Dear editor
We present a case of an 83‐year‐old woman with a history of hypertension, transient ischaemic attack (TIA), atrial fibrillation and chronic renal impairment presented to our dermatology emergency room on 9 April 2020, for evaluation of purple palpable papules and serohaematic blisters on both her lower legs, feet and toes that had appeared 5 days earlier (Fig. 1). She denied having any new medications, and she had been undergoing long‐term treatment with acenocoumarol and furosemide for her prior conditions. A month before the cutaneous lesions developed, she had a history of one week of sore throat, followed by malaise and nausea. She did not present dyspnoea, chest pain, diarrhoea, smell or taste alterations, nor fever. A clinical diagnosis of cutaneous vasculitis was made. We performed a 4‐mm punch biopsy of one palpable purple papule. A complete blood test and urinalysis were performed. The patient was started on 30 mg/day prednisone therapy with clinical improvement achieved after 10 days, after which the medication was progressively tapered. Laboratory testing showed a normal white blood cell count. Thrombocytopaenia was not observed. C‐reactive protein was 8.0 mg/L (normal value 0.0–5.9), and serum lactate dehydrogenase was 279 UI/L (normal value 100–190). Hepatic function was not impaired. Creatinine was 1.44 mg/dL (normal value 0.50–1.10), and this finding was related to prior chronic renal disease. Urinalysis showed no signs of proteinuria nor haematuria. Serum IgG, IgM and IgA levels were within normal ranges. Immunological laboratory results showed negative values for anti‐nuclear antibodies and anti‐neutrophil cytoplasmic antibodies (ANCAs). IgM cardiolipin antibodies were slightly increased: 21.29 (MPL; normal value < 20.00). Complement C3 and C4 levels were not decreased. Serum cryoglobulins were negative. Serological testing for HIV, HBV, HCV, hepatitis A virus, B19‐parvovirus, cytomegalovirus and Epstein–Barr virus ruled out infection by these pathogens. PCR for SARS‐CoV‐2 was negative. Serological qualitative rapid testing for SARS‐CoV‐2 was positive for IgM and IgG antibodies. Histopathological studies showed leukocytoclastic vasculitis (LCV) affecting dermal vessels, accompanied by extravasation of red cells, basal epidermal layer necrosis, dermal perivascular neutrophil infiltration and fibrin deposition (Fig. 2).
Figure 1.
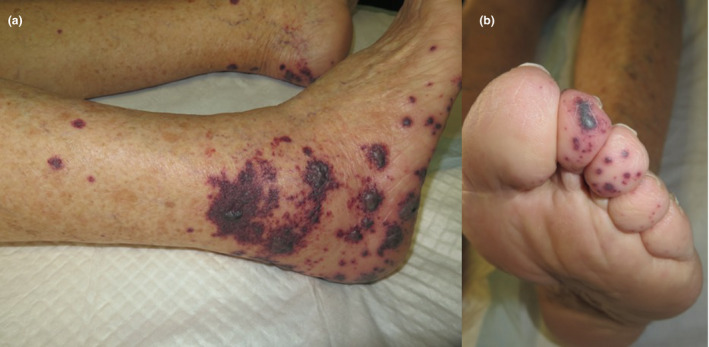
Cutaneous finding of our patient. (a) Purpuric papules on both distal legs and feet with some haematic blisters. (b) Closer look at toes showing petechiae and purpuric papules.
Figure 2.
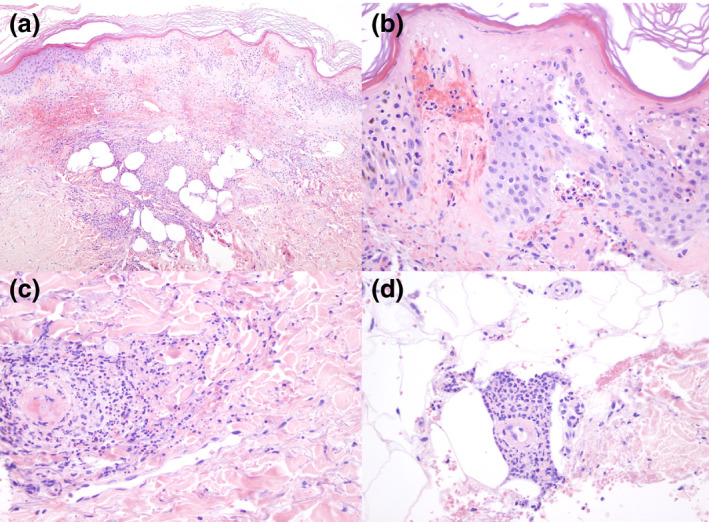
Histopathological findings with haematoxylin–eosin stain. (a) 10×. Panoramic view in which a dense inflammatory infiltration is observed extending throughout the dermis and the presence of abundant extravasated red blood cells in the superficial dermis. (b) 40×. In some areas of the sample, there is necrosis of the epidermis and accumulation of neutrophils at the tips of the dermal papillae. (c) 40×. Presence of perivascular neutrophils and fibrins in the thin vessel wall of the dermis, with the presence of leukocytoclasia. (d) 40×. Presence of neutrophils and fibrin deposits on the walls of deep thin vessels.
SARS‐CoV‐2 (COVID‐19) is a novel RNA virus that causes acute pneumonia that can lead to severe respiratory distress syndrome, which is sometimes fatal. 1 Nevertheless, most infections are mild, and asymptomatic infections are common. Cutaneous findings associated with COVID‐19 include urticarial eruptions, 2 livedo reticularis, 3 acral lesions resembling chilblains 4 and a varicella‐like exanthem. 5
CSVV represents a subgroup of vasculitis limited to the skin, usually attributed to drugs or infections. 6 Deposition of immune complexes which activate the complement cascade is thought to be the main pathogenic factor that causes small vessel wall damage in LCV. 7 Interleukin (IL)‐6 levels have also been shown to be increased in hypersensitivity and other vasculitis conditions. 8 IL‐6 elevation has been associated with the large cytokine storm that results in severe COVID‐19 infection. 9 Thus, we postulated that the CSVV in our patient might have been due to an immune response against viral antigen deposition or as a result of IL‐6 elevation during infection, which might have produced endothelial damage. The slightly increased anticardiolipin IgM antibodies could be related to acute infection. 10 We did not find any other symptoms or signs suggesting any systemic involvement due to vasculitis. Similarly, we did not suspect any thrombotic event, other than the fact that the patient was already undergoing anticoagulant therapy.
We present a case of CSVV or LCV attributable to COVID‐19, as shown by convalescent‐phase positive IgM and IgG antibodies. We conclude that cutaneous vasculitis should be considered as a possible consequence of SARS‐CoV‐2 infection during this pandemic.
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA 2020; 323: 1239. [DOI] [PubMed] [Google Scholar]
- 2. Henry D, Ackerman M, Sancelme E et al. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bouaziz JD, Duong T, Jachiet M et al. Vascular skin symptoms in COVID‐19: a french observational study. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Piccolo V, Neri I, Filippeschi C et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marzano AV, Genovese G, Fabbrocini G et al. Varicella‐like exanthem as a specific COVID‐19‐associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol 2020; 83: 280–285. 10.1016/j.jaad.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jennette JC, Falk RJ, Bacon PA et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013; 65: 1. [DOI] [PubMed] [Google Scholar]
- 7. Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology 2010; 56: 3–23. [DOI] [PubMed] [Google Scholar]
- 8. Nalbant S, Koc B, Top C et al. Hypersensitivity vasculitis and cytokines. Rheumatol Int 2002; 22: 244–248. [DOI] [PubMed] [Google Scholar]
- 9. Chen X, Zhao B, Qu Y et al. Detectable serum SARS‐CoV‐2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL‐6) level in critically ill COVID‐19 patients. Clin Infect Dis 2020. 10.1093/cid/ciaa449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McNeil HP, Chesterman CN, Krilis SA. Immunology and clinical importance of antiphospholipid antibodies. Adv Immunol 1991; 49: 193–280. [DOI] [PubMed] [Google Scholar]
Acknowledgement
The patient in this manuscript has given written informed consent to publication of their case details.


