Abstract
Coronavirus disease 2019 (COVID‐19) is highly contagious and has a variety of clinical manifestations, it can affect a number of other organs in addition to the lungs, and liver injury may occur. Severe acute respiratory syndrome coronavirus 2 can cause liver injury through systemic inflammatory response syndrome, cytokine storms, ischemia‐reperfusion injury, side effects of treatment drugs, and underlying liver disease and can attack liver cells directly via angiotensin‐converting enzyme 2. Clinical studies have found that liver injury in COVID‐19 patients mainly manifests as abnormal liver biochemical indicators, but there have been no reports of liver failure caused by this disease. The number of COVID‐19 patients with liver injury is increasing, and the incidence of liver injury in COVID‐19 patients with severe disease are higher than in patients with mild disease. Liver injury may be a risk factor, which worsens in patients with COVID‐19, and hence it is necessary to pay attention to the occurrence of liver injury in the diagnosis and treatment of COVID‐19.
Keywords: COVID‐19, liver injury, mechanism, SARS‐CoV‐2
Highlights
Liver function impairment may become a predictor of exacerbation and deterioration of patients with COVID‐19.
SARS‐CoV‐2 can cause liver injury through systemic inflammatory response syndrome (SIRS), cytokine storms, ischemia‐reperfusion injury, drugs, underlying liver disease and can attack liver cells directly via ACE2.
It is necessary to pay attention to the occurrence of liver injury in the diagnosis and treatment of COVID‐19.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is an acute respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 , 2 , 3 As it is highly contagious, people are generally susceptible, and it is mainly transmitted via respiratory droplets, COVID‐19 is spreading all over the world. It has been reported in more than 200 countries, and the number of deaths worldwide is also increasing. SARS‐CoV‐2 infection mainly affects the respiratory tract, and the main clinical manifestations are fever, dry cough, rhinorrhea, and fatigue. Many cases of COVID‐19 are acute and resolve quickly, but the disease can also be fatal, with a mortality rate of approximately 3%. 4 The fatality rate of critically ill patients admitted to the intensive care unit (ICU) is relatively high, the results from a study of 52 critically ill patients with COVID‐19 who were admitted to the ICU of Wuhan Jin Yin‐tan hospital (Wuhan, China) between late December 2019 and 26 January 2020 showed that 32 (61.5%) patients had died within 28 days, and the median duration from ICU admission to death was 7 (IQR, 3‐11) days in the non‐survivors. 5 Many clinical studies have also suggested that patients with COVID‐19 have multiple organ and multiple system damage, including circulatory system, urinary system, nervous system, and digestive system. The presence and number of these complications are high‐risk factors that aggravate a patient's condition and correlate with poor prognosis.
An increasing number of COVID‐19 patients with liver injury have been reported, but the mechanism by which COVID‐19 causes liver injury is not yet clear. This article combines the treatment experience of frontline clinicians and refers to the published literature about COVID‐19 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 to discuss the mechanism and treatment of liver injury caused by SARS‐CoV‐2 infection. The goal is to provide some reference for reducing the occurrence of liver injury complications and improving the success rate of COVID‐19 treatment.
1.1. Clinical features of COVID‐19 with liver injury
The whole genome sequencing results showed that SARS‐CoV‐2 shares 82% genome sequence similarity to SARS‐CoV, and 50% genome sequence homology to Middle East respiratory syndrome coronavirus (MERS‐CoV). 22 SARS‐CoV, MERS‐CoV and SARS‐CoV‐2 are known coronaviruses that cause severe respiratory symptoms. 11
As many as 60% of patients infected with SARS‐CoV have liver damage, 23 and some patients infected with MERS‐CoV also have liver damage. 24 Abnormal alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were first reported in 43 (43.4%) of 99 patients infected with SARS‐CoV‐2 from Wuhan Jinyintan Hospital. 6 At least 12 clinical studies based on single or multiple centers have shown that approximately 14.8%‐53% of COVID‐19 patients have liver injury, 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 indicating that liver injury is relatively common among COVID‐19 patients.
A retrospective analysis report showed that 29% of the deaths had abnormal liver function. 4 As one of the common complications of COVID‐19 patients, can liver function damage be used as one of the predictors of the aggravation and deterioration of COVID‐19 cases? A multicenter clinical study of 32 COVID‐19 patients (28 in mild or normal type, 4 in severe or critical type) showed that ALT was 22.75 (16.31‐37.25) U/L and AST was 23.63 (18.71‐26.50) U/L in 28 mild or normal patients, and ALT was 60.25 (40.88‐68.90) U/L and AST was 37.00 (20.88‐64.45) U/L in 4 cases of severe or critical type. 7 In another retrospective analysis of 333 COVID‐19 inpatients showed that 39.6% had liver injury (132 of 333) and that the proportion with liver injury in the intensive care unit (ICU) was higher than that in the general ward (45.6% [26 of 57] vs 38.4% [106 of 276]). 19 The above indicate that the incidence of liver injury in severe or critically ill cases is significantly higher than that in mild cases of COVID‐19, and the probability of patients with liver function impairment requiring ICU treatment is increased. Liver function impairment may become a predictor of exacerbation and deterioration in patients with COVID‐19.
Liver injury in COVID‐19 patients mainly manifests as abnormal liver biochemical indicators, such as increased ALT, AST, and TBIL levels and decreased albumin (ALB) levels. Can liver biochemical indicators be used as a predictor of the severity and prognosis of COVID‐19 cases? Analysis of 333 COVID‐19 patients showed that 36.9% had liver damage, that 67.4% (89 of 132) of liver injury patients had increased ALT and AST levels on admission, and that the level of ALT during hospitalization was higher than after admission ([60.28 ± 50.44] vs [42.25 ± 32.21] U/L). However, albumin levels during hospitalization were lower than after admission ([31.8 ± 5.1] vs [33.7 ± 5.4] g/L), and patients in the ICU had lower levels of ALB than did patients in general wards ([28.5 ± 3.3] vs [33.9 ± 5.5] g/L). The above differences were all statistically significant (P < .05). 19 Another single‐center study of 138 patients with COVID‐19 showed that the levels of ALT and AST in patients who required ICU treatment were significantly higher than those in patients who did not require ICU treatment (ALT: 35 [19‐57] vs 23 [19‐36] U/L, P < .007; AST: 52 [30‐70] vs 29 [21‐38] U/L, P < .001). 10 Moreover, in a large cohort of 1099 patients from 552 hospitals in 31 provinces or provincial municipalities, the total incidence of elevated total bilirubin was approximately 10%, of which the incidence in severe and nonsevere patients was approximately 13.3% and 9.9%, respectively; the incidence of increased total bilirubin in patients who needed to be moved to the ICU, needed mechanical ventilation, or died was higher than in other patients (20.8% vs 9.8%). 5 The above studies suggest that liver biochemical indicators may be used as predictors of severity and prognosis of COVID‐19 patients. Thus, clinicians should pay more attention to changes in liver biochemical indicators and also identify patients with liver damage in a timely manner.
1.2. The mechanism by which COVID‐19 causes liver injury
1.2.1. Direct damage
The results from biopsies in a death COVID‐19 patient showed moderate microvascular steatosis and mild portal and lobular activity in liver tissue. 25 In another four autopsy of COVID‐19 patients showed mild zone 3 sinusoidal dilatation, patchy hepatic necrosis, mild increase in sinusoidal lymphocytes are the main pathologic changes of liver in cases 3 and 4, and RT‐PCR showed direct evidence of the SARS‐CoV‐2 RNA sequence in the liver tissues in case 1. 26 Through learning of Tian et al 26 report, Li et al 27 observed foci of hepatic necrosis in adjacent to terminal hepatic veins and peri‐portal area, and found no significant surrounding inflammatory cellular infiltration, which was consistent with the pattern of acute liver injury. The above studies may indicate that hepatic injuries may be related to direct viral attack. It has been confirmed that SARS‐CoV‐2 enters host cells through binding of its S protein to angiotensin‐converting enzyme 2 (ACE2) on the surface of the host cell. ACE2 is the most important cell receptor that mediates the entry of SARS‐CoV‐2 into target cells. 28 , 29 However, based on single‐cell sequencing and animal model analysis of liver tissue, ChaiX's team found that the expression level of ACE2 in liver tissue was only approximately 0.31% and that specific expression of ACE2 in bile duct epithelial cells was 20 times higher than that in hepatocyte. 30 Considering limited number of autopsy cases in patients with COVID‐19 studied and the relatively low expression of ACE2 in liver, liver damage directly caused by SARS‐CoV‐2 infection of hepatocytes deserves further investigation. Moreover, biomarkers for cholangiocyte injury, such as γ‐glutamyl transferase (GGT) and alkaline phosphatase (ALP) have also been seen in some patients, 31 and consistent with injury to biliary epithelial cells, and about 10% of COVID‐19 patients have elevated total bilirubin level. These might suggest that SARS‐CoV‐2 might directly bind to cholangiocytes expressing ACE2 result in cholangiocyte injury.
Huang et al 18 found that liver injury in COVID‐19 patients differed from that in SARS patients. Liver injury as the first manifestation in COVID‐19 patients was very rare, with most being secondary liver injury. It was speculated that in addition to the virus itself causing liver injury, immune injury, systemic inflammatory response syndrome (SIRS), cytokine storms, ischemia and hypoxia reperfusion injury, and drug‐induced injury may be the main mechanisms that cause secondary liver injury in patients with COVID‐19. 11 , 12 , 14 , 27
1.2.2. Antibody‐dependent enhancement
In addition to receptor‐mediated viral infection, antibody‐dependent enhancement of infection (ADE) may occur in patients with SARS. 32 ADE refers to the interaction of a virus‐specific antibody with Fc receptor (FcR) and/or complement receptor (CR) to enhance the ability of the virus to enter granulocytes, monocytes, and macrophages. The virus constantly replicates in the above cells, resulting in increased virus production and aggravating infection. Previous studies have reported that antibodies against the SARS‐CoV spike protein trigger ADE, causing SARS‐CoV to enter immune cells that do not express ACE2 and immune damage. 33 The liver contains a large number of cells related to the immune response. Whether ADE can also mediate SARS‐CoV‐2 to infect immune cells by a non‐ACE2‐dependent pathway and participate in liver injury caused by SARS‐CoV‐2 is a concern.
1.2.3. SIRS and cytokine storms
Research has shown that the levels of inflammatory factors, including interleukin (IL), tumor necrosis factor (TNF), and endotoxin in patients with SARS with liver function impairment are significantly higher than those in patients with normal liver function, and inferred that SIRS and cytokine storms was a risk factor for liver impairment in SARS‐CoV 34 , 35 infected patients and in MERS‐CoV infected patients. 36 Limited pathological results have demonstrated that hepatocytes in patients with severe COVID‐19 exhibit nonspecific inflammatory changes, including hepatocyte swelling and steatosis, mild proliferation of hepatic sinus cells, hyperplasia of Kupffer cells, and infiltration of a small number of lymphocytes. It has also been reported that the levels of IL‐2‐receptor (IL‐2R) and IL‐6 in the serum of COVID‐19 patients are significantly increased and correlate with disease severity. 22 In addition, cytokines secreted by Th1 and Th2 cells in the serum of COVID‐19 patients, such as TNF, IL‐6, IL‐18, IL‐4, and IL‐10 were significantly increased, 5 , 18 as do peripheral blood pro‐inflammatory CCR4 + CCR6 + Th17 cells. 37 The liver has an important immune function and contains a large number of cells related to the immune response. After being infected with SARS‐CoV‐2, a large number of immune cells may be overactivated and secrete excessive cytokines, chemokines, etc, such as TNF‐α, interferon‐γ (IFN‐γ), IL‐6, IL‐8, etc, leading to acute respiratory distress syndrome and SIRS as well as inducing ischemia and hypoxia, which result in further cell damage and necrosis (Figure 1). Such a vicious cycle not only leads to lung injury but also may develop into MODS, such as the liver, myocardium, and kidney. These results suggest that the SIRS and cytokine storms caused by SARS‐CoV‐2 infection may be one of the important mechanisms of liver injury.
Figure 1.
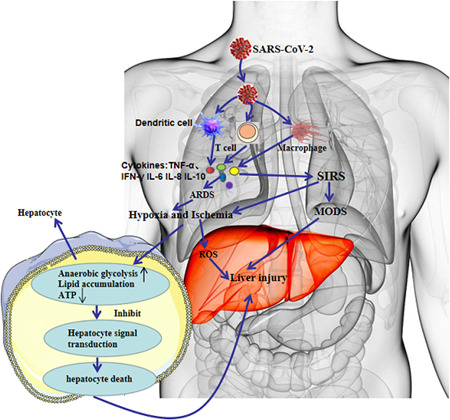
SIRS and cytokine storms in COVID‐19 with liver injury. COVID‐19, coronavirus disease 2019; IFN‐γ, interferon‐γ; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; SIRS, systemic inflammatory response syndrome; TNF‐α, tumor necrosis factor‐α
1.2.4. Ischemia and hypoxia reperfusion injury
Patients with COVID‐19 have varying degrees of hypoxemia, with more than 40% requiring oxygen therapy. 5 Complications such as aspiration distress syndrome, SIRS, and multiple organ dysfunction can cause ischemia, hypoxia, and even shock. Under the conditions of ischemia and hypoxia, lipid accumulation, glycogen consumption, and adenosine triphosphate depletion of hepatocytes can inhibit cell survival signal transduction, rapidly leading to hepatocyte death. In addition, the hypoxia of respiratory distress syndrome causes an oxidative stress response that promotes the continuous increase in levels of reactive oxygen species. Reactive oxygen species and their peroxidation products can activate transcription factors sensitive to redox and further initiate the release of various pro‐inflammatory factors to induce liver damage. The above pathophysiological changes can aggravate hepatocyte ischemia and hypoxia, affect the excretion of toxic metabolites, and further aggravate liver function injury. Therefore, ischemia and hypoxia may be among the main mechanisms for liver injury in patients with severe and critical COVID‐19 disease.
1.2.5. Drug hepatotoxicity
In China, the incidence of drug‐induced liver injury is second only to viral hepatitis and fatty liver disease (including alcoholic and nonalcoholic). Drugs commonly causing liver injury include traditional Chinese patent medicines containing components of saikosaponins, 38 , 39 antitumor drugs, antituberculosis drugs, antimalarial drugs, and antibiotics. 40 , 41 Most patients with COVID‐19 have fever, and many of them use antipyretic and analgesic drugs, which contain acetaminophen and are known to cause liver damage; overdose of these drugs can cause liver damage. Currently, there is no clearly effective antiviral drug, and many patients in the outbreak were given lopinavir, abidor, ritonavir, and other antiviral drugs. The latest study published in JCI 42 reported that CYP3A4 plays an important role in ritonavir's mediating of hepatotoxicity and the CYP3A metabolic pathways can produce electrophilic content, oxygen‐free radical, which can be covalent binding with macromolecular substances within the liver cells that cause system membrane lipid peroxidation, destruct membrane integrity and membrane Ca2+‐ATPase, disrupt cell internal and external Ca2+ homeostasis, and influence the function of critical organelles such as mitochondria, endoplasmic reticulum, and eventually lead to liver cell damage and even death. Moreover, the combination of overdose of lopinavir and ritonavir can activate the endoplasmic reticulum stress pathway in the liver, inhibit the proliferation of hepatocytes, induce apoptosis of hepatocytes through the caspase cascade system, induce inflammatory reactions, and accelerate liver injury by aggravating oxidative stress (Figure 2). 43 , 44 Some scholars believe that HIV protease inhibitors can effectively suppress SARS‐CoV‐2 replication, nevertheless, Shen's team confirmed that the risk of liver damage was increased in patients taking both hormones and HIV protease inhibitors. 45 The incidence of liver injury caused by different drugs varies, but the incidence increases with the increase in drug types. The diagnosis of drug‐induced liver injury requires the combination of medical history and relevant tests to exclude other liver diseases and assess the association between liver injury and suspected drugs by causality.
Figure 2.
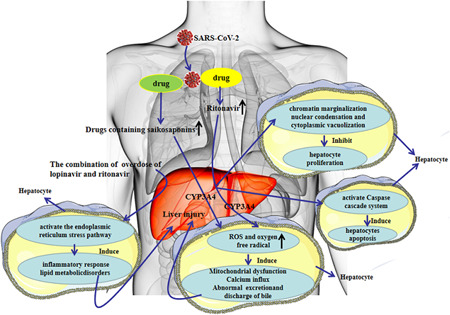
Drug hepatotoxicity in COVID‐19 patients. COVID‐19, coronavirus disease 2019; ROS, reactive oxygen species; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
1.2.6. Recurrence or aggravation of existing liver disease
Chronic liver disease is a major disease burden globally. Liver diseases, including chronic viral hepatitis, alcohol‐related liver disease, nonalcoholic fatty liver disease, and autoimmune hepatitis, affect approximately 300 million people in China. A retrospective analysis of 333 COVID‐19 inpatients 19 showed that 12 cases had a history of hepatitis B and 2 cases had a history of hepatitis C. In another study of baseline liver biochemical parameters in 324 cases in Shanghai area, the HBsAg‐positivity rate in COVID‐19 patients reached 6.5%. 46 Therefore, how different existing liver diseases influence liver injury in patients with COVID‐19 needs to be meticulously evaluated. Immune dysfunction, including lymphopenia and abnormal cytokine levels (including cytokine storms), is common in COVID‐19 patients and might be a risk factor associated with disease severity and mortality. For patients with COVID‐19 with autoimmune hepatitis, the effects of glucocorticoid administration on disease prognosis are unclear and deserve further investigation. For those patients with chronic hepatitis B who are in the immune tolerance phase, further studies are needed to confirm whether these patients have active viral replication and persistent liver injury after co‐infection with SARS‐CoV‐2. Moreover, COVID‐19 patients with systemic immunodeficiency status who have liver cirrhosis or liver cancer might be more susceptible to SARS‐CoV‐2.
From Hepatology, Chinese Medical Association, Wei 47 pointed out that for hepatitis B patients undergoing antiviral treatment, discontinuation of anti‐HBV drugs, or not receiving anti‐HBV therapy, may lead to reactivation and replication of HBV after high‐dose hormone therapy during SRSA‐CoV‐2 infection. Clinical pharmacological studies have shown that the combination of lopinavir and ritonavir can increase liver damage in patients with hepatitis B or hepatitis C infection, 48 therefore, the use of these two drugs to treat COVID‐19 patients with underlying liver disease will promote liver damage. A clinical study in Journal of Hepatology noted that long‐term administration of ribavirin for the treatment of hepatitis C infection may result in severe drug hepatotoxicity, which might be caused by drug interactions or metabolic reactions in vivo. 49
1.3. Prevention and treatment
For COVID‐19 patients with mild liver biochemical abnormalities, the primary disease should be treated actively when antiviral and supportive treatments are given to inhibit viral replication, reduce inflammation, and improve immunity, and preventive application of liver‐protecting and enzyme‐lowering drugs is not recommended. With acute liver injury, clinicians should analyze and judge the causes of liver injury and take appropriate measures, while closely monitoring ALT, AST, total bilirubin, direct bilirubin, albumin, and PTA (INR). The occurrence of acute liver failure should also be identified, and liver‐protecting and enzyme‐lowering drugs, for which the composition and mechanism of action are relatively clear, should be chosen. Nonetheless, too many drug types should not be administered (generally not more than 2), and the dosage should not be too large. For patients with severe and critical COVID‐19 disease with liver injury, which should be considered to be caused by cytokine storms and microcirculation ischemia and hypoxia, respiratory and circulatory support should be strengthened. If necessary, extracorporeal membrane oxygenation should be performed to improve the blood oxygen saturation of patients. Patients with acute liver failure should be given intensive monitoring and symptomatic and supportive treatment, and hypoproteinemia should be corrected. In cases of liver injury caused by drugs, in addition to conventional anti‐inflammatory liver protection treatment, stopping or reducing the amount of the suspected drugs should be considered, and the degree of liver damage should be assessed, followed by adjustment of the treatment plan. Anti‐HBV or anti‐HCV treatment should not be discontinued, and large doses of hormones should not be used simultaneously. Overall, the prevention and treatment experience of liver injury in SARS patients can provide some reference for COVID‐19 patients with liver injury. For example, active prevention and control of the inflammatory response in the early stage of the disease is not only conducive to reducing the nonspecific inflammation of the liver, but also prevent the occurrence of systemic inflammatory response syndrome to reduce the probability of mild disease developing into severe or critical disease.
2. CONCLUSIONS
COVID‐19 combined with liver injury is very common, and the incidence of liver injury in patients with severe or critical COVID‐19 disease is higher than that in patients with mild disease. Liver injury in patients with COVID‐19 may be caused by a variety of mechanisms, such as direct virus infection, immune injury, drug‐induced liver injury, systemic inflammatory response, ischemia and hypoxia, and recurrence or exacerbation of the underlying liver disease. As the liver is an important processing organ of the body, impaired liver function seriously affects the body's anabolism and prognosis of the disease. Therefore, clinicians should pay more attention to the occurrence of liver damage in the diagnosis and treatment of COVID‐19 and analyze comprehensively the pathogenesis of liver injury in COVD‐19 patients to develop a reasonable individualized treatment strategy.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Tian D, Ye Q. Hepatic complications of COVID‐19 and its treatment. J Med Virol. 2020;92:1818–1824. 10.1002/jmv.26036
REFERENCES
- 1. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bogoch II, Watts A, Thomas‐Bachli A, et al. Potential for global spread of a novel coronavirus from China. J Travel Med. 2020;27(2):taaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. [DOI] [PubMed] [Google Scholar]
- 4. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet. Respir Med. 2020;8(5):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu C, Jiang ZC, Shao CX, et al. Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study. Zhonghua Gan Zang Bing Za Zhi. 2020;28(2):148‐152. [DOI] [PubMed] [Google Scholar]
- 8. Chen L, Liu HG, Liu W, et al. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia]. Zhonghua Gan Zang Bing Za Zhi. 2020;28(2):148‐152. [Google Scholar]
- 9. Liu M, He P, Liu HG, et al. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(0):E016. [DOI] [PubMed] [Google Scholar]
- 10. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang C, Shi L, Wang F. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bangash MN, Patel J, Parekh D. COVID‐19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mao R, Liang J, Shen J, et al. Implications of COVID‐19 for patients with pre‐existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5(5):425–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu LL, Wang WJ, Zhu QJ, et al. Novel coronavirus pneumonia related liver injury: etiological analysis and treatment strategy. Zhonghua Gan Zang Bing Za Zhi. 2020;28(0):E001. [DOI] [PubMed] [Google Scholar]
- 15. Ren LL, Wang YM, Wu ZQ, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J. 2020;133(9):1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang SH, Han P, Xiao F, et al. Manifestations of liver injury in 333 hospitalized patients with coronavirus disease 2019. Chin J Digest. 2020;40(3):E008. [Google Scholar]
- 20. Yao N, Wang SN, Lian JQ, et al. Clinical characteristics and influencing factors of patients with novel coronavirus pneumonia combined with liver injury in Shaanxi region. Zhonghua Gan Zang Bing Za Zhi. 2020;28(0):E003. [DOI] [PubMed] [Google Scholar]
- 21. Guan WJ, Zhong NS. Clinical Characteristics of Covid‐19 in China. Reply. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PubMed] [Google Scholar]
- 22. Zhu NA, Zhang D, W W, et al. A novel coronavirus from patients with pneumonia in China, 2019. New Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chau TN, Lee KC, Yao H, et al. SARS‐associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39(2):302‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alsaad KO, Hajeer AH, Al BM, et al. Histopathology of Middle East respiratory syndrome coronovirus (MERS‐CoV) infection‐clinicopathological and ultrastructural study. Histopathology. 2018;72(3):516‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. The Lancet. Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tian SF, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID‐19) through post‐mortem core biopsies. Modern Pathology. 2020;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li YY, Xiao SY. Hepatic involvement in COVID‐19 patients: pathology, pathogenesis and clinical implications [review]. J Med Virol. 2020:1–4. [DOI] [PubMed] [Google Scholar]
- 28. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China: Life Sci. 2020;63(3):457‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS‐CoV‐2 and other lineage β‐coronaviruses. Nat Microbiol. 2020;5(4):562‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ch XQ, Hu LF, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. BioRxiv. 2020. 10.1101/2020.02.03.931766 [DOI] [Google Scholar]
- 31. Zhang Y, Zheng L, Liu L, Zhao Z, Xiao J, Zhao Q. Liver impairment in COVID‐19 patients: a retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver Int. 2020:1–9. [DOI] [PubMed] [Google Scholar]
- 32. Tirado SM, Yoon KJ. Antibody‐dependent enhancement of virus infection and disease. Viral Immunol. 2003;16(1):69‐86. [DOI] [PubMed] [Google Scholar]
- 33. Wang SF, Tseng SP, Yen CH, et al. Antibody‐dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451(2):208‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mahallawi WH, Khabour OF, Zhang Q, et al. MERS‐CoV infection in humans is associated with a pro‐inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li X, Li X, Huang N, et al. A comprehensive review and perspectives on pharmacology and toxicology of saikosaponins. Phytomedicine. 2018;50:73‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li X, Li X, Lu J, et al. Saikosaponins induced hepatotoxicity in mice via lipid metabolism dysregulation and oxidative stress: a proteomic study. BMC Complement Altern Med. 2017;17(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pan X, Zhou J, Chen Y, et al. Classification, hepatotoxic mechanisms, and targets of the risk ingredients in traditional Chinese medicine‐induced liver injury. Toxicol Lett. 2020;323:48‐56. [DOI] [PubMed] [Google Scholar]
- 41. Iorga A, Cell LD. Death in drug‐induced liver injury. Adv Pharmacol. 2019;85:31‐74. [DOI] [PubMed] [Google Scholar]
- 42. Shehu AI, Lu J, Wang P, et al. Pregnane X receptor activation potentiates ritonavir hepatotoxicity. J Clin Invest. 2019;129(7):2898‐2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zha BS, Wan X, Zhang X, et al. HIV protease inhibitors disrupt lipid metabolism by activating endoplasmic reticulum stress and inhibiting autophagy activity in adipocytes. PLOS One. 2013;8(3):e59514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cao R, Hu Y, Wang Y, et al. Prevention of HIV protease inhibitor‐induced dysregulation of hepatic lipid metabolism by raltegravir via endoplasmic reticulum stress signaling pathways. J Pharmacol Exp Ther. 2010;334(2):530‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shen T, Liu Y, Shang J, et al. Incidence and Etiology of Drug‐Induced Liver Injury in Mainland China. Gastroenterology. 2019;156(8):2230‐2241.e11. [DOI] [PubMed] [Google Scholar]
- 46. Qian ZP, Mei X, Zhang YY, et al. Analysis of baseline liver biochemical parameters in 324 cases with novel coronavirus pneumonia in Shanghai area. Zhonghua Gan Zang Bing Za Zhi. 2020;28(0):E005. [DOI] [PubMed] [Google Scholar]
- 47. Wei L. The protocol for prevention, diagnosis and treatment of corona virus infective disease 2019. Journal of Clinical Hepatology. 2020;28:E004. [Google Scholar]
- 48. Motor S, Alp H, Senol S, et al. Comparison of the chronic effects of ribavirin and caffeic acid phenethyl ester (CAPE) on pancreatic damage and hepatotoxicity. Int J Clin Exp Med. 2014;7(4):1005‐1013. [PMC free article] [PubMed] [Google Scholar]
- 49. Dyson JK, Hutchinson J, Harrison L, et al. Liver toxicity associated with sofosbuvir, an NS5A inhibitor and ribavirin use. J Hepatol. 2016;64(1):234‐238. [DOI] [PubMed] [Google Scholar]


