Summary
The emerging COVID‐19 pandemic has overwhelmed healthcare resources worldwide, and for transfusion services this could potentially result in rapid imbalance between supply and demand due to a severe shortage of blood donors. This may result in insufficient blood components to meet every patient's needs resulting in difficult decisions about which patients with major bleeding do and do not receive active transfusion support. This document, which was prepared on behalf of the National Blood Transfusion Committee in England, provides a framework and triage tool to guide the allocation of blood for patients with massive haemorrhage during severe blood shortage. Its goal is to provide blood transfusions in an ethical, fair, and transparent way to ensure that the greatest number of life years are saved. It is based on an evidence‐ and ethics‐based Canadian framework, and would become operational where demand for blood greatly exceeds supply, and where all measures to manage supply and demand have been exhausted. The guidance complements existing national shortage plans for red cells and platelets.
Keywords: blood shortage, triage, massive bleeding, guidance, National Blood Transfusion Committee
Major haemorrhage is a clinical emergency associated with most specialities, but particularly cardiothoracic and vascular surgery, obstetrics, trauma and gastroenterology. 1 Modern practice recommends balanced resuscitation with multiple components. Such early haemostatic resuscitation with blood transfusion support saves lives but is resource‐intensive. The COVID‐19 pandemic has the potential to reduce the blood supply primarily from reduced blood donations rather than increased blood usage for critically ill patients. 2 At present in the UK, the reduction in blood donations is counterbalanced by an even greater reduction in blood usage. The latter is likely associated with reduced blood requirements because of postponements of elective medical and surgical interventions and possibly reduced major trauma associated with ‘social distancing’, and because the transfusion requirements of COVID‐19‐infected patients have up to now been low. However, the potential for blood shortage remains, especially for labile cellular components such as red cells and platelets.
There is a considerable existing literature on preparing for a pandemic including the management of blood shortages. 3 , 4 , 5 , 6 , 7 , 8 Many guidelines exist for the transfusion management of major haemorrhage, including national guidelines in the UK. 9 Furthermore, the National Blood Transfusion Committee (NBTC) has recently updated its guidance to address shortages of units of red blood cells. 10 National plans for red blood cell and platelet shortages both describe three phases: green (supply generally meets demand), amber (blood inventory is insufficient to continue usual transfusion practice) and red (severe, prolonged shortage). 10 , 11 These plans provide an outline of the steps to be taken by both blood suppliers as well as hospital transfusion laboratories and hospitals, but do not address the question of how to ration blood for patients with major haemorrhage, where blood transfusion is a potentially life‐saving treatment. During this period, there should be fair, equitable, and transparent distribution of blood components to individual patients. 12 , 13
The focus of this document is to provide a framework for the development of an operational plan for triaging patients with massive haemorrhage during a red phase of shortage for red blood cells. The guidance recognises that clinical judgement and the specific context of the blood shortage are both essential to inform decision‐making for blood allocation for individual patients.
Methods
This guidance was prepared by a working group on behalf of the NBTC in England whose overall objective is to promote good transfusion practice by providing a framework to channel information and advice to hospitals and blood services in England on best practice. 14 Its content is based on existing guidelines for the management of blood shortages, 10 , 11 and the Canadian emergency framework for clinical transfusion triage for massively bleeding patients. 15 It was reviewed by members of the NBTC, which is accountable to the National Medical Director of NHS England through the Chief Scientific Officer. Its membership (see Appendix A) includes representatives of the medical Royal Colleges, specialist societies and other professional organisations with an interest in blood transfusion, the Chairs of the Regional Transfusion Committees in England, and patient representatives. Comments and suggestions were sought and received both verbally and in writing from the members of the NBTC at and after its March 30th, 2020 meeting.
Operation of the plan
Should a national red cell shortage occur, NHS Blood & Transplant (NHSBT) will notify Transfusion Laboratory Managers to implement their Emergency Blood Management Arrangements (EBMA). 10 , 11 These arrangements include establishing and convening an Emergency Blood Management Group (EBMG) or equivalent, formed of senior clinicians and hospital managers, ideally including the Chief Executive and Medical Director.
If national stocks fall to less than two days or an imminent threat to the blood supply is identified, NHSBT will communicate a move to ‘Amber phase’. 10 This may apply to either a single blood group or to all blood groups. A red phase shortage will be declared if there is a severe shortage of red cells, or if there is an imminent severe threat to the supply of blood. 10 In the red phase, very reduced blood inventory levels may require reductions in haemoglobin thresholds for elective red cell transfusions and patients with non‐elective indications such as major haemorrhage may not receive the required transfusions. Communications from NHSBT to hospitals to activate and stand down phases of shortages are provided directly to hospital blood transfusion laboratories.
Hospitals may already have established an independent clinical triage team, or even teams, to cover 24 h a day seven days/week, for resource allocation in the event of shortages, and transfusion could be included in these local arrangements during a red phase of blood shortage. If not, we recommend that senior managers and experienced physicians in roles outside the direct patient care of potentially affected patients are employed to assist with rationing decisions related to transfusion, so that front‐line clinicians are relieved of this burden and to ensure that consistent and equitable decisions are made. It is essential that senior hospital managers and clinicians support the EBMA and the arrangements for transfusion triage.
The emergency framework for rationing blood for patients predicted to need massive transfusion (see Appendices B and C)
Goal
To provide blood transfusions in an ethical, fair, and transparent way. All efforts should be made to minimise suffering and maximise the use of blood alternatives, as appropriate, for those who are triaged to ‘no transfusion’ due to insufficient resources.
Inclusion criteria
All patients needing, or predicted to need, massive transfusion due to massive haemorrhage during a red phase blood shortage, should be included. There is no agreed definition for massive transfusion, 9 and traditional definitions (e.g. transfusion of ≥10 units of red cells in 24 h) may be inappropriate in acute clinical situations, and more dynamic definitions should be used. 9 Examples include an expected blood loss of one blood volume in less than 24 h: 0.5× blood volume loss in 3 h, or transfusion of four or more units of red blood cells in 1 h.
All such patients should have access to all available blood conservation strategies as described in the local EBMA. These include (but are not limited to) intravenous/oral iron, blood components such as plasma and cryoprecipitate to manage severe haemorrhage, anti‐fibrinolytic drugs such as tranexamic acid, erythropoiesis‐stimulating agents, intraoperative cell salvage, interventional radiological procedures, rapid access to endoscopy, and non‐invasive surgery. Early surgical intervention, correction of hypothermia, attention to the management of coagulopathy and intra‐operative cell salvage may be of value in patients with massive haemorrhage.
The initial aim should be the early identification of those patients who might need massive transfusion, and to triage them for transfusion support. Guidance and tools such as those developed by National Institute for Health and Care Excellence (NICE) and the Royal College of Physicians may be helpful for triaging patients. 16 , 17 Triage is a dynamic process and patients should be actively re‐assessed based on the general and condition‐specific exclusion criteria.
Any decisions made to initiate, withdraw or withhold care must also comply with the shared decision‐making policies of the NHS. 18 This means that these decisions should include the patient and their wishes (as much as is feasible for the given situation) and, if appropriate, the patient's carers.
Reassessment of triaged patients
Patients triaged to no blood components. Patients triaged to no transfusion care should be re‐assessed at a minimum of every 24 h. A system should be in place to support physicians caring for the patient if an improvement in a patient's status would now qualify them to be re‐triaged to active transfusion management.
Patients triaged to blood components. Patients triaged to active transfusion care should be assessed at the start of massive haemorrhage resuscitation, and after a minimum of every eight units of red blood cells (adjusted for patient size, for example for children) or every 24 h for patients receiving less than eight units of blood or until cessation of haemorrhage (or more frequently – e.g. every four units if deemed necessary). If there is persistent bleeding following surgical intervention, there should be close attention to the correction of coagulopathy and consideration of return to theatre.
At each assessment, the triage team should assess and document the patient's status and overall futility of continuation of active treatment, utilising the following variables to guide their decisions regarding the value of continued transfusions:
Sequential organ failure assessment (SOFA) score. 19
Total blood components used.
Need for ongoing transfusion support.
Ability to control bleeding with either surgery or other procedure (e.g. interventional radiology, endoscopy).
Patients with a SOFA score >11, who have a continued need for large amounts of blood components, and where there is no foreseeable ability to control blood loss should be triaged to palliative care.
Ethical framework for triaging patients to active transfusion care
There is existing guidance to support decision‐making where two or more patients who equally qualify for active transfusion management require blood components at the same time. The British Medical Association 12 has drawn attention to a Government ethical framework 20 designed to support thinking through the ethical aspects of decision‐making during a pandemic. It is aimed at helping planners and strategic policy makers at national, regional and local level, both before and during a pandemic. It will also help clinicians and others (who will be guided by their own professional codes) in developing policies on clinical issues for use during a pandemic. It provided several guiding principles:
Equal respect: everyone matters and everyone matters equally, but this does not mean that everyone will be treated the same.
Respect: keep people as informed as possible; give people the chance to express their views on matters that affect them; respect people's personal choices about care and treatment.
Minimise the harm of the pandemic: reduce spread, minimise disruption, learn what works.
Fairness: everyone matters equally. People with an equal chance of benefiting from a resource should have an equal chance of receiving it — although it is not unfair to ask people to wait if they could get the same benefit later.
Working together: we need to support each other, take responsibility for our own behaviour and share information appropriately.
Reciprocity: those who take on increased burdens should be supported in doing so.
Keeping things in proportion: information communicated must be proportionate to the risks; restrictions on rights must be proportionate to the goals.
Flexibility: plans must be adaptable to changing circumstances.
Open and transparent decision‐making: good decisions will be as inclusive, transparent and reasonable as possible. They should be rational, evidence‐based, the result of a reasonable process and practical in the circumstances.
A recent article in the New England Journal of Medicine summarised the key principles as: maximization of benefits aiming at saving the most individual lives or at saving the most life years by giving priority to patients likely to survive longest after treatment, and fair allocation of resources that prioritises the value of maximizing benefits across all patients who need these resources. 13
Discussion
Transfusion support is an essential element of emergency healthcare infrastructure. Modern healthcare systems demand a safe, sufficient and timely supply of blood that is dependent on a complex interrelationship with society and is at risk of disruption when norms are threatened. The current COVID‐19 reminds us again of the need for blood services to meet the challenges posed by emergent pathogens and potential pandemics. 21 While COVID‐19 itself may not routinely demand transfusion support, the impact of the pandemic on the community could potentially reduce the supply of blood donors resulting in a rapid imbalance between blood supply and demand. In such a situation, emergency preparedness for blood services and hospitals is essential so that limited resources are rationed fairly and appropriately.
The challenge for blood services is to balance demand from users and supply from volunteer donors in a complex regulated system from donor to patient. Transfusion preparedness should therefore extend across the continuum of care and is best done in close partnership between the blood services, hospitals and patient representatives. The NBTC was established to fulfil this ambition and continues to provide a responsive source of guidance for both policy makers and practitioners. 14
The NBTC has recently issued guidance on Emergency Preparedness, Resilience and Response, 22 and shortage plans for red cells and platelets, 10 , 11 which are the most likely components to be affected by discontinuity in supply due to their limited shelf‐life. However, these documents do not provide explicit advice on how to ration blood during severe shortage for patients who are bleeding and for whom blood transfusion is a lifesaving treatment. Difficult decisions will need to be made to determine which patients receive blood. This decision‐making requires a deliberative multidisciplinary approach in hospitals to ensure that an informed, balanced and equitable allocation of blood resources is achieved.
The emergency framework produced by the Canadian National Advisory Committee on Blood and Blood Products is one valuable example that was initially developed in 2012 following Hurricane Katrina and sought to address perceived deficiencies in existing shortage plans. 15 This framework focuses on predictors of massive blood loss and mortality, ethical frameworks, and allocation protocols to guide triage working groups. The Canadian framework along with guidance from the Royal College of Physicians and the existing NHS framework for joint decision‐making formed the basis for this NBTC guidance. 17 , 18 It complements recently updated national shortage plans both in the UK and Canada, 10 , 11 , 23 and is designed to be interpreted locally depending on the circumstances at the time. We recognise the emotional challenge faced by those having to make these difficult decisions and we hope that this will serve as a tool to support them at this difficult time of the COVID‐19 pandemic and in future emergencies.
Appendix A.
Membership of the National Blood Transfusion Committee at the time of writing
Dr Jon Cort (Interim Chair); Allard Shubha (Secretary); Charles Baker; Anne Benton; Paula Bolton‐Maggs; Ruth Burey; Rebecca Cardigan; Craig Carroll; Falguni Choksey; Sammy Conran; Mike Dawe; Allistair Dodds; Graham Donald; Heidi Doughty; Angela Douglas; Kerry Dowling; Chris Elliott; Tim Ellis; Lise Estcourt; Rose Gallagher; Gabriella Gray; Sue Hill; Catherine Howell; Ant Jackson; Mervi Jokinen; Nicola Jones; Anne Kelly; Phil Kelly; Sid Khan; Alwyn Kotze; Gail Miflin; Shruthi Narayan; James Neuberger; James Reid; Chris Robbie; Susan Robinson; Rashmi Rook; Nigel Sargant; Louise Sherliker; Rhonda Skeete; Youssef Sorour; Julie Staves; Stephen Thomas; John Thompson; James Uprichard; Howard Wakeling; Helen Witham.
Appendix B.
Emergency Framework for Blood Rationing − Algorithm for Triage Team (Part 1)
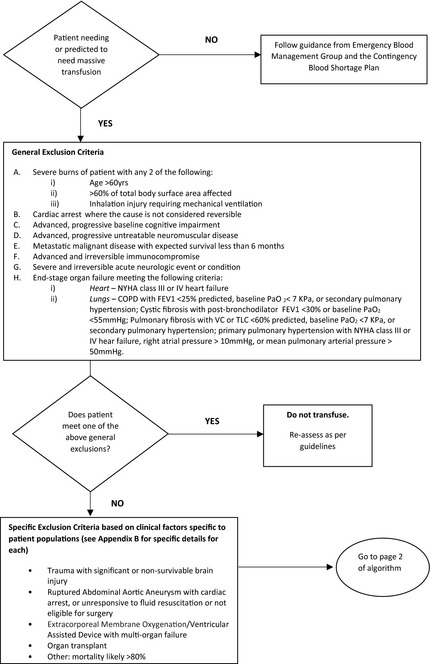
Emergency Framework for Blood Rationing − Algorithm for Triage Team (Part 2)

Figures for Appendix II have been adapted from Emergency framework for rationing of blood for massively bleeding patients during a red phase of a blood shortage. 15
Appendix C.
Senior clinical assessment is recommended for the following scenarios where the patients also have massive bleeding. We recognise that Royal Colleges and specialist societies have developed more tailored advice on ethical issues for their members.
Trauma
Children or adults considered to have significant or non‐survivable brain injury.
Clinical Consideration: CT/MRI scanning should be done as soon as possible to confirm the diagnosis of a non‐survivable brain injury.
Ruptured Abdominal Aortic Aneurysm (RAAA)
Patients with preoperative cardiac arrest.
Patients with a systolic blood pressure of less than 70 mm Hg who are unresponsive to fluid resuscitation and have lost consciousness.
Patients with RAAA that do not meet criteria for emergent vascular repair.
ECMO/VAD
Patients who require ECMO/VAD and who have multi‐organ (>1 organ) failure.
Gastroenterology
Clinical Consideration: Triage patients with gastrointestinal bleeding to centres with endoscopy to minimise the use of blood products.
Organ transplantation
Patients after the declaration of brain death who are awaiting deceased organ donation.
Patients undergoing deceased donor organ retrieval.
Deceased donor solid organ transplantation —patients and clinicians must be aware prior to the start of transplantation that blood may not be available for transfusion for massive bleeding during a 'red phase'. This needs to be included in informed consent discussions.
Living donation — during a 'red phase' it is expected that these would be deferred due to the risk that blood may not be available for transfusion if massive bleeding occurred.
Other massively bleeding situations not listed above
In a red phase, for patients with massive bleeding for reasons not listed above, do not transfuse patients for whom the triage team believes the mortality rate exceeds 80%.
Adapted from Emergency framework for rationing of blood for massively bleeding patients during a red phase of a blood shortage. 15
References
- 1. Green L, Tan J, Grist C, Kaur M, MacCallum P. Aetiology and outcome of massive transfusion in two large London teaching hospitals over a 3‐year period (2012–2014). Transfus Med. 2017;27(Suppl 5):342–7. [DOI] [PubMed] [Google Scholar]
- 2. NHS England and NHS Improvement . Clinical guide for the management of major trauma patients during the coronavirus pandemic. Specialty guides for patient management during the coronavirus pandemic. 26 March 2020 Version 1. https://www.england.nhs.uk/coronavirus/wp‐content/uploads/sites/52/2020/03/C0070‐specialty‐guide‐major‐trauma‐clinical‐guide‐27‐march‐2020.pdf [Accessed 19/04/20].
- 3. Bartlett JG, Borio L. The current status of planning for pandemic influenza and implications for health care planning in the United States. Clin Infect Dis. 2008;46:919–25. [DOI] [PubMed] [Google Scholar]
- 4. Paules CI, Eisinger RW, Marston HD, et al. What recent history has taught us about responding to emerging infectious disease threats. Ann Intern Med. 2017;167:805–11. [DOI] [PubMed] [Google Scholar]
- 5. Osterholm MT. Preparing for the next pandemic. N Engl J Med. 2005;352:1839–42. [DOI] [PubMed] [Google Scholar]
- 6. Fauci AS. Pandemic influenza threat and preparedness. Emerg Infect Dis. 2006;12:73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morse SS, Garwin RL, Olsiewski PJ. Next flu pandemic: what to do until the vaccine arrives? Science. 2006;314:929. [DOI] [PubMed] [Google Scholar]
- 8. Glynn SA, Busch MP, Schreiber GB, et al. Effect of a national disaster on blood supply and safety: the September 11 experience. JAMA. 2003;289:2246–53. [DOI] [PubMed] [Google Scholar]
- 9. Hunt BJ, Allard A, Keeling D, Norfolk D, Stanworth SJ, Pendry, K , et al. A practical guideline for the haematological management of major haemorrhage. Br J Haematol. 2015;170:788–803. [DOI] [PubMed] [Google Scholar]
- 10. National Blood Transfusion Committee . A Plan for NHS Blood and Transplant and Hospitals to Address Red Cell Shortages. Updated Version March 2020. https://www.transfusionguidelines.org/uk‐transfusion‐committees/national‐blood‐transfusion‐committee/responses‐and‐recommendations [Accessed 19/04/20].
- 11. National Blood Transfusion Committee . A Plan for NHS Blood and Transplant and Hospitals to address Platelet Shortages. Updated version January 2019. https://www.transfusionguidelines.org/uk‐transfusion‐committees/national‐blood‐transfusion‐committee/responses‐and‐recommendations [Accessed 19/04/20].
- 12. British Medical Association . COVID‐19. Ethical issues – a guidance note. https://www.bma.org.uk/media/2226/bma‐covid‐19‐ethics‐guidance.pdf [Accessed 19/04/20].
- 13. Emanuel, EJ , Persad, G & Upshar, R , et al. Fair allocation of scarce medical resources in the time of Covid‐19. N Engl J Med, 2020. 10.1056/NEJMsb2005114 [DOI] [PubMed] [Google Scholar]
- 14. The National Blood Transfusion Committee in England. https://www.transfusionguidelines.org/uk‐transfusion‐committees/national‐blood‐transfusion‐committee [Accessed 19/04/20].
- 15. National Advisory Committee on Blood and Blood products, Canada . Working group on emergency disposition of blood during a red phase blood shortage. Emergency framework for rationing of blood for massively bleeding patients during a red phase of a blood shortage. 2012. https://nacblood.ca/resources/shortages‐plan/emergency‐framework‐final.pdf [Accessed 19/04/20].
- 16. National Institute for Health and Care Excellence . COVID‐19 rapid guideline: critical care in adults. March 2020. https://www.nice.org.uk/guidance/ng159 [Accessed 19/04/20]. [PubMed]
- 17. Royal College of Physicians . Ethical dimensions of COVID‐19 for front‐line staff 31. March 2020. https://www.rcplondon.ac.uk/news/ethical‐guidance‐published‐frontline‐staff‐dealing‐pandemic?utm_source=Communications%2C%20Policy%20and%20Research&utm_medium=email&utm_campaign=11448063_Sickness%20survey%20‐%201%20April%202020&utm_content=ethical%20guidance&dm_i=1V12,6TDDR,DMK8YO,RALB5,1 [Accessed 19/04/20].
- 18. NHS England . Shared decision making. https://www.england.nhs.uk/shared‐decision‐making/ [Accessed 19/04/20].
- 19. Ferreira FL, Bota DP, Bross A, Mélot C, Vincent J. Serial evaluation of the SOFA score to predict outcome in critically Ill patients. JAMA. 2001;286:1754–8. [DOI] [PubMed] [Google Scholar]
- 20. Department of Health and Social Care . Pandemic flu. Pandemic flu planning information for England and the devolved administrations, including guidance for organisations and businesses. Ethical framework. Published 20th February 2013 and last updated 24th November 2017. https://www.gov.uk/guidance/pandemic‐flu#ethical‐framework [Accessed 19/04/20].
- 21. Zimrin AB, Hess J. Planning for pandemic influenza: effect of a pandemic on the supply and demand for blood products in the United States. Transfusion. 2007;47:1071–9. [DOI] [PubMed] [Google Scholar]
- 22. Doughty H, Chowdhury F, Ameh V, Batrick N, Baxter L, Bolton–Maggs P, et al. Emergency preparedness, resilience and response guidance for UK hospital transfusion teams. Transfus Med. 2020. https://www.transfusionguidelines.org/uk‐transfusion‐committees/national‐blood‐transfusion‐committee/responses‐and‐recommendations [Accessed 19/04/20]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The National Advisory Committee on Blood and Blood Products, Canada . National Plan for the Management of Shortages of Labile Blood Components. 2020. https://www.nacblood.ca/resources/shortages‐plan/index.html [Accessed 19/04/20].


