Abstract
On 11 March 2020, the World Health Organization (WHO) declared the coronavirus disease (COVID‐19) caused by severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) as a pandemic. Until an effective treatment or a vaccine is developed, the current recommendations are to contain the disease, and control its transmission. It is now clear that the primary mode of SARS‐CoV‐2 transmission is aerosol/droplet spread, and by contacting virus‐contaminated surfaces acting as fomites (inanimate vectors). Furthermore, recent data indicate that the live virus particles are present in saliva, and, more alarmingly, asymptomatic individuals may transmit the infection. By virtue of the nature of the practice of dentistry where intrinsically, a high volume of aerosols is produced, as well as the close proximity of dentists and patients during treatment, dentists and allied health staff are considered the highest risk health professional group for acquiring SARS‐CoV‐2 during patient management. Therefore, several organizations and specialty associations have proposed guidelines and recommendations for limiting the transmission of SARS‐COV‐2 from carriers to dentists and vice versa. This paper aims to provide a review of these guidelines, and concludes with a brief look at how the practice of dentistry may be impacted by COVID‐19, in the post‐pandemic era.
Keywords: coronavirus, COVID‐19, dentistry, oral health, SARS‐CoV2, transmission
1. BACKGROUND
As of 26 April 2020, more than two and half million cases of coronavirus disease 2019 (COVID‐2019) and over 190,000 deaths from the disease have been recorded worldwide from 210 countries (World Health Organization, 2020b). The agent of the pandemic, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is highly infectious compared with other coronaviruses such as SARS‐CoV that caused the epidemic in a relatively few countries in 2003–2004 (Samaranayake & Peiris, 2004). The high infectivity of the SARS‐CoV‐2 is thought to be due to (a) its high reproduction number (RO value between 2 and 6.75), (b) the relatively long prodromal period and (c) the probable asymptomatic carrier state (Wu, Wu, Liu, & Yang, 2020; Zhao et al., 2020).
Although up to now there has been no reported nosocomial transmission of SARS‐CoV‐2 in dental care settings, dentists are considered to be the highest risk group of healthcare workers at risk for contracting COVID‐19 according to an analysis by the O*Net Bureau of Labour Statistics of the USA (Gamio, 2020). This is likely to be due to the nature of their work that entails aerosol production, and working at very close proximity to their patients during dental treatment. Hence, a number of dental associations and societies have now promulgated several protocols to provide guidance on managing dental practices during the pandemic. This paper provides a brief overview of the etiopathology, transmission routes and symptomatology of the disease followed by a critical commentary of the various transmission control guidelines.
2. AETIOLOGY AND THE MECHANICS OF VIRAL ENTRY INTO CELLS
COVID‐19 is caused by SARS‐CoV‐2 which belongs to the family of Coronaviridae (Figures 1 and 2). Coronaviruses can be divided into four genera: alpha, beta, gamma and delta coronaviruses. Alpha and beta genera mostly infect mammals, while the gamma and delta mostly infect birds. There are six different alpha and beta variants of coronaviruses that infect humans, four of them (alpha HCoV‐229E and HCoV‐NL63, and beta HCoV‐HKU1 and HCoV‐OC43) usually cause mild symptoms similar to the common cold, while two of the beta coronaviruses can cause severe respiratory illnesses that can be fatal, such as the severe acute respiratory syndrome coronavirus (SARS‐CoV) and the Middle East respiratory syndrome coronavirus (MERS‐CoV; Ashour, Elkhatib, Rahman, & Elshabrawy, 2020; Guo et al., 2020). Indeed, recent genome sequencing and phylogenetic analyses of SARS‐CoV‐2 have shown its close resemblance to SARS‐CoV (about 79%) and MERS‐CoV (about 50%; Lu, Zhao, et al., 2020; Zhou et al., 2020). Furthermore, Zhou et al. (2020) have demonstrated the high degree of similarity in receptor‐binding domain (RBD) of SARS‐CoV, and SARS‐CoV‐2 and both appear to target angiotensin‐converting enzyme 2 (ACE2) of human cell walls. SARS‐CoV‐2 uses ACE2 as a portal of entry into host cells, thus, all cells expressing ACE2 are susceptible to infection (Hoffmann et al., 2020; Zhou et al., 2020). Therefore, several authors suggest that knowing the expression pattern of ACE2 in different organs and tissues is critical to determine the routes of entry of SARS‐CoV2, and also to understand the pathogenesis of COVID‐19 disease (Hoffmann et al., 2020; Wan, Shang, Graham, Baric, & Li, 2020; Zhang, Penninger, Li, Zhong, & Slutsky, 2020). There are several organs and tissues that express ACE2, such as the lung, heart, kidney, small and large intestine, arterial and venous endothelium, oral mucosa and salivary glands (Cano et al., 2019; Hamming et al., 2004; Liu et al., 2011; Xu et al., 2020; Zhang et al., 2020). Interestingly, a recent study used bulk RNA sequencing data extracted from oral tissues and showed a high degree of expression of ACE2 in oral tissues, such as epithelial cells of the tongue and the oral mucosa (Xu et al., 2020). Another study by Lin and colleagues used single‐cell sequencing data and found that ACE2 and FURIN (an enzyme that facilitates cellular entry of SARS‐CoV‐2) are expressed on the oral mucosa. They confirmed these results by performing immunostaining which showed high expression of these markers on the epithelial cells of tongue, lips buccal mucosa, palate and gingivae (Lin et al., 2020). These studies suggest that the oral cavity is a possible route of entry for SARS‐CoV‐2 (Xu et al., 2020).
Figure 1.
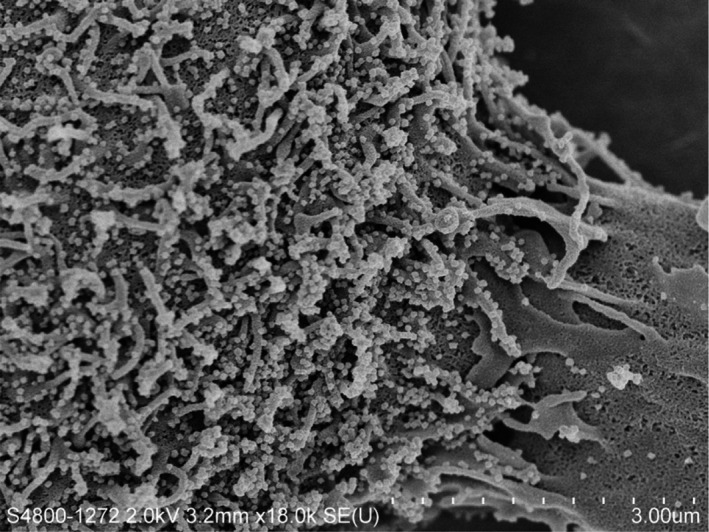
Scanning electron micrograph showing budding SARS‐CoV‐2 particles from the surface of an infected cell after 24 hr of laboratory culture. The numerous small, white spheres are the viral particle on the cell surface (Magnification 18,000×) (Image courtesy of: Professors J Nicholls LKS Faculty of Medicine, and Department of Electrical and Electronic Engineering (K. Tsia, K. Lee and Q. Lai), and Electron Microscopy Unit, The University of Hong Kong)
Figure 2.
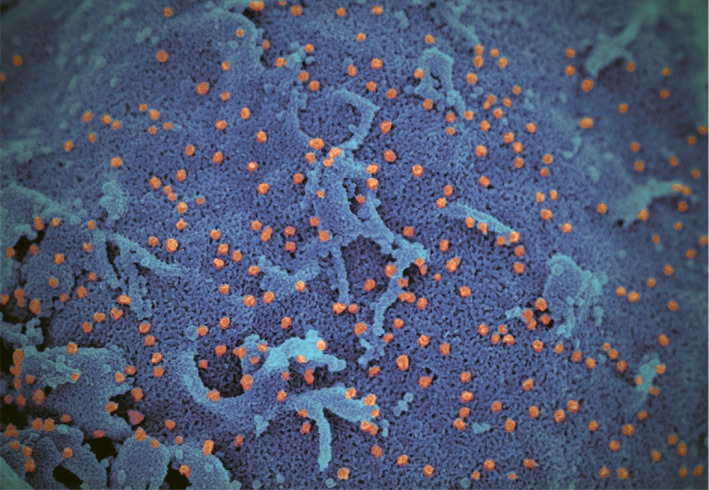
Pseudo‐colour scanning electron micrograph of SARS‐CoV‐2 in human cell culture. Figure shows the large numbers of viral particles (orange) budding from the cell surfaces (blue) Image courtesy of: Professors J. Nicholls LKS Faculty of Medicine, and Department of Electrical and Electronic Engineering (K. Tsia, K. Lee and Q. Lai), and Electron Microscopy Unit, The University of Hong Kong
3. SIGNS AND SYMPTOMS
The clinical manifestations of COVID‐19 range from early, prodromal asymptomatic cases to severe pneumonia with multiple organ failure. The most common symptoms are fever, cough, sore throat, fatigue, myalgia, headache, shortness of breath and in some cases diarrhoea. Chest computed tomographic (CT) scans might show patchy shadows and ground glass opacity in the lung (Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control, & Prevention, 2020; Guan et al., 2020; Wang, Hu, et al., 2020; Zhu et al., 2020). Authors from the Chinese Center For Disease Control and Prevention published a detailed clinical report of 72,314 of patient with COVID‐19 in mainland China and they classified the cases into mild, severe and critical (Table 1; Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control, & Prevention, 2020; Wu & McGoogan, 2020). Most of the observed cases were mild (80.9%), while only 4.7% were considered critical. They reported 2.3% mortality rate mostly in the elderly patients (>70 years) and those with co‐morbidities such as cardiovascular diseases, hypertension, diabetes, chronic respiratory disease and cancer (Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control, & Prevention, 2020). It can be concluded from the Chinese Centre for Disease Control report and other published studies that elderly patients and those with pre‐existing co‐morbidities are at a higher risk of developing severe symptoms than others (Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control, & Prevention, 2020; Guan et al., 2020; Wang, Hu, et al., 2020; Wu & McGoogan, 2020; Zhu et al., 2020). This initial observation is now confirmed by data from multiple countries where the disease is prevalent.
Table 1.
Classification of COVID‐19 cases
| Classification of COVID‐19 cases | Percentage of total observed cases | |
|---|---|---|
| Mild | Non‐pneumonia and mild pneumonia | 80.9% |
| Severe | Dyspnoea, respiratory frequency ≥ 30/min, blood oxygen saturation ≤ 93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300, and/or lung infiltrates > 50% within 24–48 hr | 13.8% |
| Critical | Respiratory failure, septic shock, and/or multiple organ dysfunction or failure | 4.7% |
In terms of the oral manifestations of the disease, there appear to be very few recognized thus far. However, recent studies have shown that loss of taste (ageusia) or taste alteration (dysgeusia/amblygeustia) is common in COVID‐19. Recently, Chen et al. (2020) conducted a survey of COVID‐19 patients, to evaluate the most common oral manifestations of the disease, and noted that more than half of their patients suffered from dysgeusia/amblygeustia. Indeed, the American Centers for Disease Control and Preventions (CDC) has now included recent loss of taste (ageusia/dysgeusia) as an early symptom of COVID‐19 (American Centers Of Disease Control & Prevention, 2020b).
The aetiopathology of this condition may be related to the fact that the cells of the salivary gland and tongue are potential targets for SARS‐CoV‐2, due to expression of ACE2.
4. ROUTES OF TRANSMISSION
The most commonly reported routes of SARS‐CoV‐2 transmission are inhalation of respiratory droplets or aerosols from the infected individuals that may occur within one metre radius of the index case, or through direct inoculation of virus‐infested particles by touching surfaces contaminated with infested respiratory droplets (fomite transmission via an inanimate vector; Guan et al., 2020; Li et al., 2020; Liu et al., 2020; Yu, Zhu, Zhang, & Han, 2020). For instance, it has been reported that SARS‐CoV‐2 can stay viable up to 24 hr on cardboard, and up to 72 hr on plastic or stainless steel surfaces (van Doremalen et al., 2020). Opinions vary as to the degree and extent of the airborne mode of SARS‐CoV‐2 transmission. There is direct and indirect evidence to indicate that aerosols with a particle size of <5 µm can be entrained in air and carried over distances of up to 1 m (Samaranayake, 2018). One recent study has reported the survival of SARS‐CoV‐2 up to 3 hr in aerosol particles, supporting the likelihood of airborne transmission (van Doremalen et al., 2020). However, another study reported the absence of SARS‐CoV‐2 in air samples collected from an actual clinical environment where symptomatic patients were admitted (Ong et al., 2020). Therefore, more studies are required to provide confirmatory evidence of airborne transmission of SARS‐CoV‐2 in both clinical as well as non‐clinical settings (World Health Organization, 2020c). Nevertheless, in clinical settings, as in dental clinics, where a large volume of aerosol is produced, airborne infection transmission is likely, and hence dentists and allied dental staff need to consider extra airborne and droplet precautions during the pandemic (World Health Organization, 2020c).
Furthermore, SARS‐CoV‐2 has been detected in saliva of infected individuals (To, Tsang, Leung, et al., 2020; To, Tsang, Yip, et al., 2020). As explained earlier, this can be attributed to the expression of ACE2 in salivary glands (Cano et al., 2019; Hamming et al., 2004; Liu et al., 2011). This is another significant factor that needs attention in dental practice, as aerosols generated during dental procedures are highly likely to be mixed with patients’ virus‐contaminated saliva (Peng et al., 2020). On the other hand, this provides an excellent opportunity to explore a non‐invasive mode of sample collection for SARS‐CoV‐2, as an alternative to the commonly used nasopharyngeal swab (Sabino‐Silva, Jardim, & Siqueira, 2020; To, Tsang, Leung, et al., 2020).
Moreover, it has been reported that stools of infected individuals are also contaminated with SARS‐CoV‐2 (Xiao et al., 2020). These findings, together with the fact that ACE2 is expressed in the gastrointestinal tract (Hamming et al., 2004) and reports of infected individuals presenting with diarrhoea (Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control, & Prevention, 2020; Guan et al., 2020), tend to imply that faecal–oral transmission route of SARS‐CoV‐2 is plausible.
5. TRANSMISSION CONTROL
As mentioned, there is a high likelihood of SARS‐CoV‐2 transmission in the dental care settings due to the dual risk of high aerosol generating procedures in dentistry, plus the saliva‐borne SARS‐CoV‐2 in both symptomatic and asymptomatic individuals. Hence, several dental societies/associations have provided guidelines to control transmission of SARS‐CoV‐2 in dental practice. The response of dental associations to curb the clinic‐associated nosocomial transmission of SARS‐CoV‐2 has been varied. Some associations have recommended complete close‐down of dental practices (British Dental Association, 2020), whilst the others have suggested restricting dental treatment to only addressing emergencies and reducing the number of routine dental check‐ups and follow‐up appointments (American Dental Association, 2020b).
These guidelines, summarized in Table 2, and described in detail below, include postponing elective dental treatment, developing new screening protocols, disease assessment through telecommunication and special additional precautions particularly during aerosol generating dental procedures.
Table 2.
Recommended measures for dental treatment during COVID‐19 pandemic
| Management of dental care |
|
| Primary care dental triage |
|
| Personal protective equipment (PPE) |
|
| Radiographs |
|
| Pre‐operative mouth rinse |
|
| Rubber dam |
|
| Type of instruments and material |
|
As per the guidelines published by American Dental Associationa, Scottish Dental Clinical Effectiveness Programmeb, New Zealand Dental Associationc and International federation of Endodontic Association ‐ Indian Endodontic Society joint statementd. American Association of Endodonticse.
5.1. Tele/online communication and evaluation of patients
Due to the alarming surge in the number of infected individuals, the American Dental Association (ADA) recommended (on 16 March 2020) to address only dental emergencies and to postpone all elective dental procedures (American Dental Association, 2020a). In addition, ADA and other dental societies have also published road maps for identifying dental emergencies in each of the dental specialties (American dental Association, 2020a, 2020c; Scotish Dental Clinical Effectiveness Programme, 2020). A list of such common dental emergencies encountered that demands the patient to visit the dental clinic in the midst of the ongoing outbreak of COVID‐19 is provided in Table 3.
Table 3.
List of commonly encountered dental emergencies that require urgent dental care
| Acute apical abscessa,b,c |
| Acute Periodontal abscess/ Endo‐Perio lesionb,c |
| Acute pericoronitisb,c |
| Necrotising ulcerative gingivitis/ periodontitisb,c |
| Reversible pulpitisa,b,c |
| Irreversible pulpitisa,b,c |
| Dentine hypersensitivityb |
| Dry socketa,b,c |
| Post‐extraction haemorrhagea,b |
| Oral ulcerationsb |
| Cracked, fractured, loose or displaced tooth fragments and restorationsa,b |
| Ill‐fitting or loose denturesa,b |
| Trauma from fractured or displaced orthodontic appliancesa,b |
| Dento‐alveolar injuriesa,b |
| Avulsed, displaced or fractured teetha,b |
| Temporary crown or bridge recementationa |
| Biopsy of abnormal tissuea |
| Removal of suturesa |
As per guidelines published by American Dental Associationa, Scottish Dental Clinical Effectiveness Programmeb and New Zealand Dental Associationc.
Virtual/telecommunication technologies are currently available to aid dental professionals to perform initial screening of patients, and identify emergencies. Such technology, in addition to taking a dental history, photographs and videos, will assist the clinician to reach a preliminary diagnosis. In regard to dental emergencies, initial management can be commenced over tele‐communication using locally available analgesics and antimicrobials; whenever appropriate. However, in the event of severe, acute signs or symptoms where the initial palliative medication therapy does not show improvement, urgent on‐site dental care has to be provided. Prior to scheduling such an appointment, a screening protocol has to be followed to obviate the potential exposure to COVID‐19.
The New Zealand Dental Association (NZDA) along with other dental societies have proposed a list of probing questions that allows the dental staff to gauge the patient's potential exposure to COVID‐19. These questions are mandatory, for all patients when they present at the dental practice. Firstly, patients should be asked whether they are positive for COVID‐19 or show signs of respiratory illness including cough, shortness of breath or sore throat. In addition, the patient should be questioned on his/her recent travel history or any close contact with infected individuals (New Zealand Dental Association, 2020a, 2020c). Furthermore, body temperature should be taken using a contact‐free forehead thermometer (World Health Organization, 2020a). If the patient does not respond affirmatively to the foregoing questions and requires emergency dental care, it must be provided in compliance with the infection protocol policies as described in the published guidelines, and outlined below.
However, if the patient says “yes” to any of the aforementioned questions, and requires emergency dental treatment, CDC has recommended immediate patient referral to a facility properly equipped to manage potentially exposed or confirmed COVID‐19 patients (American Centers Of Disease Control & Prevention, 2020). This is critical as most of the dental practices are not designed or equipped to provide the necessary droplet transmission control environment, such as an isolation room with negative pressure, and high‐efficiency particulate air (HEPA) filter systems (American Centers Of Disease Control & Prevention, 2020).
5.2. Precautions for managing dental emergencies and possible nosocomial transmission in non‐COVID19 patients
5.2.1. Precautions in waiting areas
Waiting areas in a dental practices are common zones where a higher likelihood of cross‐infection between patients and accompanying persons or dental team personnel may occur. Hence, it is important to minimize the number of individuals present in or around the waiting areas, and socially distancing them, by informing the patients to either arrive for the dental appointments unaccompanied or wait outside in a vehicle only to arrive at the dental clinic when the dental staff is ready to accommodate the patient's needs. Second, as discussed previously, due to the ability of SARS‐CoV‐2 to survive on different surfaces for a prolonged period, special changes to the design and setup of waiting area are required to limit cross‐contamination (van Doremalen et al., 2020). These include removal of all unnecessary items from the waiting rooms including unwanted furniture, magazines and toys that can harbour virus particles on their surfaces. Social distancing must also be ensured so that the sitting spaces are 2 m apart in the waiting area. Patients and visitors should be advised to wear face masks correctly and avoid touching of surfaces at all times. Further guidance includes frequent cleansing of “high‐touch” surfaces (reception counter, toilet doors, door knobs and handles etc.) using a neutral pH detergent (Ge, Yang, Xia, Fu, & Zhang, 2020). Moreover, patient‐related infographic images demonstrating optimum hand hygiene techniques, managing cough etiquette, the concept of “social distancing” that are easily comprehensible should be exhibited in the common areas (New Zealand Dental Association, 2020b). Also, as elevator buttons can be a major source of infection transmission, any such patient conveyance to the dental clinic should be regularly decontaminated as per the local guidelines (Kandel, Simor, & Redelmeier, 2014).
5.2.2. Personal protective equipment (PPE)
As discussed, inhalation of virus‐laden aerosols is a major mode of SARS‐CoV‐2 transmission (Bentley, Burkhart, & Crawford, 1994; Nejatidanesh, Khosravi, Goroohi, Badrian, & Savabi, 2013). Additionally, transmission of the virus has also been attributed to infectious droplets contaminating the conjunctival epithelium of the eyes (Lu, Liu, & Jia, 2020). Hence, PPE must be worn, to provide an effective and efficient barrier against the aerosol‐generated hazards from the operative site. These include protective eyewear, a face mask and a shield, a disposable working cap, appropriate gloves, gowns and impermeable shoe covers (Lu, Zhao, et al., 2020). If, for an unavoidable reason the dentist has to perform aerosol generating procedures, then a particulate respirator that is at least as protective as a National Institute for Occupational Safety and Health (NIOSH)‐certified N95, European Standard Filtering Face Piece 2 (EU FFP2), or equivalent has to be used (Kohn et al., 2003) together with high volume suction.
5.2.3. Radiographs
As intraoral radiographs might induce gag reflex, increase saliva secretion and coughing in patients, published guidelines recommend avoiding intraoral radiographs. Extraoral radiographic techniques such as panoramic, and cone beam computed tomography can be used as alternatives during COVID‐19 pandemic period (Ather, Patel, Ruparel, Diogenes, & Hargreaves, 2020; Indian Endodontic Society, International Federation Of Endodontic Associations, & Indian Dental Association, 2020; Meng, Hua, & Bian, 2020). However, if an intraoral radiograph is obligatory, then additional precautions are recommended such as the use of double barriers to prevent cross‐contamination through perforated attire (Hokett, Honey, Ruiz, Baisden, & Hoen, 2000).
5.2.4. Pre‐operative mouth rinse
It has been reported that the use of pre‐operative antimicrobial mouth rinse reduces the microbial count in the oral cavity and aerosols generated during dental procedures (Ather et al., 2020; Eggers, Koburger‐Janssen, Eickmann, & Zorn, 2018; Kariwa, Fujii, & Takashima, 2004; Mani, Srikanthan, Selvaraj, Menaka, & Parangimalai Diwakar, 2020; Meng et al., 2020; Peng et al., 2020). Therefore, several associations have recommended the use of a pre‐procedural mouth rinse to obviate the risk of SARS‐CoV‐2 transmission during dental treatment. For this purpose, the NZDA recommends 1% hydrogen peroxide, 0.2% chlorhexidine (CHX), 2% povidone‐iodine or 2% Listerine for 30 s prior to procedures (New Zealand Dental Association, 2020b). If a pre‐procedural mouth rinse is not possible, a swab soaked in hydrogen peroxide 1% or CHX 1% can be used alternatively (New Zealand Dental Association, 2020b). In contrast, both the Indian Endodontic Society (IES) and National Health Commission of the People's Republic of China have highlighted the poor effectiveness of 0.2% CHX against SARS‐COV‐2, and hence recommended the use of 1% hydrogen peroxide or 0.2% povidone‐iodine (Indian Endodontic Society et al., 2020; Ling & Taisheng, 2020). American Association of Endodontics recommends using a preprocedural mouth rinse of 0.2% povidone‐iodine (Ather et al., 2020). It is clear that the hydrogen peroxide and iodine are the most recommended mouth rinses in this context.
5.2.5. Rubber dam isolation
Since the virus load in human saliva is relatively high, a preprocedural mouth rinse alone may not eliminate this hazard. Hence, additional measures, such as the use of a dental rubber dam, are necessary (Spagnuolo, De Vito, Rengo, & Tatullo, 2020). For instance, it has been reported that rubber dam isolation can reduce airborne particles by up to 70%, within a 3‐foot diameter of the operational field (Peng et al., 2020; Samaranayake, Reid, & Evans, 1989). Therefore, using a rubber dam is recommended by several bodies, not only for endodontics procedures, but for almost all aerosol generating dental procedures (American Dental Association, 2020a; Ather et al., 2020; Indian Endodontic Society et al., 2020; New Zealand Dental Association, 2020b). Some have recommended rubber dam isolation as well as the use of high volume saliva ejectors during aerosol generating procedures (Indian Endodontic Society et al., 2020; Samaranayake et al., 1989). In conclusion, therefore, it is highly advisable to use dental rubber dam, high volume saliva ejectors and four‐handed dental assistance in the immediate post‐pandemic era to eliminate SARS‐CoV‐2 transmission risk posed by both symptomatic and asymptomatic patients.
5.2.6. Instruments and material
As noted, the practice of dentistry involves the use of rotary dental/surgical instruments, which create a high volume of aerosols that could contain mixture of water, saliva, blood, microorganisms and other debris. Such instrumentation includes triplex syringe (3:1 syringe), high‐ and low‐speed handpieces, ultrasonic scalers, air abrasion devices and intra‐oral sandblasters (American Centers Of Disease Control & Prevention, 2020). The NZDA along with other associations have advised to avoid using these equipments as much as possible, and stressed the use of hand instrumentation, as well as low‐speed handpieces without water spray to obviate dental aerosols. If the use of aerosol generating equipment is unavoidable, then the use of high volume saliva ejectors is recommended in addition to the other precautions mentioned above (Ather et al., 2020; New Zealand Dental Association, 2020b). Furthermore, the use of a handpiece with an anti‐retraction valve or other anti‐reflux design is recommended during the pandemic and post‐pandemic period (Peng et al., 2020). The guidelines also emphasized on the use of disposable instruments whenever possible.
5.3. Precautions for managing emergencies of potentially exposed or confirmed COVID‐19 patients
It is unlikely that symptomatic and acute COVID‐19 patients attend the dental clinic. However, if they do so due to a dental emergency, it is recommended to refer the patient to a facility equipped with airborne infection isolation rooms (AIIRs). This extra precaution is due to the potential of airborne transmission through aerosols. AIIRs are single‐patient rooms at negative pressure relative to the surrounding areas, and with a minimum of six air changes per hour. These clinics should have integrated HEPA filters in the air‐conditioning system to filter any contaminated air prior to recirculation (American Centers Of Disease Control & Prevention, 2020). Further, furniture and equipment in the room should be minimal and those essential for the operating procedure only should be present. Rooms should be always closed, and entry/exit should be minimized. Furthermore, in addition to previously discussed transmission control precautions, all dentists and allied staff should use N95 or higher‐level respirators (Kohn et al., 2003; Peng et al., 2020).
Finally, it is important to note that both the American CDC and ADA have advised that recovered COVID‐19 patients can be seen in normal clinic setting for dental emergencies only. Recovery is defined as at least 3 days (72 hr) since resolution of fever without the use of fever‐reducing medications and improvement in respiratory symptoms, for example, cough, shortness of breath, and at least 7 days since symptoms first occurred. Recovery for individuals with laboratory‐confirmed COVID‐19 is defined as individual who have not had any symptoms, at least 7 days since the date of the first positive COVID‐19 diagnostic test, and have had no subsequent illness (American Centers Of Disease Control & Prevention, 2020a; American Dental Association, 2020b).
6. POST‐PANDEMIC DENTAL PRACTICE
There is little doubt that the practice of routine dentistry will be irrevocably affected by the COVID‐19 pandemic, at least in the shorter term, until a successful antiviral agent or a vaccine is found for the causative agent. Although at this stage it is difficult to extrapolate the “new reality” of post‐pandemic dentistry, as the disease is still evolving in a majority of affected countries, there are some projections that could be made, mainly because COVID‐19 is not the first coronavirus infection the humans have encountered. Indeed, we have successfully curbed the epidemics of SARS‐CoV infection that began in 2002, and the Middle East Respiratory Syndrome (MERS) Coronavirus infection, a decade later, and thankfully, they never developed into pandemics, and the regional epidemics burnt out fairly swiftly (Samaranayake & Peiris, 2004). Only sporadic cases of MERS coronavirus are now seen, whilst it appears that the SARS‐CoV infection has disappeared altogether. So what are the lessons we could learn from the past epidemics of corona virus infections that closely resembles COVID‐19 in many respects?
In terms of post‐pandemic infection control in the dental clinic environment, it is imperative to maintain a very high degree of suspicion by all dental personnel whilst strictly adopting the standard infection control precautions. Fortunately, the latter appears to be the norm in most, if not all, dental practices, and hence additional precautions appear to be unnecessary for routine patients. All dental personnel must maintain vigilance for any individuals entering the clinical premises with symptoms of acute respiratory infections (e.g. cough, cold, sneezing etc). Further, it may be highly desirable to record the temperature of each patient immediately after entering the premises, and before the patient enters the patient waiting area. For instance, this could be performed by a trained receptionist using a non‐contact thermometer gun. Additionally, the patient history questionnaire should include recent travel abroad, as the COVID‐19 pandemic may be smouldering in some parts of the world for the foreseeable future. As diarrhoea is also a relatively common in COVID‐19, a recent or current history of this symptom may also have to be included in the questionnaire (Wang, Fang, et al., 2020) together with the newly described recent loss of taste and/or smell (American Centers Of Disease Control & Prevention, 2020b). Routine wearing of face masks by both patients, visitors, para‐dental personnel such as the receptionists could be the norm until the foreseeable future. Above all, the environmental disinfection of the clinic including the waiting rooms should be meticulously adhered to with a rigorous, supervised protocol.
The foregoing is a provisional and a rather brief account of the plausible, key features of post‐pandemic patient management. Definitive recommendations and guidelines on this subject should be developed by local and regional health authorities and are beyond the remit of this article.
7. DRUGS AND VACCINES FOR COVID‐19
Many researchers and scientists, the world over, are working on potential drugs, and vaccines to manage COVID‐19. Repurposed drugs are being either experimentally used or planned for the management of the condition, and these include the anti‐retroviral drugs lopinavir and ritonavir successfully used to manage HIV disease, anti‐malarial drug hydroxychloroquine and the antibiotic azithromycin (Cao et al., 2020; Rosa & Santos, 2020). There is also a current Australian trial of the BCG (Bacille Calmette Guerin vaccine which contains attenuated Mycobacterium bovis bacilli) for the condition (Miller et al., 2020).
Other possible products in the pipe‐line include massive screening of millions of compounds using in silico research for their ability to destroy the SARS‐CoV‐2. As these are all in the early experimental stages and human trials are still ongoing, conclusive data are yet unavailable. Although a promise of a quick cure for the disease using the above approaches appears to be waning, several vaccines are under various developmental stages in many countries and should be available within the next 18 months or so, after appropriate human trials. There are recent, promising reports of patients showing improvement when they are administered hyperimmune sera (containing antibodies to SARS‐CoV2) from recovered COVID‐19 patients (Cunningham, Goh, & Koh, 2020). This implies that the vaccination for preventing COVID‐19 is the most promising approach to obviate a recurrence of the pandemic. If this was the case, then mass vaccination for COVID‐19 could be the future, a situation akin to the annual vaccination required to prevent seasonal influenza amongst susceptible population cohorts, including dental care workers. Predictably then, one could foresee, the COVID‐19 vaccine to be the next new addition to the armamentarium of recommended vaccinations for all dental health careworkers.
8. SUMMARY
Since the announcement of COVID‐19 as a pandemic and the reported transmission risk to dental professionals, several dental societies, the world over, have published guidelines for transmission control and management of dental emergencies during the current pandemic. In this review, we summarize and outline the common themes and principles that emerge from these recommendations. There is universal concurrence on directives such as postponing elective dental treatment, developing appropriate screening protocols through telecommunication, applying special additional, droplet precautions when treating patients with dental emergencies, and sequestrated treatment of infected or suspect patients in specially fitted suites with negative pressure. We also discuss the recommended equipment and settings for clinics that can receive confirmed COVID‐19 patients. Finally, we briefly outline the practice of dentistry in the post‐pandemic era, and potential developments in antiviral drugs and vaccines that may be used in future to manage COVID‐19. It should, however, be noted that due to the highly fluid, dynamic and the evolving nature of the pandemic, and the unfolding natural history of the disease process, the foregoing recommendations are likely to change and all dental personnel should constantly keep abreast of the new developments and pronouncements on infection control in dentistry, issued by the local and regional health authorities.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTION
Mohamed Jamal: Conceptualization; Validation; Writing‐original draft; Writing‐review & editing. Maanas Shah: Validation; Writing‐original draft; Writing‐review & editing. Sameeha Hussain Almarzooqi: Validation; Writing‐original draft; Writing‐review & editing. Hend Aber: Validation; Writing‐original draft; Writing‐review & editing. Summayah Khawaja: Validation; Writing‐original draft; Writing‐review & editing. Rashid El Abed: Writing‐original draft; Writing‐review & editing. Zuhair Alkhatib: Conceptualization; Writing‐original draft; Writing‐review & editing. Lakshman Perera Samaranayake: Validation; Writing‐original draft; Writing‐review & editing.
Jamal M, Shah M, Almarzooqi SH, et al. Overview of transnational recommendations for COVID‐19 transmission control in dental care settings. Oral Dis.2021;27(Suppl. 3):655–664. 10.1111/odi.13431
Contributor Information
Mohamed Jamal, Email: mohamed.jamal@mbru.ac.ae.
Lakshman Perera Samaranayake, Email: lsamaranayake@sharjah.ac.ae.
REFERENCES
- American Centers Of Disease Control and Prevention (2020a). Interim Infection Prevention and Control Guidance for Dental Settings During the COVID‐19 Response. Retrieved from https://www.cdc.gov/coronavirus/2019‐ncov/hcp/dental‐settings.html
- American Centers Of Disease Control and Prevention (2020b). Coronavirus Disease 2019 (COVID‐19), Symptoms of Coronavirus. Retrieved from https://www.cdc.gov/coronavirus/2019‐ncov/symptoms‐testing/symptoms.html
- American Dental Association (2020a). ADA adds frequently asked questions from dentists to coronavirus resources. Retrieved from https://www.ada.org/en/publications/ada‐news/2020‐archive/march/ada‐adds‐frequently‐asked‐questions‐from‐dentists‐to‐coronavirus‐resources
- American Dental Association (2020b). ADA Interim Guidance for Management of Emergency and Urgent Dental Care. Retrieved from https://www.ada.org/~/media/CPS/Files/COVID/ADA_Int_Guidance_Mgmt_Emerg‐Urg_Dental_COVID19.pdf
- American Dental Association . (2020c, 1 April). Summary of ADA Guidance During the COVID‐19 Crisis. Retrieved from https://success.ada.org/~/media/CPS/Files/COVID/COVID‐19_Int_Guidance_Summary.pdf?utm_source=cpsorg&utm_medium=cpsalertbar&utm_content=cv‐pm‐summary‐guidance&utm_campaign=covid‐19
- American Dental Association . (2020d). What Constitutes a Dental Emergency? Retrieved from https://success.ada.org/~/media/CPS/Files/Open%20Files/ADA_COVID19_Dental_Emergency_DDS.pdf?utm_source=adaorg&utm_medium=covid‐resources‐lp&utm_content=cv‐pm‐emerg‐def&utm_campaign=covid‐19&_ga=2.222051098.343913536.1585559595‐1007660533.1561873549
- Ashour, H. M. , Elkhatib, W. F. , Rahman, M. M. , & Elshabrawy, H. A. (2020). Insights into the recent 2019 novel coronavirus (SARS‐CoV‐2) in light of past human coronavirus outbreaks. Pathogens, 9(3), 186. 10.3390/pathogens9030186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ather, A. , Patel, B. , Ruparel, N. B. , Diogenes, A. , & Hargreaves, K. M. (2020). Coronavirus disease 19 (COVID‐19): Implications for clinical dental care. Journal of Endodontics, 46(5), 584–595. 10.1016/j.joen.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley, C. D. , Burkhart, N. W. , & Crawford, J. J. (1994). Evaluating spatter and aerosol contamination during dental procedures. Journal of the American Dental Association, 125(5), 579–584. 10.14219/jada.archive.1994.0093 [DOI] [PubMed] [Google Scholar]
- British Dental Association . (2020, 27 April). Coronavirus: Your FAQs. Retrieved from https://bda.org/advice/Coronavirus/Pages/faqs.aspx
- Cano, I. P. , Dionisio, T. J. , Cestari, T. M. , Calvo, A. M. , Colombini‐Ishikiriama, B. L. , Faria, F. A. C. , … Santos, C. F. (2019). Losartan and isoproterenol promote alterations in the local renin‐angiotensin system of rat salivary glands. PLoS One, 14(5), e0217030. 10.1371/journal.pone.0217030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, B. , Wang, Y. , Wen, D. , Liu, W. , Wang, J. , Fan, G. , … Wang, C. (2020). A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. New England Journal of Medicine, 382(19), 1787–1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Zhao, J. , Peng, J. , Li, X. , Deng, X. , Geng, Z. , … Wang, S. (2020). Detection of 2019‐nCoV in saliva and characterization of oral symptoms in COVID‐19 patients. SSRN, 10.2139/ssrn.3557140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, A. C. , Goh, H. P. , & Koh, D. (2020). Treatment of COVID‐19: Old tricks for new challenges. Critical Care, 24(1), 91. 10.1186/s13054-020-2818-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers, M. , Koburger‐Janssen, T. , Eickmann, M. , & Zorn, J. (2018). In vitro bactericidal and virucidal efficacy of povidone‐iodine gargle/mouthwash against respiratory and oral tract pathogens. Infectious Diseases and Therapy, 7(2), 249–259. 10.1007/s40121-018-0200-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention (2020). The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. Zhonghua Liu Xing Bing Xue Za Zhi, 41(2), 145–151. 10.3760/cma.j.issn.0254-6450.2020.02.003 [DOI] [PubMed] [Google Scholar]
- Gamio, L. (2020, 15 March). The Workers Who Face the Greatest Coronavirus Risk. The New York Times. Retrieved from https://www.nytimes.com/interactive/2020/03/15/business/economy/coronavirus‐worker‐risk.html
- Ge, Z.‐Y. , Yang, L.‐M. , Xia, J.‐J. , Fu, X.‐H. , & Zhang, Y.‐Z. (2020). Possible aerosol transmission of COVID‐19 and special precautions in dentistry. Journal of Zhejiang University‐SCIENCE B, 21(5), 361–368. 10.1631/jzus.B2010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W.‐J. , Ni, Z.‐Y. , Hu, Y. U. , Liang, W.‐H. , Ou, C.‐Q. , He, J.‐X. , … Zhong, N.‐S. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382(18), 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y.‐R. , Cao, Q.‐D. , Hong, Z.‐S. , Tan, Y.‐Y. , Chen, S.‐D. , Jin, H.‐J. , … Yan, Y. (2020). The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak – An update on the status. Military Medical Research, 7(1), 11. 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming, I. , Timens, W. , Bulthuis, M. L. , Lely, A. T. , Navis, G. , & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology, 203(2), 631–637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , … Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokett, S. D. , Honey, J. R. , Ruiz, F. , Baisden, M. K. , & Hoen, M. M. (2000). Assessing the effectiveness of direct digital radiography barrier sheaths and finger cots. Journal of the American Dental Association, 131(4), 463–467. 10.14219/jada.archive.2000.0202 [DOI] [PubMed] [Google Scholar]
- Indian Endodontic Society, International Federation Of Endodontic Associations, & Indian Dental Association . (2020). Joint position statement on dental practice during COVID‐19. Retrieved from https://www.ies.org.in/pdf_server.php?file=dental‐practice‐covid‐19 [Google Scholar]
- Kandel, C. E. , Simor, A. E. , & Redelmeier, D. A. (2014). Elevator buttons as unrecognized sources of bacterial colonization in hospitals. Open Med, 8(3), e81–e86. [PMC free article] [PubMed] [Google Scholar]
- Kariwa, H. , Fujii, N. , & Takashima, I. (2004). Inactivation of SARS coronavirus by means of povidone‐iodine, physical conditions, and chemical reagents. Japanese Journal of Veterinary Research, 52(3), 105–112. [PubMed] [Google Scholar]
- Kohn, W. G. , Collins, A. S. , Cleveland, J. L. , Harte, J. A. , Eklund, K. J. , & Malvitz, D. M. (2003). Guidelines for infection control in dental health‐care settings–2003. MMWR – Recommendations and Reports, 52(Rr‐17), 1–61. [PubMed] [Google Scholar]
- Li, Q. , Guan, X. , Wu, P. , Wang, X. , Zhou, L. , Tong, Y. , … Feng, Z. (2020). Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. New England Journal of Medicine, 382(13), 1199–1207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, B.‐P. , Zhong, M. , Gao, H.‐B. , Wu, K.‐B. , Liu, M.‐X. , Liu, C. … Wang, L.‐J. (2020). Significant expression of FURIN and ACE2 on oral epithelial cells may facilitate the efficiency of 2019‐nCov entry. bioRxiv, 2020.2004.2018.047951. 10.1101/2020.04.18.047951 [DOI] [Google Scholar]
- Liu, J. , Liao, X. , Qian, S. , Yuan, J. , Wang, F. , Liu, Y. … Zhang, Z. (2020). Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, 2020. Emerging Infectious Diseases, 26(6), 1320–1323. 10.3201/eid2606.200239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Wei, Q. , Alvarez, X. , Wang, H. , Du, Y. , Zhu, H. , … Chen, Z. (2011). Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. Journal of Virology, 85(8), 4025–4030. 10.1128/jvi.02292-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, C. W. , Liu, X. F. , & Jia, Z. F. (2020). 2019‐nCoV transmission through the ocular surface must not be ignored. Lancet, 395(10224), e39. 10.1016/s0140-6736(20)30313-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, R. , Zhao, X. , Li, J. , Niu, P. , Yang, B. O. , Wu, H. , … Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet, 395(10224), 565–574. 10.1016/s0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani, S. , Srikanthan, S. , Selvaraj, B. , Menaka, V. , & Parangimalai Diwakar, M. (2020). Effectiveness of 0.2% chlorhex plus and 0.1% turmix as preprocedural mouthrinses on aerosol contamination produced by ultrasonic scalers: An interventional study. Journal of Dental Research and Review, 7(1), 5–9. 10.4103/jdrr.jdrr_7_20 [DOI] [Google Scholar]
- Meng, L. , Hua, F. , & Bian, Z. C. (2020). Coronavirus disease 2019 (COVID‐19): Emerging and future challenges for dental and oral medicine. Journal of Dental Research, 99(5), 481–487. 10.1177/0022034520914246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A. , Reandelar, M. J. , Fasciglione, K. , Roumenova, V. , Li, Y. , & Otazu, G. H. (2020). Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID‐19: an epidemiological study. medRxiv, 2020.2003.2024.20042937. 10.1101/2020.03.24.20042937 [DOI] [Google Scholar]
- Ling, L. , & Taisheng, L. (2020). Interpretation of "Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019‐nCoV) Infection by the National Health Commission (Trial Version 5)". National Medical Journal of China, 100, E001‐E001. 10.3760/cma.j.issn.0376-2491.2020.0001 [DOI] [PubMed] [Google Scholar]
- Nejatidanesh, F. , Khosravi, Z. , Goroohi, H. , Badrian, H. , & Savabi, O. (2013). Risk of contamination of different areas of dentist's face during dental practices. International Journal of Preventive Medicine, 4(5), 611–615. [PMC free article] [PubMed] [Google Scholar]
- New Zealand Dental Association (2020a). COVID‐19 Response Interim Advice for Oral Health Practitioners. Retrieved from https://www.dcnz.org.nz/assets/Uploads/COVID/NZDA‐COVID‐19‐Response‐Interim‐advise‐for‐oral‐health‐practitioners‐20Mar20.pdf
- New Zealand Dental Association (2020b). COVID‐19 Safety Standards. Retrieved from , https://www.nzda.org.nz/assets/files, https://www.nzda.org.nz/assets/files/Standards__Guidelines/COVID‐19_Safety_Standards.pdf
- New Zealand Dental Association (2020c, 26 March).Medical Management of Acute Dental Pain. Retrieved from https://www.nzda.org.nz/assets/files/Standards__Guidelines/Medical_Management_of_Acute_Dental_Pain.pdf
- Ong, S. W. X. , Tan, Y. K. , Chia, P. Y. , Lee, T. H. , Ng, O. T. , Wong, M. S. Y. , & Marimuthu, K. (2020). Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) from a symptomatic patient. JAMA, 323(16), 1610. 10.1001/jama.2020.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, X. , Xu, X. , Li, Y. , Cheng, L. , Zhou, X. , & Ren, B. (2020). Transmission routes of 2019‐nCoV and controls in dental practice. International Journal of Oral Science, 12(1), 9. 10.1038/s41368-020-0075-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa, S. G. V. , & Santos, W. C. (2020). Clinical trials on drug repositioning for COVID‐19 treatment. Revista Panamericana De Salud Publica, 44, e40. 10.26633/rpsp.2020.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino‐Silva, R. , Jardim, A. C. G. , & Siqueira, W. L. (2020). Coronavirus COVID‐19 impacts to dentistry and potential salivary diagnosis. Clinical Oral Investigations, 24(4), 1619–1621. 10.1007/s00784-020-03248-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake, L. P. (2018). Essential microbiology for dentistry. Philadelphia, PA: Elsevier Health Sciences. [Google Scholar]
- Samaranayake, L. P. , & Peiris, M. (2004). Severe acute respiratory syndrome and dentistry: A retrospective view. Journal of the American Dental Association, 135(9), 1292–1302. 10.14219/jada.archive.2004.0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake, L. P. , Reid, J. , & Evans, D. (1989). The efficacy of rubber dam isolation in reducing atmospheric bacterial contamination. ASDC Journal of Dentistry for Children, 56(6), 442–444. [PubMed] [Google Scholar]
- Scotish Dental Clinical Effectiveness Programme . (2020). Management of acute dental prolems during COVID‐19 pandemic.
- Spagnuolo, G. , De Vito, D. , Rengo, S. , & Tatullo, M. (2020). COVID‐19 outbreak: An overview on dentistry. International Journal of Environmental Research and Public Health, 17, 2094. 10.3390/ijerph17062094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To, K.‐W. , Tsang, O.‐Y. , Leung, W.‐S. , Tam, A. R. , Wu, T.‐C. , Lung, D. C. , … Yuen, K.‐Y. (2020). Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐ 2: An observational cohort study. The Lancet Infectious Diseases, 20(5), 565–574. 10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To, K. K. , Tsang, O. T. , Chik‐Yan Yip, C. , Chan, K. H. , Wu, T. C. , Chan, J. , … Yuen, K. Y.. (2020). Consistent detection of 2019 novel coronavirus in saliva. Clinical Infectious Diseases: An official publication of the Infectious Diseases Society of America, ciaa149. Advance online publication. 10.1093/cid/ciaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen, N. , Bushmaker, T. , Morris, D. H. , Holbrook, M. G. , Gamble, A. , Williamson, B. N. , … Lloyd‐Smith, J. O. (2020). Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. New England Journal of Medicine, 382(16), 1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, Y. , Shang, J. , Graham, R. , Baric, R. S. , & Li, F. (2020). Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade‐long structural studies of SARS coronavirus. Journal of Virology, 94(7), e00127‐20. 10.1128/jvi.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Hu, B. O. , Hu, C. , Zhu, F. , Liu, X. , Zhang, J. , … Peng, Z. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA, 323(11), 1061. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Fang, J. , Zhu, Y. , Chen, L. , Ding, F. , Zhou, R. , … Zhao, Q. (2020). Clinical characteristics of non‐critically ill patients with novel coronavirus infection (COVID‐19) in a Fangcang Hospital. Clinical Microbiology and Infection: The official publication of the European Society of Clinical Microbiology and Infectious Diseases, S1198‐743X(20)30177‐4. Advance online publication. 10.1016/j.cmi.2020.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2020a). Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected.
- World Health Organization . (2020b, 26 April). Coronavirus disease 2019 (COVID‐19) Situation Report – 97. Retrieved from https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200426‐sitrep‐97‐covid‐19.pdf?sfvrsn=d1c3e800_6
- World Health Organization . (2020c, 29 March). Modes of transmission of virus causing COVID‐19:implications for IPC precaution recommendations. Retrieved from https://www.who.int/publications‐detail/modes‐of‐transmission‐of‐virus‐causing‐covid‐19‐implications‐for‐ipc‐precaution‐recommendations
- Wu, D. , Wu, T. , Liu, Q. , & Yang, Z. (2020). The SARS‐CoV‐2 outbreak: What we know. International Journal of Infectious Diseases, 94, 44–48. 10.1016/j.ijid.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z. , & McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA, 323(13), 1239. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- Xiao, F. , Tang, M. , Zheng, X. , Liu, Y. , Li, X. , & Shan, H. (2020). Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology, 158(6), 1831–1833.e3. 10.1053/j.gastro.2020.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H. , Zhong, L. , Deng, J. , Peng, J. , Dan, H. , Zeng, X. , … Chen, Q. (2020). High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science, 12(1), 8. 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, P. , Zhu, J. , Zhang, Z. , & Han, Y. (2020). A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person‐to‐person transmission during the incubation period. The Journal of Infectious Diseases, 221(11), 1757–1761. 10.1093/infdis/jiaa077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Penninger, J. M. , Li, Y. , Zhong, N. , & Slutsky, A. S. (2020). Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Medicine, 46(4), 586–590. 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, S. , Lin, Q. , Ran, J. , Musa, S. S. , Yang, G. , Wang, W. , … Wang, M. H. (2020). Preliminary estimation of the basic reproduction number of novel coronavirus (2019‐nCoV) in China, from 2019 to 2020: A data‐driven analysis in the early phase of the outbreak. International Journal of Infectious Diseases, 92, 214–217. 10.1016/j.ijid.2020.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X.‐L. , Wang, X.‐G. , Hu, B. , Zhang, L. , Zhang, W. , … Shi, Z.‐L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, N. A. , Zhang, D. , Wang, W. , Li, X. , Yang, B. O. , Song, J. , … Tan, W. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine, 382(8), 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]


