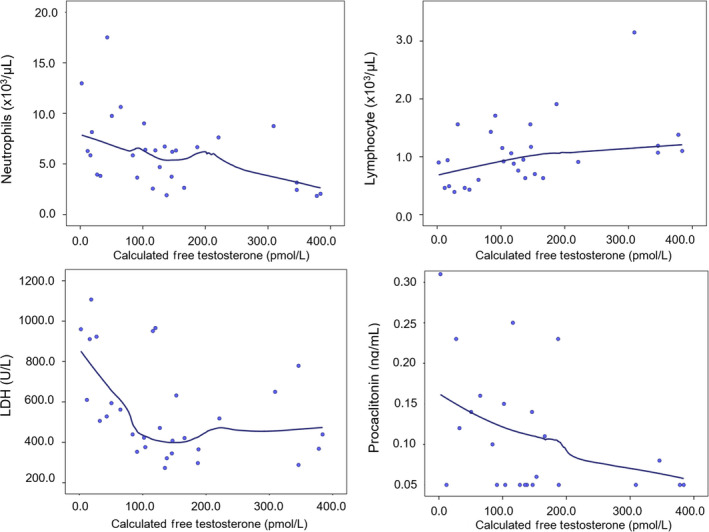
Relationship between calculated free testosterone and blood inflammatory markers of severity of SARS‐CoV‐2 pneumonia. The smooth curves were carried out by locally weighted scatterplot smoothing (LOWESS) analysis. Abbreviations: LDH = lactate dehydrogenase
Table 3.
Confirmation of thresholds for total and free testosterone toward blood inflammatory markers using linear regressions with linear spline functions
| Total testosterone | Calculated free testosterone | |||||
|---|---|---|---|---|---|---|
| Linear | <5 nmol/L | ≥5 nmol/L | Linear | <100 pmol/L | ≥100 pmol/L | |
| Neutrophils (103/µL) |
B = −0.23 [−0.40;‐0.06] P = .012 |
B = −0.63 [−1.53;0.27] P = .161 |
B = −0.15 [−0.40;0.09] P = .200 |
B = −0.14 [−0.25;‐0.03] P = .012 |
B = −0.32 [−0.73;0.09] P = .121 |
B = −0.10 [−0.25;0.05] P = .191 |
| Lymphocytes (103/µL) |
B = 0.03 [0.01;0.06] P = .023 |
B = 0.07 [−0.08;0.22] P = .355 |
B = 0.03 [−0.01;0.07] P = .175 |
B = 0.02 [0.00;0.04] P = .017 |
B = 0.04 [−0.03;0.11] P = .234 |
B = 0.02 [−0.01;0.042] P = .160 |
| Procalcitonin (ng/mL) |
B = −0.03 [−0.07;0.00] 0.084 |
B = −0.18 [−0.35;‐0.01] P = .042 |
B = −0.00 [−0.05;0.05] 0.920 |
B = −0.02 [−0.04;0.00] P = .069 |
B = −0.09 [−0.16;‐0.01] P = .026 |
B = −0.00 [−0.03;0.03] P = .884 |
| LDH (U/L) |
B = −14.49 [−26.49;‐2.49] P = .020 |
B = −72.72 [−130.02;‐15.43] P = .015 |
B = −3.39 [−18.98;12.20] 0.659 |
B = −8.06 [−15.78;‐0.35] P = .041 |
B = −41.46 [−67.18;‐15.73] P = .003 |
B = 0.71 [−8.81;10.22] P = .880 |
| CRP (mg/L) |
B = −3.00 [−6.67;0.68] P = .106 |
B = −10.02 [−28.78;8.75] P = .283 |
B = −1.66 [−6.76;3.45] 0.511 |
‐‐‐ | ‐‐‐ | ‐‐‐ |
| Ferritin (ng/mL) |
B = −21.68 [−80.77;37.40] P = .458 |
B = −232.17 [−397.37;‐66.97] P = .010 |
B = −49.76 [−196,47;96,94] P = .474 |
‐‐‐ | ‐‐‐ | ‐‐‐ |
Data are reported as B coefficients and 95% confidence interval. Data derived from linear regressions (“Linear” columns) with total and free testosterone used as continuous variables using the whole range of values. Columns reporting thresholds (< and ≥ 5 nmol/L; < and ≥ 100 pmol/L) report data derived from linear regressions linear spline functions. Significant associations from linear regressions with the whole range of testosterone values without significant associations with spline functions denote that linearity could be assumed. Significant and non‐significant associations from linear regressions with the whole range of testosterone values with significant associations with spline functions denote that linearity could not be assumed and confirm the indicated threshold value. Non‐significant associations from linear regressions with the whole range of testosterone values with non‐significant associations with spline functions denote a lack of association.
Abbreviations: CRP, C‐reactive protein; LDH, lactate dehydrogenase.
Bold values denote statistically significant P‐values.
A threshold effect for cFT of 100 pmol/L was confirmed for PCT and LDH that decreased on average of 0.09 ng/mL and 41.46 U/L, for each 10 pmol/L increase in cFT, respectively; again, these values were higher than those predicted by assuming linearity. Conversely, linearity could be assumed for the relationship between cFT and neutrophil and lymphocyte counts. After adjustment for age and comorbidities, all the above‐mentioned linear relationships were confirmed (neutrophils: B= −0.10 [−0.21;0.00], P = .064; lymphocyte: B = 0.02 [0.00;0.03]; P = .045; PCT: B= −0.02 [−0.04; 0.00]; P = .135; LDH: B= −10.93 [−18.58;‐3.29], P = .007).
After confirming the association between TT and cFT with several markers of disease severity, the relationship of these parameters with the clinical outcomes was evaluated. Ordinal linear regressions showed that for each nmol/L decrease in TT and for each 10 pmol/L decrease in cFT, the probability of having worse outcomes significantly increased (OR = 1.42 [1.06;1.89]; P = .017 and OR = 1.25 [1.06;1.48]; P = .007 for TT and cFT, respectively) being unaffected by the adjustment for age and CCI (OR = 1.35 [1.03;1.76]; P = .029 and OR = 1.23 [1.04;1.46]; P = .015 for TT and cFT, respectively). The estimated probability of being transferred to IM and ICU, or to die based on T levels is reported in Figure 4. A non‐linear change in the probability of different outcomes is well recognizable. When applying the aforementioned thresholds, the probability of being transferred to IM was 36.51% [29.28‐52.86] and 95.44% [78.05‐99.09] below and above TT 5 nmol/L, respectively (P < .0001). The probability of being transferred to the ICU or dying below and above TT 5 nmol/L was 14.18% [8.89‐17.03] or 0.60% [0.12‐3.32], P < .0001 or 12.40% [6.77‐16.43] and 0.39% [0.07‐2.26], P < .0001, respectively.
Figure 4.
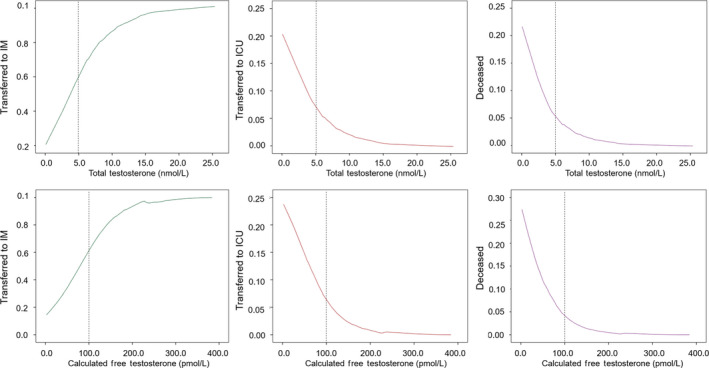
Relationship between total or calculated free testosterone and estimated probability of different clinical outcomes in SARS‐CoV‐2 pneumonia patients based on T levels. The smooth curves were carried out by locally weighted scatterplot smoothing (LOWESS) analysis. Abbreviations: IM = internal medicine inpatient clinics; ICU = intensive care unit
As for actual cases, eight out of 21 (38.1%) transferred to IM, two out of two (100%) transferred to ICU, and two out of two (100%) deceased had TT < 5 nmol/L (P = .035). As for cFT, the probability of being transferred to IM, ICU, or dying in men with cFT below and above 100 pmol/L was 25.58% [19.35‐41.76] vs 84.08% [74.58‐99.45], 18.43% [11.80‐21.43] vs 2.10% [0.06‐3.65], and 16.39% [8.59‐21.93] vs 1.26% [0.04‐2.25], respectively; all P < .0001. As for actual cases, five out of 21 (23.8%) transferred to IM inpatient clinics, two out of two (100%) transferred to ICU, and two out of two (100%) deceased had cFT < 100 pmol/L (P = .010).
4. DISCUSSION
Our study demonstrates for the first time that lower levels of TT and cFT (assessed the first day after the admission in RICU) are novel predictors of poor prognosis in SARS‐CoV‐2 men admitted in RICU for pneumonia. Noteworthy, we found a longitudinal relationship between lower TT and cFT levels and a higher risk of clinical deterioration, thus leading to ICU transfer or to death. Accordingly, we found that lower TT and cFT are significantly associated with higher serum LDH, ferritin, PCT, as well as to an increased level of neutrophils and decrease in lymphocyte count. This latter finding confirms the relevant clinical importance of our observations as all the above‐mentioned inflammatory biomarkers had emerged as poor prognostic factors for SARS‐CoV‐2 infection. 20 Of note, no differences in D‐dimer, an important parameter for the diagnosis of disseminated intravascular coagulation, were observed. Interestingly, for several of these markers (ie, PCT, LDH, and ferritin) a non‐linear association with T was found with an apparent threshold effect (namely, TT at 5 nmol/L or cFT at 100 pmol/L). For TT < 5 nmol/L, the increase in PCT, LDH, or ferritin associated with one nmol/L decline in TT emerged to be sixfold, fivefold, and 10‐fold higher than the linear prediction. Similar results were obtained for cFT below 100 pmol/L for PCT and LDH. Overall, this means that below these threshold values, a further decrease in TT or cFT of 0.5‐1.3 nmol/L or 10‐23 pmol/L, respectively, could be sufficient to cause an increase in these prognostic markers from the lower limit of normal range above the levels that were found associated with SARS‐CoV‐2‐associated in‐hospital death. 2
Also, in this case, statistical modeling for estimating probability demonstrated a non‐linear relationship with mortality or ICU transfer risk. Below TT 5 nmol/L or cFT 100 pmol/L, a steep increase in mortality or ICU transfer risk was observed, with 20‐ to 30‐fold or 10‐ to 15‐fold higher estimated average risk of adverse outcomes as compared to TT or cFT above thresholds, respectively. In contrast, a gradual improvement of clinical outcomes (transfer to non‐intensive care IM) with increasing TT or cFT levels was observed.
We also showed that low baseline TT and cFT levels are related to a more severe ARDS at RICU admission. Noteworthy, these data highlight a novel potential mechanism of frailty and mortality by identifying low T as a risk factor for the severe respiratory failure and inflammatory storm in SARS‐CoV‐2 infections. Our observations are further substantiated by several epidemiological evidence demonstrating that male hypogonadism represents a risk factor for a higher morbidity and mortality. 10 , 21 In particular, it has been well demonstrated that the so‐called functional hypogonadism is associated with conditions like obesity and inflammation in males. 22 Therefore, it could be also speculated that obesity and low T could even foster the cytokine storm aggravating further the clinical condition.
Consistently, over the last decades it has become clear that T is involved in a multitude of biological processes in males, other than reproduction and sexuality. In particular, a novel aspect of the physiology of T is its anti‐inflammatory role. 23 , 24 Several preclinical and clinical evidence showed that low T boosted pro‐inflammatory cytokines and that T treatment blunted their levels. 23 , 25 , 26 There is evidence for an immunomodulatory and protective effect of T by regulating differentiation of T lymphocytes. 23 , 27 , 28 Interestingly, we observed that lymphocyte count increased, while neutrophil levels decreased, as a function of increasing TT and cFT.
Accumulating evidence suggests that SARS‐CoV‐2 infection severity is influenced by a dysregulation of the immune response. A drastic lymphopenia with reduction in numbers of CD4 + T cells, CD8 + T cells, B cells, and natural killer (NK) cells is a common feature in patients with severe SARS‐CoV‐2 infection, but not in patients with mild disease, 29 with neutrophil‐to‐lymphocyte ratio reflecting an enhanced inflammatory process and a poor prognosis. 30 AR is expressed on CD4 + T lymphocytes, CD8 + T lymphocytes, and macrophages supporting the possibility of direct action of T on these cells. 31
A worsening of clinical status was coupled not only with reduced T level, but also with increased LH levels, even though the latter association did not maintain significance when the four groups were compared, thus supporting the presence of a primary hypogonadism. Accordingly, an orchitis‐like syndrome has been hypothesized in SARS‐CoV‐2 men. 32 Several pathogenic mechanisms occurring during the SARS‐CoV‐2 infection might be responsible for the impairment of testicular function. First, ACE2 is highly expressed within the human testis, being a constitutive product of adult‐type Leydig cells. 33 , 34 Because angiotensin II reduced both basal and LH‐stimulated testosterone synthesis by Leydig cells, ACE2 has been hypothesized to modulate the steroidogenic activity of these cells and to shield testis by limiting angiotensin II detrimental effects. 35 , 36 ACE2 on Leydig cells could also alter local microvascular flow and permeability 33 and favoring inflammation. 37 Moreover, increased LH levels could contribute further to testicular alterations by modulating testicular blood flow, endothelial cell permeability, and fluid accumulation within the testis. 38 Finally, in our study, SARS‐CoV‐2 men are affected by a severe form of hypogonadism associated with a primary testicular impairment. A number of well‐designed longitudinal studies have shown that late‐onset hypogonadism (LOH) represents a common clinical entity among aging males. In most men, there is a slow decline in serum TT levels with aging, even in the absence of disease. 39 However, in our cohort of men T level was sharply reduced without showing any significant age‐dependent modulation, further supporting the view of a potential direct effect of SARS‐CoV‐2 on testicular function.
A study on 6 men (mean age 39 years; ranged from 20 to 58 years old) has previously reported LH and T levels in SARS men. 35 The authors compared SARS men with age‐matched healthy men, showing a rise of LH similar to our study, but associated with normal T level. 37 Our study is the first to report novel evidence of a clear‐cut reduction in T level along with an LH increase in SARS‐CoV‐2 men; however, while TT and cFT were associated with a higher risk of clinical deterioration, increased LH levels tended to have a similar relationship, without reaching a statistical significance. We would like to recognize that low serum T levels might also be a consequence and not a reason for the patients' condition. However, the causal effect relationship could only be tested by randomized trials with testosterone treatment vs placebo in hypogonadal men upon submission to the ICU. Also, the presence of either a psychiatric disorder or cardiac arrhythmias was associated with a higher risk of a poor prognosis, whereas obese patients were paradoxically less prone to worse outcomes.
Some limitations should be recognized. Firstly, the sample size is relatively small with a limited number of adverse outcomes. However, a small sample size represents a concern for lack of significant associations, more than for significant ones. Secondly, six patients were still in charge of the RICU at the time of the present analysis; hence, we do not know their definitive outcome. However, the days passed in RICU are comparable among the groups. Unfortunately, information on the onset of infection before RICU was not collected. Moreover, this study lacks of a control group of patients not affected by SARS‐CoV‐2 but hospitalized and evaluated in the same way. This comparison could have strengthen our study as the prevalence of hypogonadism in elderly male patients admitted to the hospital for acute illness is reported to be close to 50%. 16 , 17 Finally, T was assessed only one time and not by the gold standard method (such as mass spectrometry, which was not available in this clinical setting) but it was measured by a commercially available immunoassay used in a high‐volume hospital and undergoing quality control programs. Free T was calculated rather than measured.
5. CONCLUSIONS
Our study demonstrated that, besides a clear pattern of inflammatory, hematologic, biochemical, and immune biomarker abnormalities, lower TT and cFT levels enable significant discrimination between SARS‐CoV‐2 patients with or without poor clinical outcomes. Therefore, both TT and cFT level assessment may potentially aid in terms of risk stratification modeling to predict severe and even fatal SARS‐CoV‐2 infection. To the best of our knowledge, this is the first study unraveling the prognostic role of T levels toward either severity or mortality associated with SARS‐CoV‐2 pneumonia. Whether testosterone therapy could theoretically be beneficial, mitigating the steep increase in clinical deterioration among severely hypogonadal SARS‐CoV‐2 men may be worth further studies.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest in connection with this article.
AUTHORS’ CONTRIBUTIONS
Giulia Rastrelli, Vincenza Di Stasi, Linda Vignozzi, Mario Maggi, and Giuseppe De Donno conceived and designed the study. Vincenza Di Stasi, Francesco Inglese, Massimiliano Beccaria, Martina Garuti, Domenica Di Costanzo, Fabio Spreafico, Graziana Francesca Greco, Giulia Cervi, Antonietta Pecoriello, and Giuseppe De Donno acquired the data. Giulia Rastrelli, Vincenza Di Stasi, Angela Magini, Mario Maggi, Giuseppe De Donno, and Linda Vignozzi analyzed and interpreted the data. Giulia Rastrelli, Vincenza Di Stasi, and Linda Vignozzi drafted the article. Giulia Rastrelli, Andrea Salonia, Andrea Lenzi, Mario Maggi, and Linda Vignozzi revised the article for intellectual contents. Giulia Rastrelli, Vincenza Di Stasi, Francesco Inglese, Massimiliano Beccaria, Martina Garuti, Domenica Di Costanzo, Fabio Spreafico, Graziana Francesca Greco, Giulia Cervi, Antonietta Pecoriello, Angela Magini, Tommaso Todisco, Sarah Cipriani, Elisa Maseroli, Giovanni Corona, Andrea Salonia, Andrea Lenzi, Mario Maggi, Giuseppe De Donno, and Linda Vignozzi provided the final approval of the completed article.
Rastrelli G, Di Stasi V, Inglese F, et al. Low testosterone levels predict clinical adverse outcomes in SARS‐CoV‐2 pneumonia patients. Andrology.2021;9:88–98. 10.1111/andr.12821
Giulia Rastrelli and Vincenza Di Stasi equally contributed to this study.
REFERENCES
- 1. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. JAMA Intern Med. 2020:e200994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev. 2020:e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wynants L, Van Calster B, Bonten MMJ, et al. Prediction models for diagnosis and prognosis of covid‐19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Porcheddu R, Serra C, Kelvin D, et al. Similarity in case fatality rates (CFR) of COVID‐19/SARS‐COV‐2 in Italy and China. J Infect Dev Ctries. 2020;14:125‐128. [DOI] [PubMed] [Google Scholar]
- 6. Wenham C, Smith J, Morgan R, Gender and COVID‐19 Working Group . COVID‐ 19: the gendered impacts of the outbreak. Lancet. 2020;395:846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salonia A, Corona G, Giwercman A, et al. SARS‐CoV‐2, Testosterone and frailty in males (PROTEGGIMI): A multidimensional research project. Andrology. 2020. [DOI] [PubMed] [Google Scholar]
- 8. Pozzilli P, Lenzi A. Testosterone, a key hormone in the context of COVID‐19 pandemic. Metabolism. 2020;108:154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dimopoulou C, Goulis DG, Corona G, Maggi M. The complex association between metabolic syndrome and male hypogonadism. Metabolism. 2018;86:61‐68. [DOI] [PubMed] [Google Scholar]
- 10. Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Endogenous testosterone levels and cardiovascular risk: meta‐analysis of observational studies. J Sex Med. 2018;15:1260‐1271. [DOI] [PubMed] [Google Scholar]
- 11. Chen J, Jiang Q, Xia X, et al. Individual Variation of the SARS‐CoV2 Receptor ACE2 Gene Expression and Regulation. Preprints, 2020; 2020030191. [DOI] [PMC free article] [PubMed]
- 12. Li G, He X, Zhang L, et al. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID‐19. J Autoimmun. 2020:102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehra MR, Desai SS, Kuy SreyRam, et al. Cardiovascular disease, drug therapy, and mortality in Covid‐19. N Engl J Med. 2020:NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Tajar A, Forti G, O'Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810‐1818. [DOI] [PubMed] [Google Scholar]
- 15. Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241‐4247. [DOI] [PubMed] [Google Scholar]
- 16. Iglesias P, Prado F, Macías MC, et al. Hypogonadism in aged hospitalized male patients: prevalence and clinical outcome. J Endocrinol Invest. 2014;37:135‐141. [DOI] [PubMed] [Google Scholar]
- 17. Nakashima A, Ohkido I, Yokoyama K, et al. Associations between low serum testosterone and all‐cause mortality and infection‐related hospitalization in male hemodialysis patients: a prospective cohort study. Kidney Int Rep. 2017;2:1160‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Definition Task Force ARDS , Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526‐2533. [DOI] [PubMed] [Google Scholar]
- 19. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666‐3672. [DOI] [PubMed] [Google Scholar]
- 20. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan. China. Intensive Care Med. 2020;46:846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Araujo AB, Dixon JM, Suarez EA, et al. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta‐analysis. J Clin Endocrinol Metab. 2011;96:3007‐3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corona G, Goulis DG, Huhtaniemi I,, et al. European Academy of Andrology (EAA) guidelines* on investigation, treatment and monitoring of functional hypogonadism in males. Andrology. 2020. [DOI] [PubMed] [Google Scholar]
- 23. Vignozzi L, Cellai I, Santi R, et al. Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J Endocrinol. 2012;214:31‐43. [DOI] [PubMed] [Google Scholar]
- 24. Vignozzi L, Morelli A, Sarchielli E, et al. Testosterone protects from metabolic syndrome‐associated prostate inflammation: an experimental study in rabbit. J Endocrinol. 2012;212:71‐84. [DOI] [PubMed] [Google Scholar]
- 25. Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217:R25‐45. [DOI] [PubMed] [Google Scholar]
- 26. Mohamad N‐V, Wong SK, Wan Hasan WN, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22:129‐140. [DOI] [PubMed] [Google Scholar]
- 27. Fijak M, Schneider E, Klug J, et al. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol. 2011;186:5162‐5172. [DOI] [PubMed] [Google Scholar]
- 28. Vignozzi L, Gacci M, Cellai I, et al. Fat boosts, while androgen receptor activation counteracts, BPH‐associated prostate inflammation. Prostate. 2013;73(8):789‐800. [DOI] [PubMed] [Google Scholar]
- 29. Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lagunas‐Rangel FA. Neutrophil‐to‐lymphocyte ratio and lymphocyte‐to‐C‐reactive protein ratio in patients with severe coronavirus disease 2019 (COVID‐19): A meta‐analysis. J Med Virol. 2020:jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL‐10 production. J Immunol. 2001;167(4):2060‐2067. [DOI] [PubMed] [Google Scholar]
- 32. Isidori AM, Corona G, Aversa A, et al. The SIAMS‐ED trial: A national, independent, multicentre study on cardiometabolic and hormonal impairment of men with erectile dysfunction treated with vardenafil. Int J Endocrinol. 2014;2014:858715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Douglas GC, O’Bryan MK, Hedger MP, et al. The novel angiotensin‐converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703‐4711. [DOI] [PubMed] [Google Scholar]
- 34. Wang Z, Xu X. scRNA‐seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS‐CoV‐2 infection in spermatogonia, leydig and sertoli cells. Cells. 2020;9:E920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dufau ML, Khanum A, Winters CA, et al. Multistep regulation of Leydig cell function. J Steroid Biochem. 1987;27:343‐350. [DOI] [PubMed] [Google Scholar]
- 36. Khanum A, Dufau ML. Angiotensin II receptors and inhibitory actions in Leydig cells. J Biol Chem. 1988;263:5070‐5074. [PubMed] [Google Scholar]
- 37. Xu J, Qi L, Chi X, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006;74:410‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Widmark A, Damber JE, Bergh A. Relationship between human chorionic gonadotrophin‐induced changes in testicular microcirculation and the formation of testicular interstitial fluid. J Endocrinol. 1986;109:419‐425. [DOI] [PubMed] [Google Scholar]
- 39. Wu FCW, Tajar A, Pye SR, et al. Hypothalamic‐pituitary‐testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737‐2745. [DOI] [PubMed] [Google Scholar]


