Abstract
The global response to finding therapeutics for coronavirus disease 2019 (COVID‐19) is chaotic even if well intentioned. There is an opportunity, but more importantly, an obligation for the global clinical and quantitative pharmacology community to come together and use our state‐of‐the‐art tools and expertise to help society accelerate therapeutics to fight COVID‐19. This brief commentary is a call to action and highlights how the global pharmacology community should contribute to the COVID‐19 pandemic and prepare for future pandemics.
We are currently in the grip of the coronavirus disease 2019 (COVID‐19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). As of April 24, 2020, this pandemic has resulted in > 3.5 million cases and 250,000 deaths globally. 1 We are now in an acceleration phase of the pandemic with increases in both cases and deaths projected for several months.
The global response to finding therapeutics for COVID‐19 is chaotic even if well intentioned. It is troubling that there have been > 590 clinical trials conducted or in progress to date with no definitive confidence in the regimens tested. 2 For example, there are 94 clinical trials completed or ongoing to evaluate the role of hydroxychloroquine or chloroquine in treating or preventing COVID‐19, including many that are underpowered, duplicative, and not likely to be pooled for secondary analysis. 2 Many of these trials are wasting important resources in terms of scientists, clinicians, and patients devoting their efforts to such studies. There is also the opportunity cost that these resources could instead be channeled to other opportunities that are more likely to provide additional new information in the quest to find new therapeutics or vaccines.
There is an opportunity, but more importantly, an obligation for the global clinical and quantitative pharmacology community to come together and use our state‐of‐the‐art tools to efficiently and accurately identify the right drug (or drug combinations) at the right dose(s) in the right person at the right stage of disease to give society therapeutic tools to fight COVID‐19. In doing so, this work will lay the groundwork to prepare for the next pandemic. This brief commentary is a call to action and highlights how the global pharmacology community should contribute to accelerating identification of effective treatments for this pandemic and prepare for a more efficient approach for the future.
Critical triage points for accelerating a therapeutic for COVID‐19 include: demonstrating proof of pharmacological plausibility (e.g., mechanism of action, preclinical activity against targets including SARS‐CoV‐2 or host‐target); establishing a proof of hope (e.g., expectation that pharmacologically relevant concentrations at the target site can be achieved clinically, a relevant therapeutic index exists, and no issues exist for other clinical pharmacology considerations); developing clinical proof of concept and dose determination (e.g., demonstration that the proof of hope translates into an established clinical dose rationale); and ultimately clinical confirmation and real‐world validation (e.g., conduct of randomized controlled trials and evaluation of real‐world evidence; Figure 1 ). Failing to adequately address any of these steps means risk is carried forward, and it is the patients who bear the brunt of this risk. The cumulative carryover of avoiding these critical stage‐gates in drug development is the amplification of poor decision quality and society will pay the ultimate a price with global health and economic impact as we wait for effective therapeutics and vaccines.
Figure 1.
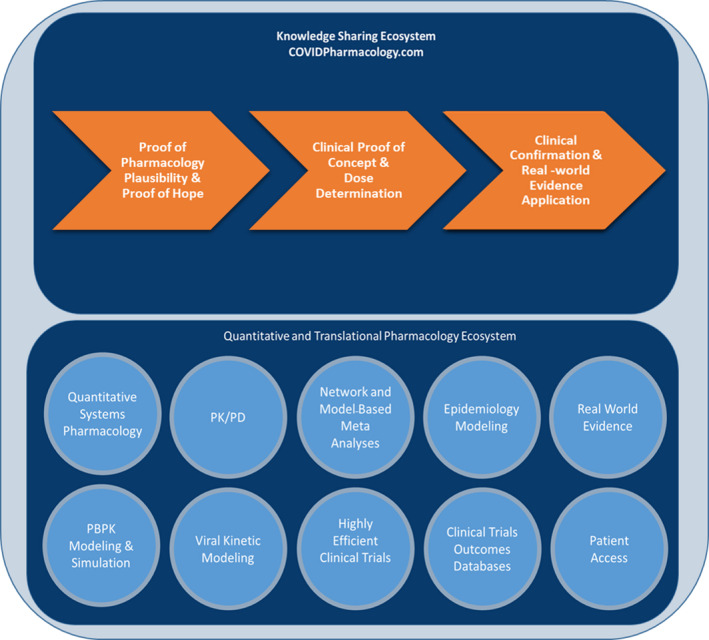
Critical areas of activity that support decision points for coronavirus disease 2019 (COVID‐19) candidate therapeutics and examples of the quantitative and translational pharmacology ecosystem that can support the triage process. PBPK, physiologically‐based pharmacokinetic; PK/PD, pharmacokinetic/pharmacodynamic.
For COVID‐19, we are developing therapeutics in an environment where every milligram above optimal is considered wasted, meaning fewer patients are treated and extra manufacturing capacity is required, and where every milligram under optimal provides the virus an opportunity to overcome our interventions. Given the understandable urgency for therapeutic interventions, the usual rigor for “clinical proof of concept and dose determination” appears to be overlooked, with many situations where a single dose regimen is rushed into an randomized controlled trial. 3 This is a significant factor driving duplication of clinical trials efforts globally. At best, these trials will be inefficient at determining the optimal dose, if they can be used in pooled analyses. At worst, they will be uninformative. In light of the increased urgency and less stringent gating criteria for candidate therapeutics for COVID‐19, the global clinical and quantitative pharmacology community has much to offer and should be more vocal in making this known (Figure 1 ). Our toolbox allows developing smarter trial designs, including approaches such as umbrella designs, incorporating dose regimen diversity; pharmacokinetic/pharmacodynamic (PK/PD) evaluations, even in real‐time, leveraging biosimulation to optimize dose regimens that may not have been formally studied, and applying model‐based meta‐analysis approaches to explore the impact of dose on efficacy from across multiple dose arms and trials. Our PK/PD toolbox has relevance even in compassionate use situations and can provide supportive information as evidenced by the compassionate use protocol developed for linezolid. 4
Without taking a structured approach to developing therapeutics in a pandemic, it is easy to put hope in an early experimental finding that is not translatable. As an example, Caly et al. reported the in vitro activity of ivermectin against SARS‐CoV‐2. 5 In a letter to the editor, Yeo et al. showed that even with the most generous assumptions for clinical translation, the in vitro half‐maximal inhibitory concentration is > 9‐fold and > 21‐fold higher than the day 3 plasma and lung tissue simulated peak plasma concentration (Cmax), respectively, following a high‐dose ivermectin regimen of 600 µg/kg dose daily for 3 days. 5 This position was echoed in a public statement by the Mectizan Donation Program for Onchocerciasis. 6 The Caly paper resulted in US Food and Drug Administration (FDA) warnings over veterinary use of ivermectin. 7 A recent editorial by Chaccour underscored the importance of rigor in uncertain times, cautioned the application of the data, acknowledged the unlikely attainment of relevant concentrations, and, yet, ultimately called for clinical trials. 8 When does a “proof of hope” triage point gate administering to patients? In any other situation, the lack of available relevant information would not support moving to clinical studies. In this case, it is supplemented by a knowledge of human PKs that casts doubt on the ability to achieve relevant concentrations.
Taken in isolation, in vitro promises lead to in vivo failure in most cases. In the volatile environment of this pandemic, the global clinical and quantitative pharmacology community must unite and address these translational pharmacology issues. We have much to offer from our quantitative and translational pharmacology ecosystem, including physiologically‐based pharmacokinetic, PK/PD modeling, and quantitative systems pharmacology that can drive better decisions in the “proof of hope” triage point (Figure ). 1 Our time and resources are precious, and we must apply our resources to those compounds most likely to succeed against COVID‐19.
The quantitative and translational pharmacology ecosystem includes other approaches that elevates the entire patient journey, such as “clinical confirmation and real‐world evidence application.” Population PK methods can support dosing regimens for subpopulations, viral kinetic modeling can inform the target treatment window, 9 epidemiological models can help to determine optimal dose, duration, and treatment strategies to mitigate spread of infection, and physiologically‐based pharmacokinetic models can illuminate optimal approaches to manage problematic drug‐drug interactions. Clinical trial outcomes databases enable comparing treatments and doses and real‐world evidence interventions, such as registry studies, that can be conducted to maximize the ability to tease out comparative treatment effects. Furthermore, appropriate linkage of PK/PD and epidemiology to health economic models can help payers define procurement and deployment approaches that optimize a therapeutic intervention’s place within the healthcare system. 10 These tools can also inform potential therapeutic combinations against COVID‐19. However, one cannot overstate the added complexity of proving the benefit of a combined regimen vs. a monotherapy during a pandemic nor the additional challenges with procuring, deploying, and embedding combined treatment approaches into clinical practice in a pandemic setting.
Many significant consortia announcements for coordinating and speeding development of COVID‐19 therapeutics have been announced, such as the COVID‐19 Therapeutics Accelerator sponsored by the Gates Foundation, Wellcome, Mastercard, and a range of other donors, and also a National Institutes of Health (NIH)‐driven public‐private partnership involving the FDA and European Medicines Agency (EMA), the foundation for the NIH, and 15 industry partners.
This unprecedented situation presents opportunities far beyond improved coordination, faster review times, and parallel processing of clinical trials, which can only incrementally improve the speed of a drug development process that is traditionally measured in years. We do not have that time. We need the best thinking from the clinical and quantitative pharmacology community with visibility at the tables where decisions on COVID‐19 therapeutics are being made. The international clinical and quantitative community, spearheaded by its professional bodies, should be at the forefront advocating for the application of translational pharmacology approaches to improve efficiency in development and certainty in critical drug development decisions.
To establish a knowledge‐sharing ecosystem for advancing clinical and quantitative pharmacology approaches to accelerate therapeutics for COVID‐19, the COVID‐19 Pharmacology Resource Center (https://www.covidpharmacology.com/) was established with funding from the Gates Foundation (Figure 1 ). It features an in silico workbench with pharmacological information and PK and PD simulation tools to inform dosing strategies and clinical study designs or provide the rationale that may dissuade further clinical investigations. Currently, there are in silico workbenches for hydroxychloroquine, chloroquine, lopinavir/ritonavir, azithromycin, and pharmaco‐epidemiological modeling. Simulation tools for tocilizumab, indinavir, and darunavir will be available Q2 2020. Researchers can join and contribute to the Center’s forums to advance critical insights in clinical and quantitative pharmacology and help create new or improve existing models as more evidence evolves. The Center also shares clinical pharmacology tables that contain pertinent information on these compounds relevant to dosing in special patient populations as well as general PK and PD characteristics. The virtual Center will continue to grow and link with other information resources, including COVID‐19 clinical trials databases and the COVID‐19 Therapeutics Accelerator Clinical Studies Proposal Portal. With the global clinical pharmacology community contributing, it is the intention that the Center will become a collaborative resource that allows anyone to learn from and contribute to the clinical pharmacology knowledge of the COVID‐19 response community.
Now is the time for our community to step up and share what we can do with the full repertoire of our approaches within the clinical and quantitative pharmacology ecosystem for COVID‐19 and for the future therapeutics and pandemics. We must share best practices and in silico tools, advocate for integrating quantitative approaches in consortia efforts, inform to influence by ensuring contextualized translation of in vitro information to the clinic, and bring to the forefront the highest priority of determining the optimal dose for patients.
Now is the opportunity to not just think but work without borders, so as a community, we can accelerate getting the right drug at the right dose in the right patient at the right time for COVID‐19.
Conflict of Interest
D.H. and S.K. work for the Bill & Melinda Gates Foundation and are members of the COVID‐19 Therapeutics Accelerator. F.B., S.K.M., and C.R.R. work for Certara, a consulting firm in integrated drug development, and have directly consulted with a variety of not‐for‐profit global health organizations, biotechnology, and pharmaceutical companies, and governments with an interest in medical countermeasures against respiratory virus infections.
Funding
The COVID‐19 Pharmacology Resource Center (http://www.covidpharmacology.com) is funded by the Bill & Melinda Gates Foundation.
References
- 1. Johns Hopkins Coronavirus Resource Center. COVID‐19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. <https://coronavirus.jhu.edu/map.html>. Accessed May 5, 2020.
- 2. Global Coronavirus COVID‐19 Clinical Trial Tracker. <https://www.covid19‐trials.com>. Accessed April 20, 2020.
- 3. Smith, P.F. , Dodds, M. , Bentley, D. , Yeo, K. & Rayner, C. Dosing will be a key success factor in repurposing antivirals for COVID‐19. Br. J. Clin. Pharmacol. 10.1111/bcp.14314. [DOI] [PubMed] [Google Scholar]
- 4. Rayner, C.R. , Forrest, A. , Meagher, A.K. , Birmingham, M.C. & Schentag, J.J. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin. Pharmacokinet. 42, 1411–1423 (2003). [DOI] [PubMed] [Google Scholar]
- 5. Bray, M. , Rayner, C.R. , Noël, F. , Jans, D. & Wagstaff, K. Ivermectin and COVID‐19: a report in antiviral research, widespread interest, an FDA warning, two letters to the editor and the authors' responses. Ant. Res. 178, 104805 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mectizan donation program. <https://mectizan.org>. Accessed April 24, 2020.
- 7. Solomon, S. FDA letter to stakeholders: do not use ivermectin intended for animals as treatment for COVID‐19 in humans. US Food and Drug Administration. <https://www.fda.gov/animal‐veterinary/product‐safety‐information/fda‐letter‐stakeholders‐do‐not‐use‐ivermectin‐intended‐animals‐treatment‐covid‐19‐humans>. Accessed April 20, 2020.
- 8. Chaccour, C. , Hammann, F. , Ramón‐García, S. & Rabinovich, N.R. Ivermectin and novel coronavirus disease (COVID‐19): keeping rigor in times of urgency. Am. J. Trop. Med. Hygiene 102, 1156–1157 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goncalves, A. et al Timing of antiviral treatment initiation is critical to reduce SARS‐Cov‐2 viral load. medRxiv. 10.1101/2020.04.04.20047886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamal, M.A. et al Interdisciplinary pharmacometrics linking oseltamivir pharmacology, influenza epidemiology and health economics to inform antiviral use in pandemics. Br. J. Clin. Pharmacol. 83, 1580–1594 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


