Abstract
The coronavirus disease (COVID‐19) has created a variety of challenges for health care professionals, including ambulatory care clinical pharmacists. High‐quality remote and minimal‐contact care has become a necessity. Ambulatory care clinical pharmacists around the nation have adjusted their practice. In many cases, this included implementation of telehealth programs for comprehensive medication management. The redesign of ambulatory care Advanced Pharmacy Practice Experiences (APPE) also required quick adaptation. In this paper, we describe the clinical practice and experiential education challenges encountered by an ambulatory care clinical pharmacist workgroup in a COVID‐19 “hotspot,” with an emphasis on solutions and guidance. We discuss how to adapt ambulatory care clinical pharmacy practices including methods of minimal‐contact care, reimbursement opportunities, tracking outcomes, and restructuring ambulatory care APPE. As ambulatory care clinical pharmacists continue to expand the services they provide in response to COVID‐19, we also describe opportunities to promote pharmacists as providers during times of pandemic and into the future.
Keywords: ambulatory care, clinical pharmacist, COVID‐19, experiential learning
1. BACKGROUND
The first case of coronavirus disease 2019 (COVID‐19) in the United States (US) was reported in Washington state in January 2020. 1 Cases in the United States continue to increase exponentially, with several “hotspots” emerging throughout the country, particularly within counties of New Jersey, New York, Michigan, and Washington. 2 The surge of COVID‐19 cases has created numerous unprecedented challenges in the health care field. In most areas across the country, non‐essential procedures have been halted and health systems are encouraged to provide remote patient care activities when possible both to promote social distancing and help flatten the curve. 3 In the absence of clear consensus guidelines, ambulatory practices have varied in their approaches to restructuring the delivery of care. Some practices have temporarily closed their doors, while others remain open. Many of these open establishments are limiting face‐to‐face care to urgent visits only; thus, non‐urgent visits require another method of care delivery. At the same time, a lack of access to routine health care during national emergencies and pandemics may lead to higher mortality rates for patients with chronic diseases. 4 , 5 Therefore, swift and prudent implementation of practice changes have been important in providing continued medication management during the coronavirus pandemic.
Ambulatory care clinical pharmacists provide accessible and coordinated health care services in various outpatient settings (community pharmacies, physicians' offices/clinics). 6 Positive clinical and financial outcomes across many chronic disease states have been associated with directed patient care services offered by ambulatory care clinical pharmacists. 7 , 8 , 9 , 10 Direct patient care services are often delivered under collaborative practice agreements and include comprehensive medication management (CMM); whereby pharmacists ensure each patient's medications are appropriate, effective, achieving therapeutic goals, safe, and that medication regimens can be adhered to and taken as intended. 11 As part of the CMM process, pharmacists can ensure medication access, and thereby may enroll patients into medication access programs. Furthermore, pharmacists may conduct medication stewardship activities.
During the pandemic, clinical pharmacy practice has required adaptability to provide direct patient care services, while meeting the changing demands across health care systems. 12 Pharmacists in ambulatory care practices received rapid rates of information exchange regarding the virus and its transmission, executive orders, and reimbursement guides. In addition to changes in clinical practice, many simultaneously adapted their students' Advanced Pharmacy Practice Experiences (APPE) as educators. Collaborations between pharmacists across different ambulatory care practices can assist with modifying both clinical practices and APPE. As such, a group of ambulatory care clinical pharmacists in Detroit, Michigan quickly convened to form a workgroup. This workgroup enabled timely information exchange, support, and guidance for redesigning ambulatory care practice models and APPE. The purpose of this paper is to highlight clinical and experiential challenges that ambulatory care clinical pharmacists have been facing, generate discussion, and provide examples of potential solutions that can serve as a framework for COVID‐19 ambulatory care practices and experiential sites.
2. MODIFYING AMBULATORY CARE PHARMACY PRACTICE
During the COVID‐19 era, ambulatory care clinical pharmacists are ensuring patient access to care, supporting their care teams, and easing the burden on the health care system. These roles have become increasingly important following the institution and extension of “stay‐at‐home” orders. Ambulatory care clinical pharmacists are providing COVID‐19 education and triaging, medication stewardship, medication coordination, and insurance coordination. 13 To ensure continuity of care and provision of CMM, pharmacists have quickly adapted to the urgent need for practice changes, with a shift to telehealth that consist of video and/or telephone encounters.
2.1. Telehealth services
Telehealth has been an emerging form of care delivery for pharmacists in recent years. Telehealth is a way to provide long‐distance health care and education to patients by using electronic information and telecommunication technologies. 14 , 15 This mode of service has been linked to a positive impact on outcomes related to clinical disease management, patient self‐management, and adherence in the management of chronic diseases. 16 Numerous pharmacy organizations support the use of telehealth and recognize the future relevance of this practice for the profession. The National Association of Boards of Pharmacy and the American Pharmacists Association both have definitions of “telehealth” or “telepharmacy” that address operations and regulatory considerations. 17 , 18 The American College of Clinical Pharmacy published guidance for clinical pharmacists providing CMM remotely via telehealth in alignment with the Standards for Practice of Clinical Pharmacists. 19 , 20
Amidst COVID‐19, telehealth services have been scaled‐up and have become the main mode of facilitating CMM services. 3 , 21 Given the guidance and experience many pharmacists have with telehealth, they are well‐positioned to meet the increased demands during this time of need. Ambulatory care clinical pharmacists can conduct either scheduled or ad hoc telehealth visits, which typically include communication with patients via phone or video. The scope of the pharmacists' services provided should be consistent with the scope of practice in their respective states, within the health system, and as written in a collaborative practice agreement. Such services may include adjusting medication therapy, ordering self‐monitoring devices and laboratory monitoring, providing education, and designing a follow‐up care plan.
2.2. Monitoring and managing patients with minimum contact
In order to decrease the risk of COVID‐19 exposure and transmission, ambulatory care clinical pharmacists can utilize telehealth to promote and provide more frequent follow‐up on patient self‐monitoring; this can serve as a short‐term alternative to office‐based and laboratory monitoring. While some patients may already have access to monitoring devices, such as scales, home blood pressure cuffs, home blood glucose monitors, and peak flow meters, pharmacists can help facilitate access to these tools. Other devices, such as point‐of‐care machines, “smart devices,” and continuous blood glucose monitors may provide additional information for patients who have access and meet specified criteria. In cases where telehealth guided by self‐monitoring is not sufficient, or not an option, ambulatory care clinical pharmacists have implemented solutions such as drive‐up testing.
Drive‐up point‐of‐care monitoring and medication administration can keep patients from entering the clinic and minimize person‐to‐person exposure risk. Ambulatory care clinical pharmacists can offer this service to increase access and provide faster testing and treatment for patients. One of the most widely implemented drive‐up services being conducted during the pandemic is point‐of‐care international ratio (INR) testing for patients on warfarin therapy. High‐quality anticoagulation management is important for patient safety and efficacy. Frequent INR monitoring, often performed at intervals of 1 month or less, is common for patients taking warfarin. 22 Therefore, drive‐up INR monitoring can offer timely evaluation for less stable patients where extended interval INR monitoring is not optimal. There are additional recommended strategies for safe warfarin management during the pandemic. These strategies are dependent on factors such as therapeutic stability, clinical, and economic feasibility of switching to an agent that does not require routine monitoring, reasonable access to a clinical laboratory that can offer limited patient exposure, or eligibility and access to home INR self‐testing (Figure 1). 23
FIGURE 1.
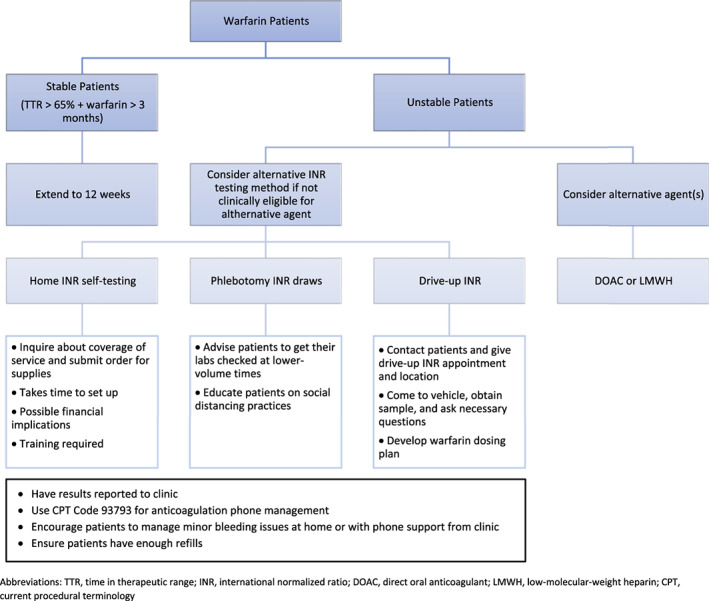
Warfarin Management During the COVID‐19 Pandemic. 20 CPT, current procedural terminology; DOAC, direct oral anticoagulant; INR, international normalized ratio; LMWH, low‐molecular‐weight heparin; TTR, time in therapeutic range;
In addition to drive‐up INR testing, other monitoring or medication‐related services may be delivered in a drive‐up manner. The Public Readiness and Emergency Preparedness Act authorized pharmacists to order and administer COVID‐19 tests, including serology tests, during the national public‐health emergency. 24 Therefore, COVID‐19 testing is an important drive‐up or on‐site service that pharmacists can provide at this time. Blood pressure monitoring for those needing antihypertensive titration can also be performed through drive‐up services. Furthermore, patients requiring scheduled injections, such as denosumab, epoetin, colony‐stimulating factors, or other such medications are good candidates for drive‐up minimized contact care.
3. TELEHEALTH BILLING AND REIMBURSEMENT OPPORTUNITIES
In response to COVID‐19, there have been expanded approvals of existing telehealth billing codes, as well as the addition of new codes. The Coronavirus Preparedness and Response Supplemental Appropriations Act, the largest economic relief bill in US history, became law on March 6, 2020. 25 The intention of this legislation was, in part, to loosen restrictions on telehealth and promote patient access to care. As a result, on March 17, 2020, the Centers for Medicare & Medicaid Services (CMS) introduced a waiver to ensure access to telehealth for COVID‐19 care, as well as management of other chronic conditions. 26 Medicare has provided guidance on billing for telehealth, which often serves as a standard for Medicaid and commercial payors (Table 1). Medicare specifically groups these opportunities into three categories: telehealth using video, virtual check‐ins using phone or other technologies, and e‐visits using an online patient portal. More recently, CMS has also allowed reimbursement for telephonic discussions without the use of video. 26 , 27 Telehealth video visits require real‐time audio and video for communication. Of note, during the pandemic, these visits are currently being paid at the same rate as in‐person visits; co‐insurance and deductibles may still apply, although Medicare has indicated that co‐insurance can be waived. 26 Virtual check‐ins refer to brief patient‐initiated communication with a practitioner via telephone, video, secure text messaging, email, shared images, or use of the patient portal. Patient consent is required for virtual check‐ins. Last, e‐visits refer to non‐face‐to‐face patient‐initiated communication using an online patient portal. Codes are available for physicians and non‐physicians to be billed for time spent over a 7‐day period. While both virtual check‐ins and e‐visits are intended to be patient‐initiated, practitioners can educate the patient on the service availability prior to patient agreement. Co‐insurance and deductibles apply to both types of interactions. Reimbursement opportunities for telehealth services for Federally Qualified Health Centers also exist. 28 Each of the described services can be furnished by the pharmacist and billed “incident to” a recognized provider. New patients can engage in telehealth visits without a prior established relationship with a Medicare‐recognized provider. 26 Nevertheless, “incident to” billing roles for pharmacists stipulate that the patient must have an established relationship with the provider; this language has not been changed in response to COVID‐19 telehealth changes. 29 The CMS requires documentation of duration of patient contact for time‐based billing codes but has removed requirements to document history and physical exam. 27 Ambulatory care clinical pharmacists can also continue to utilize pre‐COVID‐19 Chronic Care Management codes offered by CMS for non‐face‐to‐face telephonic and care coordination efforts for Medicare beneficiaries. 30 Existing reimbursement opportunities for non‐face‐to‐face anticoagulation management are also available. 31 Of note, the American Society of Health System Pharmacists (ASHP) continues to seek clarification from CMS on additional billing stipulations specific to pharmacists. Therefore, pharmacists should be mindful of additional guidance as it becomes released. 32
TABLE 1.
Summary of Medicare billing opportunities
| Mode and description of service | CPT/HCPCS Code | Guidance for Pharmacists | Documentation |
|---|---|---|---|
| Expanded Medicare reimbursement opportunities | |||
| Telehealth using Video 23 |
|
|
|
| Audio and video capabilities for two‐way, real‐time interactive communication |
|
||
| Examples permitted include: Facetime, Skype, Zoom | |||
| Telehealth using Telephone 24 |
|
|
|
| Evaluation and management billing code for telephonic outreach |
|
||
|
Brief check in via telephone or other telecommunication |
|
|
|
|
Discussion through online patient portal |
Cumulative time over 7 days:
Non‐physicians:
|
|
|
| Examples of pre‐existing Medicare reimbursement opportunities | |||
| Chronic Care Management 27 Non‐face‐to‐face care coordination efforts and/or telephonic care |
|
|
|
|
Anticoagulation (Warfarin) Management 28 Home and Outpatient International Normalized Ratio (INR) Monitoring Services |
|
|
|
Pharmacists must adhere to incident‐to billing rules which requires that the patient has an established relationship with the provider.
Established patients only.
While Medicare billing codes are typically uniform across states, pharmacists should review Medicaid and commercial telehealth billing codes in their respective states. 33 Commercial payers each have varying requirements for telehealth during the COVID‐19 crisis but are generally allowing flexibility as well. Unique opportunities exist to provide remote care via these payors. For example, in Michigan, pharmacists who have previously received training as “care managers” are able to utilize telehealth billing codes from select commercial payors that are particularly advantageous for CMM services. 34
The Office for Civil Rights at the Department of Health and Human Services and CMS have relaxed restrictions surrounding telehealth and supporting technologies. 26 , 35 While some Medicare telehealth billing codes were available prior to COVID‐19, access was limited to a select group of patients (ie, rural areas) or under specific situations. Geographical site restrictions were waived by CMS to allow patients to receive Medicare telehealth services regardless of the patient's location. 26 Furthermore, CMS has waived penalties for HIPAA violations to provide flexibility for the different modes of care described. 35 Technologies with audio and video capabilities are authorized for delivering Medicare telehealth visits. Use of popular applications, including FaceTime or Skype, is permitted without risk of HIPAA violation penalties. Applications that are public facing, such as Facebook Live or Twitch, should not be used in the provision of telehealth. 35 Pharmacists and students working remotely can consider using cellular devices linked to their clinic phone number, call forwarding, or apps that disguise the caller's personal number and displays the clinic number instead (I.e. Doximity[R]). 36
The telehealth billing mechanisms described can be utilized for CMM, anticoagulation specific services, and other non‐face‐to‐face care efforts. Last, while most telehealth encounters can be reimbursed, pharmacists should be mindful that some brief communications may not necessarily be linked to reimbursement.
4. MONITORING CLINICAL AND FINANCIAL OUTCOMES
A comprehensive evaluation program is essential for the success and quality improvement of telehealth services. 19 A systematic review found traditional pharmacist telehealth programs in ambulatory care had an overall positive impact on outcomes related to laboratory value monitoring and achieving therapeutic goals. 16 For commonly managed disease states like hypertension, diabetes, hyperlipidemia, and anticoagulation, outcomes measured have included change in blood pressure, A1c, LDL, INR, and time in the therapeutic range. Other outcomes such as hospitalizations or emergency department visits, readmissions, and mortality have also been measured by programs. Patient self‐reported questionnaire scores find positive patient satisfaction with pharmacist telehealthcare. 37
Ambulatory care clinical pharmacists providing telehealth during COVID‐19, similarly, should track both clinical and non‐clinical outcomes, such as number of patient outreach encounters and billing codes submitted. Documentation and evaluation of such outcomes should be determined on a practice‐specific basis, but nevertheless should be pre‐planned. Both clinical and financial outcomes tracked during the provision of telehealth during COVID‐19 can also help to provide justification for ongoing program maintenance beyond this public emergency.
5. EXPANDING THE TRADITIONAL ROLE OF THE AMBULATORY CARE CLINICAL PHARMACIST
Patient care opportunities for pharmacists may expand far beyond traditional roles when in a crisis such as the COVID‐19 pandemic. For example, ambulatory care clinical pharmacists could play a larger role managing chronic conditions or providing point‐of‐care testing for infectious diseases to reduce the burden on other health care providers. 38 To encourage this expansion during the COVID‐19 outbreak, national pharmacy organizations joined to create policy recommendations which urged policymakers and national institutions to recognize and authorize the expanded role of pharmacists to support the COVID‐19 response. 39 Several governors enacted executive orders which enhanced “operational capacity, flexibility, and efficiency of pharmacies” and permitted licensed pharmacists to provide direct patient care without direct supervision by a physician. 40 , 41 Additional guidance from the Department of Health and Human Services encouraged pharmacists to aid in COVID‐19 testing. 42 These declarations reduced regulatory barriers, allowing pharmacists to use clinical judgement without physician directives.
Examples of pharmacist role expansion were abundant among the aforementioned ambulatory care clinical pharmacist workgroup. An ambulatory care clinical pharmacist practicing in an outpatient pulmonary clinic managed all incoming medication requests and triaged/treated patients presenting with pulmonary symptoms to prevent acute care utilization while physicians were redeployed to intensive care units. Additionally, a psychiatric ambulatory care clinical pharmacist, using updated clozapine COVID‐19 guidelines and updated FDA recommendations, was able to make clinical decisions about whether it was appropriate for delayed absolute neutrophil count laboratory monitoring for clozapine Risk Evaluation and Mitigation Strategy. 43 If the decision was made by the pharmacist to delay laboratory monitoring due to COVID‐19 concerns, the associated risks were also communicated to the patient by the pharmacist via a telephonic visit. The pharmacist also ensured access to the medication. Other role expansions include authorizing refills for medications, substituting therapeutically equivalent medications, and administering necessary vaccines/medications without a physician's order.
6. REDESIGNING AMBULATORY CARE APPE DURING COVID‐19
Changes to clinical practice during the COVID‐19 pandemic have required adjustments in the experiential training of health care students. Early in the pandemic, the American Association for Medical Colleges (AAMC) swiftly called for a pause to all medical student clinical rotations, and later supported their voluntary participation in direct patient care experiences. 44 The Accreditation Council for Pharmacy Education and the American Association of Colleges of Pharmacy issued some guidance and suggestions to pharmacy school Deans regarding the experiential training of pharmacy students. Clinical rotations were not paused for pharmacy students. As such, pharmacy schools and health‐systems were required to strike a balance between providing adequate learning experiences while ensuring the safety of the students, staff, patients, and community.
State pharmacy organizations, such as the Michigan Pharmacists Association (MPA), advocated for the expanded role of student pharmacists in clinical practice to ease the care burden. 45 Students were enabled to support pharmacists' efforts by administering COVID‐19 testing or dispensing medications without direct oversight. 45 Additionally, some pharmacy schools elected to graduate students early to assist with the pandemic efforts. 46 , 47 One effective approach to modifying inpatient APPE rotations was described in the literature. Pharmacist preceptors assessed students virtually as they conducted modified rotational activities, such as presenting on de‐identified patients, answering drug information questions, and drafting therapeutic protocols. 48 There is a paucity of published literature providing guidance for the training of students in the ambulatory care practice during a pandemic. Since the pandemic is expected to continue well into the early summer with a potential for resurgence, a cohesive approach to delivering APPE was necessary for present and future planning. In response, the previously mentioned ambulatory care workgroup convened to address challenges being faced including how to provide effective orientation, shadowing and training, assessment, and feedback.
The COVID‐19 pandemic presented many challenges to delivering experiential APPE in ambulatory clinics. The strategies developed in a COVID hotspot may serve as a framework for other pharmacy schools and health‐systems around the country. Our workgroup devised a structured plan to help adapt ambulatory care APPE rotations during the COVID‐19 pandemic (Figure 2). The plan focused on how to remotely orient students to complete tasks and demonstrate competence in the learning objectives of the experience. The workgroup also identified opportunities to modify traditional experiential activities using remote virtual platforms to host video meetings, share clinical files, and conduct experiential activities. Consideration was given to determining which virtual meeting platforms would comply with the Health Insurance Portability and Accountability Act. 35 Additionally, the workgroup modified data collection, patient communication, and documentation templates to support clinical activities of the students. There was also a consensus that once shadowing and training components were satisfied preceptors may supervise student‐patient communication using methods such as three‐way calling and virtual audio/video visits. Following the first trial of remote APPE, the workgroup modified the systematic approach to include acceptable clinical activities for students. Some of these activities include, but are not limited to, conducting patient interviews, obtaining medication histories, completing prior authorizations, ensuring patient access to medications, assisting with refill requests, developing clinical protocols, providing remote in‐services to the care team, participating in multi‐disciplinary patient discussions, answering drug information questions, and collecting clinical outcome data.
FIGURE 2.
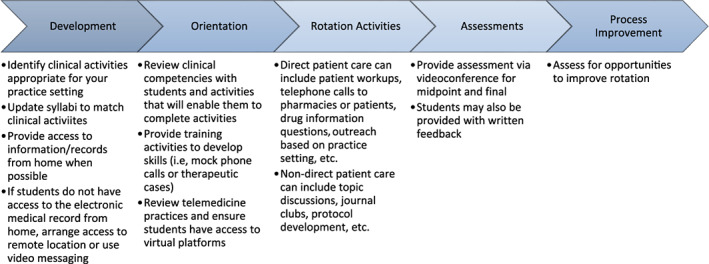
Systematic approach to delivering ambulatory care rotations remotely
7. OPPORTUNITIES AND FUTURE THOUGHTS
The coronavirus pandemic has abruptly changed almost every aspect of health care. In outpatient clinics, operations have slowed or even suspended scheduling of face‐to‐face visits. Telehealth and limited contact forms of patient care have become key modes of ambulatory care delivery. At the same time, many outpatient health care workers have been redeployed to other areas of demand. Thus, ambulatory care clinical pharmacists are practicing at the top of their licenses, filling gaps as health care provider colleagues are redeployed due to the pandemic, especially by managing medication therapy for chronic conditions. Economic, regulatory, and logistical barriers for pharmacists to assume provider roles have shifted, or even evaporated for the time being. 26 , 41 Ambulatory care clinical pharmacists have been ready, agile, and able to develop best practices to meet the rapidly evolving need for increased provider access.
While being flexible and strategic during the COVID‐19 crisis, pharmacists, health care organizations, and regulators should be forward thinking. After the crisis is over, health care will most likely look different. Social distancing is expected to linger beyond the re‐opening of businesses and outpatient care. Pharmacists can be providers and leaders in COVID‐19 testing that will likely become a key component in re‐opening the country to return to work. Telehealth is likely to become a prevailing form of health care delivery. It will be important for pharmacists to sustain heightened levels of patient‐centered care in various modes of delivery, including telehealth. Furthermore, provision of telehealth by pharmacists can increase access to and quality of care, which is prudent as health care continues to transition from traditional payment models to value‐based care. 49 Advocacy for maintaining expanded scopes of pharmacy practice will be important in removing barriers that hinder the optimization of medication therapy through pharmacists' care. Patient's access to pharmacists' care must be sustainable as well. Medicare, Medicaid, and commercial payors should institute systems to ensure access to pharmacist services. Specifically, a joint group of pharmacy organizations recommended immediate legislative action to include the Pharmacy and Medically Underserved Areas Enhancement Act. 39 , 50 This crisis has magnified the need for pharmacists to fill provider roles. Pharmacists must be ready to act and advocate for appropriate coverage and reimbursement for their services. Reimbursement should include screening, testing, immunization, and medication management under Medicare B, C, and D.
8. CONCLUSION
The COVID‐19 crisis has created a need for ambulatory care clinical pharmacists to be problem‐solvers and make quick adjustments. It has highlighted a need to collaboratively share information between ambulatory care clinical pharmacists across practices, to recreate workflow and care delivery, and to expand our roles. Collaboration as practitioners and experiential educators aids in efficiency and helps ambulatory care clinical pharmacists effectively assist health care teams, provide care for patients, and protect communities both during times of pandemic and into the future.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Mohammad I, Berlie HD, Lipari M, et al. Ambulatory care practice in the COVID‐19 era: Redesigning clinical services and experiential learning. J Am Coll Clin Pharm. 2020;3:1129–1137. 10.1002/jac5.1276
REFERENCES
- 1. Centers for Disease Control and Prevention . Coronavirus Disease 2019 (COVID‐19). 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html#2019coronavirus-summary. Accessed April 18, 2020.
- 2. Johns Hopkins University . Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed April 18, 2020.
- 3. Myers US, Birks A, Grubaugh AL, Axon RN. Flattening the Curve by Getting Ahead of It: How the VA Healthcare System Is Leveraging Telehealth to Provide Continued Access to Care for Rural Veterans. J Rural Heal. 2020;1–6. 10.1111/jrh.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mokdad AH, Mensah GA, Posner SF, et al. When chronic conditions become acute: prevention and control of chronic diseases and adverse health outcomes during natural disasters. Prev Chronic Dis. 2005;2:1–4. [PMC free article] [PubMed] [Google Scholar]
- 5. Saulnier DD, Brolin Ribacke K, von Schreeb J. No Calm After the Storm: A Systematic Review of Human Health Following Flood and Storm Disasters. Prehosp Disaster Med. 2017;32(5):568–579. [DOI] [PubMed] [Google Scholar]
- 6. Board of Pharmacy Specialties . bpsweb.org. 2020. Accessed April 18, 2020.
- 7. Chisholm‐Burns MA, Kim Lee J, Spivey CA, et al. US Pharmacists' Effect as Team Members on Patient Care. Med Care. 2010;48(10):923–933. [DOI] [PubMed] [Google Scholar]
- 8. Santschi V. Impact of pharmacist care in the management of cardiovascular disease risk factors. Arch Intern Med. 2011;171(16):1441–1453. [DOI] [PubMed] [Google Scholar]
- 9. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carter BL, Rogers M, Daly J, Zheng S, James PA. The Potency of Team‐Based Care Interventions for Hypertension. Arch Intern Med. 2009;169(19):1748–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American College of Clinical Pharmacy . Comprehensive medication management in team‐based care. https://www.accp.com/docs/positions/misc/CMM%20Brief.pdf. Accessed May 12, 2020. 2020.
- 12. Gross AE, MacDougall C. Roles of the clinical pharmacist during the COVID‐19 pandemic. J Am Coll Clin Pharm. 2020;3(3):564–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhat S, Kehasse A. Additional Clinical Pharmacists Roles During COVID‐19. J Am Coll Clin Pharm. 2020;3(4):825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Telemedicine Association . About Telemedicine. http://legacy.americantelemed.org/main/about/about-telemedicine/telemedicine-faqs. Accessed December 5, 2020. 2020.
- 15. Health Resources & Services Administration . Telehealth Programs. https://www.hrsa.gov/ruralhealth/telehealth/index.html. 2019. .
- 16. Niznik JD, He H, Kane‐Gill SL. Impact of clinical pharmacist services delivered via telemedicine in the outpatient or ambulatory care setting: A systematic review. Res Soc Adm Pharm. 2018;14(8):707–717. [DOI] [PubMed] [Google Scholar]
- 17. National Association of Boards of Pharmacy . Model Pharmacy Act/Rules. https://nabp.pharmacy/publications-reports/resource-documents/model-pharmacy-act-rules/. Accessed April 18, 2020.
- 18. American Pharmacist Association . Telehealth. https://www.pharmacist.com/telehealth. Accessed April 19, 2020.
- 19. Badowski ME, Wright EA, Bainbridge J, et al. Implementation and evaluation of comprehensive medication management in telehealth practices. J Am Coll Clin Pharm. 2020;3(2):520–531. [Google Scholar]
- 20. American College of Clinical Pharmacy . Standards of practice for clinical pharmacists. Pharmacotherapy. 2014;34(8):794–797. [DOI] [PubMed] [Google Scholar]
- 21. Nagata JM. Rapid Scale‐Up of Telehealth during the COVID‐19 Pandemic and Implications for Subspecialty Care in Rural Areas. J Rural Heal. 2020;21(8):622–629. [DOI] [PubMed] [Google Scholar]
- 22. Holbrook A, Schulman S, Witt DM, et al. Evidence‐based management of anticoagulant therapy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 SUPPL):e152S–e184S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaatz S, Clark N, Allen A, et al. Management of Warfarin patients who have difficulty getting INR testing during COVID‐19 pandemic: practical experiences and advice from the experts. Anticoagulation Forum. 2020. https://acforum.org/web/webinars/AC Forum Webinar COVID 19 March 25_Final.pdf. [Google Scholar]
- 24. World Health Organization . Coronavirus disease (COVID‐19) outbreak: rights, roles and responsibilities of health workers, including key considerations for occupational safety and health. Geneva, Switzerland: WHO, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/health-workers. Accessed April 18, 2020. [Google Scholar]
- 25. United States Congress . Coronavirus Aid, Relief, and Economic Security (CARES) Act; 2020. https://www.congress.gov/116/bills/hr748/BILLS-116hr748enr.pdf.
- 26. Centers for Medicare and Medicaid Services . Medicare Telemedicine Health Care Provider Fact Sheet. 2020. https://www.cms.gov/newsroom/fact‐sheets/medicare‐telemedicine‐health‐care‐provider‐fact‐sheet. Accessed April 18, 2020.
- 27. American Academy of Family Physicians . Using Telehealth to Care for Patients During the COVID‐19 Pandemic. https://www.aafp.org/patient-care/emergency/2019-coronavirus/telehealth.html. 2020. Accessed April 19, 2020.
- 28. Centers for Medicare and Medicaid Services . Federally Qualified Health Centers (FQHC) Center. https://www.cms.gov/Center/Provider‐Type/Federally‐Qualified‐Health‐Centers‐FQHC‐Center. 2020. Accessed April 20, 2020.
- 29. Dietrich E, Gums JG. Incident‐to billing for pharmacists. J Manag Care Spec Pharm. 2018;24(12):1273–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centers for Medicare and Medicaid Services . Chronic Care Management Services; 2020. https://www.cms.gov/Outreach‐and‐Education/Medicare‐Learning‐Network‐MLN/MLNProducts/Downloads/ChronicCareManagement.pdf. Accessed April 18, 2020.
- 31. Medicare Learning Network . Guidance on Coding and Billing Date of Service on Professional Claims; 2019. https://www.cms.gov/Outreach‐and‐Education/Medicare‐Learning‐Network‐MLN/MLNMattersArticles/downloads/SE17023.pdf.
- 32. Issue Brief : COVID‐19 and Telehealth Changes.; 2020. https://www.ashp.org/Advocacy-and-Issues/Key-Issues/Other-Issues/Issue-Brief-COVID-19.
- 33. Centers for Medicare and Medicaid Services . Medicaid State Plan Fee‐for‐Service Payments for Services Delivered Via Telehealth; 2020. https://www.medicaid.gov/medicaid/benefits/downloads/medicaid-telehealth-services.pdf.
- 34. Michigan Institute for Care Management and Transformation . Complex Care Management Course. 2019. https://micmt-cares.org/sites/default/files/sustainability and billing v3_0.pdf.
- 35. U.S Department of Heath and Human Services . Notification of Enforcement Discretion for Telehealth Remote Communications During the COVID‐19 Nationwide Public Health Emergency. https://www.hhs.gov/hipaa/for‐professionals/special‐topics/emergency‐preparedness/notification‐enforcement‐discretion‐telehealth/index.html. 2020. Accessed April 18, 2020.
- 36. Doximity . 2020. https://www.doximity.com/about/company.
- 37. Hatton J, Chandra R, Lucius D, Ciuchta E. Patient Satisfaction of Pharmacist‐Provided Care via Clinical Video Teleconferencing. J Pharm Pract. 2018;31(5):429–433. [DOI] [PubMed] [Google Scholar]
- 38. American Society of Health‐System Pharmacists . Pharmacy Readiness for Coronavirus Disease. 2020. https://www.ashp.org/‐/media/assets/advocacy‐issues/docs/Pharmacy‐Readiness‐for‐Coronavirus‐Disease‐2019‐COVID‐19‐STATE.ashx?la=en&hash=6420DD319DEF9C0C008B161D36615C8E3229532B. Accessed April 18, 2020.
- 39. Consultant Pharmacist Forum . Executive Summary: Pharmacists as Front‐Line Responders for COVID‐19 Patient Care. 2020. https://www.pharmacist.com/sites/default/files/files/APHA Meeting Update/PHARMACISTS_COVID19‐Final‐3‐20‐20.pdf. Accessed April 18, 2020.
- 40. Whitmer, Gretchen; GilChrist II G . Executive Order #2020–25: Temporary Enhancements to Operational Capacity, Flexibility, and Efficiency of Pharmacies. Lansing; 2020. https://www.michigan.gov/whitmer/0,9309,7‐387‐90499_90705‐523301‐,00.html.
- 41. Whitmer, Gretchen; GilChrist II G . Executive Order #2020–30: Temporary Relief from Certain Restrictions and Requirements Governing the Provision of Medical Services. Lansing; 2020. https://www.michigan.gov/whitmer/0,9309,7-387-90499_90705-523481-,00.html.
- 42. U.S Deptartment of Health and Human Services . Guidance for Licensed Pharmacists, COVID‐19 Testing, and Immunity under the PREP Act; 2020. https://www.hhs.gov/sites/default/files/authorizing-licensed-pharmacists-to-order-and-administer-covid-19-tests.pdf.
- 43. U.S. Department of Health and Human Services . Policy for Certain REMS Requirements During the COVID‐19 Public Health Emergency: Guidance for Industry and Health Care Professionals; 2020. https://www.fda.gov/emergency‐preparedness‐and‐response/mcm‐issues/coronavirus‐disease‐2019‐covid‐19.
- 44. American Association for Medical Colleges . Important Guidance for Medical Students on Clinical Rotations During the Coronavirus (COVID‐19) Outbreak. https://www.aamc.org/news‐insights/press‐releases/important‐guidance‐medical‐students‐clinical‐rotations‐during‐coronavirus‐covid‐19‐outbreak. Published 2020. Accessed April 13, 2020.
- 45. Michigan Pharmacist Association . Michigan Pharmacist Association: COVID‐19 Update. https://myemail.constantcontact.com/MPA‐COVID‐19‐Update.html?soid=1107842212368&aid=0FxGi4kj_‐U. Published 2020. Accessed April 18, 2020.
- 46. Pharmacy students to graduate early in preparation for COVID‐19 impact . Purdue University News. https://www.purdue.edu/newsroom/releases/2020/Q2/pharmacy-students-to-graduate-early-in-preparation-for-future-covid-19-impact.html. Published April 9, 2020.
- 47. Makin B. Coronavirus: Rutgers to graduate pharmacy students early to help on front lines. My Central Jersey. https://www.mycentraljersey.com/story/news/education/college/rutgers/2020/04/02/coronavirus‐nj‐rutgers‐school‐pharmacy‐move‐up‐graduation/5117180002/. Published April 2, 2020.
- 48. Badreldin HA, Alshaya O, Saleh KB, Alshaya AI. Restructuring the Inpatient Advanced Pharmacy Practice Experience to Reduce the Risk of Contracting COVID‐19: Lessons from Saudi Arabia. J Am Coll Clin Pharm. 2020;3(4):771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Littauer SL, Dixon DL, Mishra VK, Sisson EM, Salgado TM. Pharmacists providing care in the outpatient setting through telemedicine models: a narrative review. Pharm Pract (Granada). 2017;15(4):1134–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. American Pharmacist Association . Pharmacists Provide Care. pharmacistsprovidecare.com. 2020. Accessed April 13, 2020.


