Abstract
Clinically approved PARP inhibitors (PARPi) have a mild adverse effect profile and are well tolerated as continuous daily oral therapy. We review the evidence that justifies the repurposing of PARPi to block the proliferation of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and combat the life‐threatening sequelae of coronavirus disease 2019 (COVID‐19) by several mechanisms. PARPi can effectively decrease IL‐6, IL‐1 and TNF‐α levels (key interleukins in SARS‐CoV‐2‐induced cytokine storm) and can alleviate subsequent lung fibrosis, as demonstrated in murine experiments and clinical trials. PARPi can tune macrophages towards a tolerogenic phenotype. PARPi may also counteract SARS‐CoV‐2‐induced and inflammation‐induced cell death and support cell survival. PARPi is effective in animal models of acute respiratory distress syndrome (ARDS), asthma and ventilator‐induced lung injury. PARPi may potentiate the effectiveness of tocilizumab, anakinra, sarilumab, adalimumab, canakinumab or siltuximab therapy. The evidence suggests that PARPi would benefit COVID‐19 patients and trials should be undertaken.
Abbreviations
- ARDS
acute respiratory distress syndrome
- AIF
apoptosis‐inducing factor
- COPD
chronic obstructive pulmonary disease
- CoVs
coronaviruses
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- HRR
homologous recombination repair
- ssRNA+
positive‐sense single‐stranded RNA
- RNS
reactive nitrogen species
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TMPRSS2
type II transmembrane serine protease
- VILI
ventilation‐induced lung damage
- WHO
World Health Organization
1. CHARACTERISTICS OF THE SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 (CORONAVIRUS DISEASE 2019) INFECTION
The newly emerging coronavirus disease, coronavirus disease 2019 (COVID‐19), is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) (Zhu et al., 2020). The SARS‐CoV‐2 belongs to the Coronaviridae family, being genetically related to the human pathogen SARS‐CoV‐1 and MERS‐CoV and to a number of bat‐origin coronaviruses (CoVs) (Lu et al., 2020). SARS‐CoV‐2 is an enveloped virus that has a positive‐sense single‐stranded RNA (ssRNA+) genome of nearly 30,000 nucleotide in length (Zhu et al., 2020).
The first site of viral infection is the upper respiratory tract. At a later stage of infection, the virus may disseminate to and replicate in the lower respiratory tract (Zhu et al., 2020). Viral RNA and infectious virus can be found in the nasopharyngeal swab and the sputum. In addition, infection of the gastrointestinal tract has been reported and infectious viruses can be isolated from faecal specimens (Xiao et al., 2020). The virus uses the ACE2 as cellular receptor and a type II transmembrane serine protease, TMPRSS2, as co‐factor that activates the attachment protein of SARS‐CoV‐2 (Lu et al., 2020) to aid viral entry into epithelial cells of the respiratory and in the gastrointestinal tract (Xiao et al., 2020). In the majority of the cases, patients have very mild symptoms. However, a considerable portion of the patients develop severe symptoms and require intensive care and mechanical ventilation; this patient group shows an increased risk for death (Chen et al., 2020;Guan et al., 2020; Huang et al., 2020). The risk factors for complications and mortality include pre‐existing cardiovascular, metabolic or neoplastic diseases and older age (Nikolich‐Zugich et al., 2020; Zhou et al., 2020). Men have higher chance for developing severe symptoms, as well as, for fatal outcome (Guan et al., 2020; Huang et al., 2020).
Although the pathogenesis of COVID‐19 is not fully understood, immunopathological mechanisms are thought to play an important role. A detrimental sequel of SARS‐CoV‐2 virus infection is a cytokine storm (also called cytokine release syndrome or macrophage overactivation syndrome) that occurs after the elimination of the virus and culminates in multiple organ failure (Mehta et al., 2020; Shi et al., 2020). Cytokine storm may be responsible for a considerable fraction of adverse outcomes in SARS‐CoV‐2 infection (Shi et al., 2020). The reason for the absence of the adaptive response is unknown. In clinical practice, tocilizumab, an antibody against IL‐6 (Luo et al., 2020; McGonagle, Sharif, O'Regan, & Bridgewood, 2020; Toniati et al., 2020) and anakinra, an IL‐1 receptor antagonist (Adam Monteagudo, Boothby, & Gertner, 2020; McGonagle et al., 2020), were used successfully to cope with SARS‐Cov‐2‐induced cytokine storm suggesting the involvement of these interleukins. Other drugs, primarily used in rheumatological settings, are also suggested to be used to block cytokine storm (Ceribelli et al., 2020).
The current treatment options in SARS‐CoV‐2 infection are limited to drugs empirically tested in the clinical setting. The unmet medical need posed by SARS‐CoV‐2 infection calls for rapid repositioning of available drugs. These drugs are mostly antiviral drugs (e.g. lopinavir–ritonavir combinational therapy and remdesivir) or anti‐inflammatory drugs, as noted above. None of the current COVID‐19 treatments are supported by prospective randomized clinical trials and therefore, all of these treatments should be considered with caution. The World Health Organization (WHO) initiated a large‐scale drug repositioning study to rapidly assess the most promising candidates to fight SARS‐CoV‐2 pandemic. In this position review, we are reviewing the applicability of poly (ADP‐ribose) polymerase inhibitors (PARPi) and their possible interactions with known therapeutic options in SARS‐CoV‐2 infection.
2. BRIEF INTRODUCTION TO THE BIOLOGY OF POLY (ADP‐RIBOSE) POLYMERASES (PARPs)
PARP/ARTD enzymes constitute a family of 17 members in humans (PARP1–PARP17) (Ame, Spenlehauer, & de Murcia, 2004). When activated, PARPs cleave their substrate, NAD+, and couple the resulting ADP‐ribose units onto acceptor proteins forming monomers, oligomers or polymers of ADP‐ribose. The polymer of ADP‐ribose is called PAR. The large, negatively charged monomers, oligomers or polymers heavily impact on the behaviour of target proteins and a huge number of proteins were shown to be ADP‐ribosylated or PARylated on multiple different amino acids (Bai, 2015). In addition, ADP‐ribose units can serve as binding surface for proteins (Tartier et al., 2003).
Macrodomain‐containing proteins constitute a small protein family (Karlberg, Langelier, Pascal, & Schuler, 2013). Macrodomains can bind to ADP‐ribose units and some can hydrolyse ADP‐ribose units upon binding (Karlberg et al., 2013). Through binding or hydrolysing ADP‐ribose, macrodomain‐containing proteins can translate ADP‐ribosylation signals into cellular adaptation programmes. Macrodomains can be found in all domains of life, including viruses (Fehr, Jankevicius, Ahel, & Perlman, 2018) and, more specifically, CoVs (Grunewald et al., 2019).
PARP1 is responsible for ~85–90% of cellular PARP activity, PARP2 is responsible for 10–15%, while the rest of the enzymes share the remainder of all cellular PARP activity (Schreiber et al., 2002). Active PARPs represent a large burden on cellular NAD+ levels (Sims, Berger, & Berger, 1983). Although, originally, excess PARP activation is linked to DNA damage‐associated pathologies, recent advances showed that PARP activity under physiological conditions also represents a large burden on cellular NAD+ levels (Bai et al., 2011; Bai et al., 2011; Mohamed, Hajira, Pardo, & Boriek, 2014).
PARP1, PARP2 and PARP3 can be activated by binding to irregular or damaged DNA (Bai, 2015) that is often the result of ROS or reactive nitrogen species (RNS) production under inflammatory conditions (Bai & Virag, 2012). The activation of PARP1, PARP2 and PARP3 is vital for initiating DNA repair and the resolution of irregular DNA structures and modulating chromatin structure. Through these, certain PARP isoforms (chiefly, PARP1) are involved in recombination and transcription events that encompass changes to DNA structure (Bai, 2015). Importantly, PARP2 can bind to RNA, which may activate the enzyme (Leger, Bar, Savic, Santoro, & Hottiger, 2014). There are other pathways to activate PARPs involving signal transduction pathways (Bai, 2015). PARP enzymes were shown to be involved in transcription, mRNA handling and polypeptide elongation (for comprehensive reviews, see Kim, Challa, Jones, & Kraus, 2020).
PARP enzymes are involved in a wide variety of cellular processes; among these, their impact in cell death, immune function (Bai & Virag, 2012; Fehr et al., 2020), antiviral response (Fehr et al., 2018; Fehr et al., 2020), transcription, translation (Kim et al., 2020) and autophagy (Rodríguez‐Vargas, Oliver‐Pozo, & Dantzer, 2019) are relevant for our discussion. PARPi that are in current clinical use (olaparib, rucaparib, niraparib and talazoparib) are inhibitors of PARP1, PARP2 and PARP3 (Wahlberg et al., 2012).
3. THERAPEUTIC POTENTIAL OF PARPi
3.1. Anti‐inflammatory effects of PARPi and modulation of cell death—Combatting the cytokine storm
The protective effect of PARPi, which is applicable to non‐oncological models of inflammation, was first suggested by Nathan Berger's group nearly 40 years ago (Sims et al., 1983). They noted that DNA damage‐induced cell death was associated with PARP activation, resulting in massive depletion of its substrate, NAD+, and consequently ATP that resulted in necrosis. Depletion of these pools was prevented in the presence of a PARPi and necrosis reduced. Subsequently, it has also been shown that the product of the PARP reaction, that is, PAR, can also trigger apoptosis‐inducing factor (AIF) release that promotes a specific programmed cell death pathway called “parthanatos” (Fatokun, Dawson, & Dawson, 2014). Relevant to inflammatory normal tissue damage in general and acute respiratory distress syndrome (ARDS) in particular is the role of oxidative and nitrosative stress, which is integral to inflammation and causes DNA strand breakage that massively activates PARP (Bai & Virag, 2012; Ke et al., 2019). Indeed, PARP can actively increase and prolong inflammation through a vicious cycle of ROS/RNS‐induced DNA damage, PARP‐mediated necrosis and increase in inflammatory cytokines (Figure 1). PARPi have been shown to limit inflammation‐induced normal tissue damage, including acute lung injury, in animal models (reviewed in Pazzaglia & Pioli, 2019; Virág, 2005).
FIGURE 1.
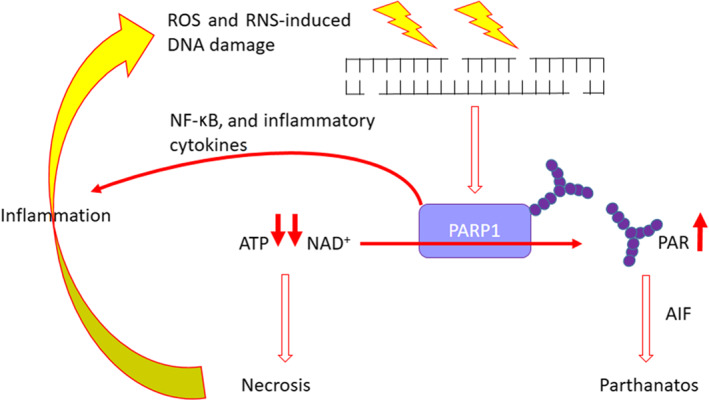
The role of PARP in inflammation‐induced tissue damage. Infection‐induced ROS and reactive nitrogen species (RNS) cause DNA strand breakage. This activates PARP1 and PARP2, which generates PAR that can lead to apoptosis‐inducing factor (AIF) release leading to cell death by parthanatos. This reaction also depletes NAD+ and subsequently ATP leading to necrosis, which causes further inflammation and activation of immune cells (e.g. macrophages) leading to more ROS and RNS generation and, so, a vicious cycle of further PARP activation and downstream consequences. PARP activation also stimulates the production of inflammatory cytokines, such as IL‐6, further contributing to this vicious cycle. All of this can essentially be blocked by the use of a PARPi
Reactive species production increases in SARS‐CoV‐2 (COVID‐19) infection (Kouhpayeh et al., 2020; Sawalha, Zhao, Coit, & Lu, 2020; Wang et al., 2020; Zhang, Wu, Li, Zhao, & Wang, 2020) that makes PARP activation and PARP activation‐mediated cell death likely. Pharmacological PARP inhibition is likely to reduce cell death under these conditions as suggested by the study on another ssRNA(+) virus, the Zika virus. Zika virus‐induced cell death was effectively blocked by PARP inhibition (Xu et al., 2019). The source of the reactive species can be diverse, stemming from activated immune (e.g. macrophages) or dysfunctional cells. Interestingly, the nucleocapsid protein of SARS‐CoV‐1 can induce ROS production in non‐immune cells, so that can be a pathway in the case of SARS‐Cov‐2 as well (Zhang et al., 2007). In fact, a pre‐publication paper (Heer et al., 2020) already suggested that SARS‐CoV‐2 infection induces PARP activity.
PARP1 is the best characterized member of the PARP family from the perspective of immune processes. PARP1 seems to have mostly pro‐inflammatory properties in terms of Th1‐ and Th2‐mediated processes (Bai & Virag, 2012; Fehr et al., 2020). PARP2 has limited role in pro‐inflammatory processes (Bai & Virag, 2012). Pharmacological PARP inhibition is generally anti‐inflammatory. PARP1 is involved in regulating innate and adaptive immunity (Fehr et al., 2020) through mediating signal transduction and transcription events in multiple immune cell lines that is translated to differential expression of cytokines and chemokines, as well as their receptors (Bai & Virag, 2012; Fehr et al., 2020). In humans, there is evidence that the polymorphism in PARP1 (V762A) that confers reduced activity is associated with reduced risk of asthma (Ozaydin et al., 2014). PARP activity is increased in the lung tissues of asthmatic patients and in mouse models, the PARPi, olaparib, prevents asthma, indicating that the extrapolations from preclinical models to human conditions are valid (Ghonim et al., 2015). Also, PARP inhibition confers protection to the lungs upon acute lung injury inflicted by various noxae (burns, smoke inhalation, bacterial infection, etc.) (Hamahata et al., 2012; Murakami et al., 2004) in an NF‐κB‐dependent fashion (Ahmad et al., 2015; Wang et al., 2013). Various PARPi (3‐AB, PJ‐34 and INO‐1001) have been shown to be protective in various preclinical models of acute respiratory distress syndrome by reducing the production of inflammatory mediators and preventing the depletion of NAD+ and ATP and also the deterioration of barrier function that may contribute to exudate formation in acute respiratory distress syndrome (reviewed in Virág, 2005). The most common animal model of human acute lung injury/acute respiratory distress syndrome is intratracheal instillation of LPS. PARP plays an important role in the pathogenesis of LPS‐mediated acute respiratory distress syndrome damage by a variety of mechanisms. Both genetic disruption of PARP1 and inhibition of PARP ameliorate acute respiratory distress syndrome and acute respiratory distress syndrome‐associated tissue damage (reviewed in Sethi, Dharwal, & Naura, 2017). Following LPS administration, olaparib not only reduced inflammatory cell infiltration in the lung and pulmonary oedema and exudate but also reduced secondary kidney injury (Kapoor, Singla, Sahu, & Naura, 2015). Relevance to human lung disease comes from the observation that in cases of chronic obstructive pulmonary disease (COPD), plasma NAD+ is low and DNA damage, PARP activity and the percentage of PAR positive lymphocytes were higher than in control subjects (Hageman et al., 2003; Oit‐Wiscombe, Virag, Soomets, & Altraja, 2013).
PARP inhibition has an additional lung protective feature. PARPi are able to reduce lung fibrosis, a common sequel of SARS‐Cov‐2 lung inflammation, in different preclinical animal models (Carlile et al., 2016; Hoyt & Lazo, 1992, 1993; Lucarini et al., 2017). PARP activity in idiopathic lung fibrosis patients was higher than in healthy controls (Hu et al., 2013), suggesting that the findings of the animal models can be translated to the human situation.
As we noted above, a major reason of mortality in SARS‐CoV‐2 (COVID‐19) infection is the macrophage overactivation that leads to cytokine storm and, consequently, to multi‐organ failure (Vaninov, 2020). To date, IL‐6 and IL‐1 were implicated in SARS‐CoV‐2 (COVID‐19)‐induced cytokine storm (Conti et al., 2020; McGonagle et al., 2020), however the involvement of TNF‐α is also likely. PARPi, including olaparib, can reduce the expression of IL‐6 in multiple organs, including the lung (Ghonim et al., 2015; Kim et al., 2008; Liaudet et al., 2002; Pagano et al., 2007; Sahu, Narota, & Naura, 2020) in animal models. Importantly and in good agreement with the previous dataset, pharmacological PARP inhibition using INO‐1001 in humans reduced serum IL‐6 and C‐reactive protein (CRP) levels (Morrow et al., 2009), further reinforcing the notion that data from preclinical studies can be translated to humans.
Another interleukin implicated in eliciting the cytokine storm in SARS‐CoV‐2 (COVID‐19) infection is IL‐1 (Conti et al., 2020; McGonagle et al., 2020). PARPi, including olaparib, can decrease IL‐1β expression in animal models (Liaudet et al., 2002; Mabley et al., 2001; Sahu et al., 2020; Sethi, Sharma, & Naura, 2019) that, again points out the applicability of PARPi to dampen IL‐1 expression in humans. Finally, pharmacological PARP inhibition, including with the clinically approved PARPi, olaparib, reduced TNF‐α levels in the lungs in various animal models of lung inflammation (Cuzzocrea et al., 2002; Kim et al., 2008; Liaudet et al., 2002; Sahu et al., 2020; Virag et al., 2004).
The data we presented suggest that PARP inhibition can counteract inflammation‐induced cell death and possibly counteracts the acute respiratory distress syndrome‐like features and cytokine storm through blocking macrophage overactivation (cytokine storm) through down‐regulating the expression of IL‐6, IL‐1, and TNF‐α in humans. These events represent a vicious circle that is sustained by PARP1 activation. Therefore, PARPi can break the chain of events at multiple loci (as suggested in Jagtap & Szabo, 2005) and exert cytoprotective effects on the pulmonary epithelial and endothelial cells.
3.2. Ventilator‐induced lung injury
SARS‐Cov‐2‐infected patients requiring intensive care often need mechanical ventilation over extended periods (Huang et al., 2020). Pharmacological inhibition of PARP reduced the inflammatory component of ventilation‐induced lung damage in a murine model (Kim et al., 2008). Furthermore, in another rat preclinical model of severe acute lung injury, the PARPi PJ‐34 protected the kidney from injury following mechanical ventilation (Vaschetto et al., 2010). Similar findings were obtained in an ovine model, where acute lung injury was induced by smoke inhalation and local Pseudomonas aeruginosa colonization (Murakami et al., 2004). It appears that in ventilation‐induced lung damage (VILI), PARP inhibition breaks a similar vicious cycle as in acute respiratory distress syndrome or other inflammatory models (Jagtap & Szabo, 2005). The similarity of the findings in rodent and large animal models supports the potential for translating these findings to the human situation.
3.3. Antiviral effects of PARPs and its interactions with CoVs
Several members of the PARP family are involved in host–virus interactions, and by our current understanding, most PARP enzymes have antiviral properties (Fehr et al., 2020; Grunewald, Shaban, Mackin, Fehr, & Perlman, 2020). PARPs impact on multiple steps of the viral life cycle. These steps are mostly related to the nucleic acid‐binding properties of PARPs. PARPs can interfere with viral integration, recombination, and transcription (Fehr et al., 2020); however, these are not relevant in the context of CoVs.
PARP13 was shown to be induced upon orthomyxovirus or alphavirus infection and to have protective function (Fehr et al., 2020). Interferon (IFN) treatment induces a set of PARP enzymes PARP9, PARP10, PARP12 and PARP14 in cells (Grunewald et al., 2019). These PARP enzymes inhibit viral translation probably through ADP‐ribosylating key cellular proteins (Grunewald et al., 2019).
Members of the virus families Coronaviridae, Togaviridae and Hepeviridae encode macrodomain proteins that bind to and hydrolyse ADP‐ribose from proteins and are critical for optimal replication and virulence (Grunewald et al., 2019; Grunewald et al., 2020). The conserved CoV macrodomain in SARS‐CoV was found to play a role in viral replication and to suppress IFN and cytokine production (Grunewald et al., 2019; Grunewald et al., 2020). Interestingly, the nucleocapsid protein of SARS‐CoV and a number of other CoVs are ADP‐ribosylated (Grunewald, Fehr, Athmer, & Perlman, 2018), yet the significance of this post‐translational modification is unexplored.
Since PARPi have preference towards PARP1, PARP2 and PARP3 (Wahlberg et al., 2012), it is unlikely that PARPi treatment would interfere with the antiviral effects of the minor PARP isoforms. An interesting and potentially relevant finding is that another PARPi, PJ34, can form a complex with the nucleocpasid protein of the human CoV CoV‐OC43 and, hence, can hinder its RNA‐binding affinity (Lin et al., 2014).
3.4. Interactions with drugs proposed for COVID‐19 therapy
The WHO initiated a large‐scale drug repurposing clinical trial entitled “Solidarity” (WHO, 2020). In the frame of this trial, four treatment schemes will be assessed:‐ (a) remdesivir, (b) lopinavir–ritonavir combination, (c) chloroquine/hydroxychloroquine and (d) IFN‐β. Empirical clinical data suggest the applicability of these biological therapy drugs to combat cytokine storm. Here, we will evaluate the potential for PARPi in combination with the drugs investigated in the Solidarity trial. Combination therapy is particularly advantageous, since it requires lower doses with better tolerability of the components. As we noted earlier, none of the current COVID‐19 treatments are supported by prospective randomized clinical trials, and therefore, all of these treatments should be considered with caution.
To date, PARPi have not been reported to interact with ritonavir, lopinavir, or remdesivir. IFN‐β has pleiotropic antiviral actions, and therefore, probably, IFN‐β can induce the antiviral function of PARPs.
Chloroquine and hydroxychloroquine are antimalarial and antirheumatic drugs that were assessed in small‐scale cohorts in France and China (Gao, Tian, & Yang, 2020; Gautret et al., 2020). Although criticism was raised concerning the available studies and the potential cardiotoxicity of both drugs, the WHO included chloroquine and hydroxychloroquine to kick off a multicentre, large population study. Chloroquine and hydroxychloroquine probably block the processing of SARS‐CoV‐2 by blocking the acidification of the vesicles containing the virus, in a process biochemically similar to the process of autophagy. Of note, the genetic or pharmacological inhibition of PARP1 blocks the early steps of autophagy (Rodríguez‐Vargas et al., 2019). Furthermore, the genetic or pharmacological inhibition of PARP2 blocks the degradation of the cargo of autophagic vesicles mimicking the action of chloroquine (Janko et al., 2020). From these observations, one may extrapolate that PARPi could potentiate the antiviral effects of chloroquine or hydroxychloroquine and could be exploited to achieve dose reduction of chloroquine or hydroxychloroquine. It is also of note that ADP‐ribosylation‐mediated autophagic processes were found to be important in the pathogenesis of other microorganisms such as Legionella in high‐profile studies (e.g. Kalayil et al., 2018). Nevertheless, links between PARPi and chloroquine are purely hypothetical.
IL‐6 is a key interleukin in sustaining cytokine storm following SARS‐CoV‐2 infection (Zhang et al., 2020). Studies have shown that anti‐IL‐6 immunotherapy using tocilizumab was beneficial for patients (Luo et al., 2020; McGonagle et al., 2020). As we noted earlier, PARP inhibition was shown to reduce IL‐6 expression in humans; therefore, it is possible that PARP inhibition could also potentiate the effects of anti‐IL‐6 (tocilizumab and siltuximab) or anti‐IL‐6 receptor (sarilumab) treatment.
Preclinical data suggest that PARPi can reduce IL‐1 (Liaudet et al., 2002; Mabley et al., 2001; Sethi et al., 2019) and TNF‐α expression (Kim et al., 2008; Liaudet et al., 2002; Virag et al., 2004). It is possible therefore that PARPi could potentiate canakinumab (IL‐1β antibody), anakinra (IL‐1 receptor antagonist) and adalimumab (TNF‐α antibody) therapy.
4. AVAILABLE PARPi AND THEIR CURRENT SCOPE OF USE
Four PARPi are approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) for cancer therapy. Although the original rationale for their development was to overcome DNA repair‐mediated resistance to DNA damaging anticancer therapy, their approval has been as single agents exploiting tumour‐specific defects in the cDNA repair pathway homologous recombination repair (HRR) by a process known as synthetic lethality.
Currently, PARPi are only approved for cancer therapy as single agents (Table 1). The first to be approved was olaparib, now called Lynparza®. Originally approved by the FDA at the end of 2014 at a dose of 400 mg twice daily in capsule formulation, it is now approved in tablet formulation at 300 mg twice daily for maintenance (including front‐line) therapy in ovarian cancer. It is also approved at the same dose and schedule for metastatic breast and pancreatic cancer in individuals with germline BRCA mutations. Also approved in ovarian cancer are rucaparib (Rubraca®) at 600 mg p.o. twice daily and niraparib (Zejula®) 300 mg p.o. once daily. Talazoparib (Talzenna®) is the most potent of all the PARPi and has been approved at dose of only 1 mg daily for germline BRCA‐mutated metastatic breast cancer. Veliparib is a less potent PARPi that has so far failed to show sufficient single‐agent activity for approval but has been in advanced clinical trial alone and in combination with other anticancer agents for over a decade.
TABLE 1.
Human studies involving Phase IV PARP inhibitors
| Drug name | Dose and schedule | Indication | FDA approval | NCT# and trial details | Reference |
|---|---|---|---|---|---|
| Olaparib | 400 mg capsules b.i.d. | Maintenance therapy in platinum‐sensitive relapsed serous ovarian cancer | December 2014 | NCT00753545 Phase II | (Ledermann et al., 2012) |
| Tablets 300 mg b.i.d. | First‐line maintenance therapy in BRCA‐mutated ovarian cancer after platinum therapy | August 2017 for tablet formulation and December 2018 for maintenance | NCT01874353 SOLO‐2 Phase III and NCT01844986 SOLO‐1 Phase III | (Moore et al., 2018; Pujade‐Lauraine et al., 2017) | |
| Tablets 300 mg b.i.d. | BRCA‐mutated breast cancer | January 2018 | NCT02000622 Phase III | (Robson et al., 2017) | |
| Tablets 300 mg b.i.d. | First‐line maintenance therapy in gBRCA‐mutated pancreatic cancer | December 2019 | NCT02184195 POLO Phase III | (Golan et al., 2019) | |
| Rucaparib | Oral 600 mg b.i.d. | Maintenance therapy in platinum‐sensitive, relapsed ovarian, fallopian tube, or primary peritoneal cancer | Accelerated December 2016 with companion diagnostic and April 2018 independent of companion diagnostic | NCT01482715 Study 10 Phase I/II, NCT01891344 Ariel 2 Phase III, and NCT01968213 Ariel 3 Phase III | (Coleman et al., 2017; Kristeleit et al., 2017; Oza et al., 2017; Swisher et al., 2017) |
| Niraparib | Oral 300 mg daily | Maintenance therapy in platinum‐sensitive ovarian cancer | March 2017 additionally October 2019 for maintenance after 4 chemotherapy regimes | NCT01847274 ENGOT‐OV16/NOVA Phase III | (Mirza et al., 2016) |
| Single‐agent oral 300 mg daily | Maintenance therapy in ovarian cancer after 4 previous chemotherapy regimens | October 2019 | NCT02354586 QUADRA Phase III | (Moore et al., 2019) | |
| Talazoparib | 1 mg daily | gBRCA HER2 negative locally advanced or metastatic breast cancer | October 2018 | NCT01945775 EMBRCA Phase III | (Litton et al., 2018) |
| Veliparib | Combination with carboplatin and paclitaxel | Non‐small‐cell lung cancer | Orphan status November 2016 | NCT02106546 | (Ramalingam et al., 2017) |
Note: The table encompasses only the clinically approved PARPi.
Abbreviation: FDA, Food and Drug Administration.
Due to their tumour‐specific synthetic lethality, their toxicity as monotherapies is mild and manageable. The common adverse effects are fatigue, gastrointestinal (nausea/vomiting, abdominal pain, and diarrhoea) and haematological (neutropenia for olaparib, anaemia for rucaparib and talazoparib, and thrombocytopenia for niraparib), mostly Grade 1–2 (LaFargue, Dal Molin, Sood, & Coleman, 2019; Murthy & Muggia, 2019). Myelodysplastic syndrome and acute myeloid leukaemia have also been noted, but this may have been caused mainly by prior chemotherapy, particularly platinum‐based therapies (Murthy & Muggia, 2019). In combination with DNA damaging therapies, the safe dose is much lower and the tolerated schedules are much shorter. For example, the approved dose of rucaparib (Rubraca®) is 600 mg p.o. 2× daily continuously and the safe dose in combination with carboplatin is 240 mg once daily for 14 days every 21 days and only 18 mg·m−2 i.v. (equivalent to 60 mg p.o.) for 5 days every 28 days in combination with temozolomide (Curtin, 2020).
It is anticipated that the doses needed for non‐oncological diseases, for example, acute respiratory distress syndrome, are likely to be lower (and hence less toxic) for two reasons. Firstly, the doses found to be effective preclinically in non‐oncological models are 1–2 orders of magnitude lower than the monotherapy doses used in cancer models (reviewed in Berger et al., 2018). This is likely to reflect the intracellular concentrations needed to maintain NAD+ pool from excessive depletion versus those needed to completely block repair for a sustained period. Secondly, the approved doses are largely based on dose escalation Phase I trials where the endpoint is tolerability, which may not be appropriate for a tumour‐specific drug and may be well in excess of the effective dose.
5. CONCLUDING REMARKS
The evidence we describe above indicates that PARPi may have beneficial effects in SARS‐CoV‐2 infection and its sequelae by preventing macrophage overactivation and the subsequent cytokine storm, as well as by protecting cells against cell death. It is also of note that PARPi were protective against risk factors for bad clinical outcomes of SARS‐CoV‐2 infection, such as cardiovascular and metabolic diseases (Bai, 2015). Importantly, PARPi are cytoprotective in the central nerve system (Fatokun et al., 2014) and the cardiovascular system (Bai, 2015), the systems that are damaged in COVID‐19 patients with bad clinical outcome.
Of particular interest regarding COVID‐19 is the sex bias of the mortality statistics. Men are more than twice as likely to die as women (Guan et al., 2020; Huang et al., 2020). Previous research has shown that men have on average 40% higher PARP activity than women, at least in their peripheral blood mononuclear cells (PBMCs), with similar sex differences in mice (Mabley et al., 2005; Zaremba et al., 2011). In most preclinical studies where the protective effects of PARPi were evaluated, only male animals have been used. However, in those studies where both sexes were included, the protective effects of PARPi were less pronounced in females (reviewed in Berger et al., 2018; Curtin & Szabo, 2013). There are no data for sex differences in PARP activity in human lung tissue due to the difficulty of obtaining viable normal lung tissue. However, in humans following traumatic brain injury, men were 2.6 times more likely than women to have elevated PAR‐modified proteins in their CSF after comparable levels of trauma and strikingly similar to the studies in PBMCs, the mean PAR level was approximately 40% higher in males than females (Sarnaik et al., 2010).
Currently, there are over 600 registered clinical trials investigating COVID‐19, not one of which includes a PARPi. The preclinical evidence suggests that 5–10% of the currently approved PARPi doses would be sufficient/effective and tolerable. Finally, given there has been no FDA‐approved therapy for acute respiratory distress syndrome (reviewed in Standiford & Ward, 2016), we believe that the preclinical data and scientific rationale justify investigations with (low dose?) PARPi.
6. SEARCH STRATEGY AND SELECTION CRITERIA
References to this review were identified through the prior knowledge of the authors that was complemented by systematic search of PubMed by using the combinations “PARP IL‐6,” “PARP IL‐1,” “PARP TNFa/alpha,” “PARP virus,” “PARP SARS,” “virus macrodomain,” “PARP fibrosis lung,” “PARP remedsivir,” “PARP ritonavir,” “PARP lopinavir,” “PARP chloroquine,” and “PARP hydroxychloroquine.” Articles published in English were included with no restriction on publication date.
6.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
CONFLICT OF INTEREST
N.C. reports grants from AICR during the conduct of the study. In addition, N.C. has a patent WO 2005/012305 A2 with royalties paid to CRUK and Newcastle University and a patent WO/2006/033006 with royalties paid to CRUK and Newcastle University. The other authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
Our work was supported by grants from the Hungarian Scientific Research Fund NKFIH (K123975, GINOP‐2.3.2‐15‐2016‐0000, EFOP‐3.6.2‐16‐2017‐00008, GINOP‐2.3.2 STAY ALIVE, and 20765‐3/2018/FEKUTSTRAT) and by the Faculty of Medicine at the University of Debrecen. The research was financed by the Higher Education Institutional Excellence Program (NKFIH‐1150‐6/2019) of the Ministry of Innovation and Technology in Hungary, within the framework of the Biotechnology thematic programme of the University of Debrecen.
Curtin N, Bányai K, Thaventhiran J, Le Quesne J, Helyes Z, Bai P. Repositioning PARP inhibitors for SARS‐CoV‐2 infection(COVID‐19); a new multi‐pronged therapy for acute respiratory distress syndrome? Br J Pharmacol. 2020;177:3635–3645. 10.1111/bph.15137
REFERENCES
- Adam Monteagudo, L. , Boothby, A. , & Gertner, E. (2020). Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol, 2(5), 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, S. F. , Zoheir, K. M. , Ansari, M. A. , Korashy, H. M. , Bakheet, S. A. , Ashour, A. E. , … Attia, S. M. (2015). The role of poly (ADP‐ribose) polymerase‐1 inhibitor in carrageenan‐induced lung inflammation in mice. Molecular Immunology, 63(2), 394–405. 10.1016/j.molimm.2014.09.009 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , … Southan, C. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Introduction and other protein targets. British Journal of Pharmacology, 176(Suppl 1), S1–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ame, J. C. , Spenlehauer, C. , & de Murcia, G. (2004). The PARP superfamily. BioEssays, 26(8), 882–893. 10.1002/bies.20085 [DOI] [PubMed] [Google Scholar]
- Bai, P. (2015). Biology of poly (ADP‐ribose) polymerases: The factotums of cell maintenance. Molecular Cell, 58(6), 947–958. 10.1016/j.molcel.2015.01.034 [DOI] [PubMed] [Google Scholar]
- Bai, P. , Canto, C. , Brunyánszki, A. , Huber, A. , Szántó, M. , Cen, Y. , … Auwerx, J. (2011). PARP‐2 regulates SIRT1 expression and whole‐body energy expenditure. Cell Metabolism, 13(4), 450–460. 10.1016/j.cmet.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, P. , Cantó, C. , Oudart, H. , Brunyánszki, A. , Cen, Y. , Thomas, C. , … Auwerx, J. (2011). PARP‐1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metabolism, 13(4), 461–468. 10.1016/j.cmet.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, P. , & Virag, L. (2012). Role of poly (ADP‐ribose) polymerases in the regulation of inflammatory processes. FEBS Letters, 586(21), 3771–3777. 10.1016/j.febslet.2012.09.026 [DOI] [PubMed] [Google Scholar]
- Berger, N. A. , Besson, V. C. , Boulares, A. H. , Burkle, A. , Chiarugi, A. , Clark, R. S. , … Szabo, C. (2018). Opportunities for the repurposing of PARP inhibitors for the therapy of non‐oncological diseases. British Journal of Pharmacology, 175(2), 192–222. 10.1111/bph.13748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlile, G. W. , Robert, R. , Matthes, E. , Yang, Q. , Solari, R. , Hatley, R. , … Birault, V. (2016). Latonduine analogs restore F508del–cystic fibrosis transmembrane conductance regulator trafficking through the modulation of poly‐ADP ribose polymerase 3 and poly‐ADP ribose polymerase 16 activity. Molecular Pharmacology, 90(2), 65–79. 10.1124/mol.115.102418 [DOI] [PubMed] [Google Scholar]
- Ceribelli, A. , Motta, F. , De Santis, M. , Ansari, A. A. , Ridgway, W. M. , Gershwin, M. E. , & Selmi, C. (2020). Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. Journal of Autoimmunity, 109, 102442 10.1016/j.jaut.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N. , Zhou, M. , Dong, X. , Qu, J. , Gong, F. , Han, Y. , … Zhang, L. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. The Lancet, 395(10223), 507–513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, R. L. , Oza, A. M. , Lorusso, D. , Aghajanian, C. , Oaknin, A. , Dean, A. , … Ledermann, J. A. (2017). Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet, 390(10106), 1949–1961. 10.1016/S0140-6736(17)32440-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti, P. , Ronconi, G. , Caraffa, A. , Gallenga, C. E. , Ross, R. , Frydas, I. , & Kritas, S. K. (2020). Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): Anti‐inflammatory strategies. Journal of Biological Regulators & Homeostatic Agents, 34(2). 10.23812/CONTI-E [DOI] [PubMed] [Google Scholar]
- Curtin, N. , & Szabo, C. (2013). Therapeutic applications of PARP inhibitors: Anticancer therapy and beyond. Molecular Aspects of Medicine, 6, 1043–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin, N. J. (2020). The development of Rucaparib/Rubraca®: A story of the synergy between science and serendipity. Cancers, 12(3). 10.3390/cancers12030564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea, S. , McDonald, M. C. , Mazzon, E. , Dugo, L. , Serraino, I. , Threadgill, M. , … Thiemermann, C. (2002). Effects of 5‐aminoisoquinolinone, a water‐soluble, potent inhibitor of the activity of poly (ADP‐ribose) polymerase, in a rodent model of lung injury. Biochemical Pharmacology, 63(2), 293–304. 10.1016/S0006-2952(01)00864-4 [DOI] [PubMed] [Google Scholar]
- Fatokun, A. A. , Dawson, V. L. , & Dawson, T. M. (2014). Parthanatos: Mitochondrial‐linked mechanisms and therapeutic opportunities. British Journal of Pharmacology, 171, 2000–2016. 10.1111/bph.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr, A. R. , Jankevicius, G. , Ahel, I. , & Perlman, S. (2018). Viral macrodomains: Unique mediators of viral replication and pathogenesis. Trends in Microbiology, 26(7), 598–610. 10.1016/j.tim.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr, A. R. , Singh, S. A. , Kerr, C. M. , Mukai, S. , Higashi, H. , & Aikawa, M. (2020). The impact of PARPs and ADP‐ribosylation on inflammation and host–pathogen interactions. Genes & Development, 34(5–6), 341–359. 10.1101/gad.334425.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, J. , Tian, Z. , & Yang, X. (2020). Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Bioscience Trends, 14(1), 72–73. 10.5582/bst.2020.01047 [DOI] [PubMed] [Google Scholar]
- Gautret, P. , Lagier, J. C. , Parola, P. , Hoang, V. T. , Meddeb, L. , Mailhe, M. , … Raoult, D. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID‐19: Results of an open‐label non‐randomized clinical trial. International Journal of Antimicrobial Agents, 105949 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghonim, M. A. , Pyakurel, K. , Ibba, S. V. , Al‐Khami, A. A. , Wang, J. , Rodriguez, P. , … Boulares, A. H. (2015). PARP inhibition by olaparib or gene knockout blocks asthma‐like manifestation in mice by modulating CD4+ T cell function. Journal of Translational Medicine, 13, 225 10.1186/s12967-015-0583-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghonim, M. A. , Pyakurel, K. , Ibba, S. V. , Wang, J. , Rodriguez, P. , Al‐Khami, A. A. , … Boulares, A. H. (2015). PARP is activated in human asthma and its inhibition by olaparib blocks house dust mite‐induced disease in mice. Clinical Science (London, England), 129(11), 951–962. 10.1042/CS20150122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan, T. , Hammel, P. , Reni, M. , Van Cutsem, E. , Macarulla, T. , Hall, M. J. , … Kindler, H. L. (2019). Maintenance olaparib for germline BRCA‐mutated metastatic pancreatic cancer. The New England Journal of Medicine, 381(4), 317–327. 10.1056/NEJMoa1903387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald, M. E. , Chen, Y. , Kuny, C. , Maejima, T. , Lease, R. , Ferraris, D. , … Fehr, A. R. (2019). The coronavirus macrodomain is required to prevent PAsRP‐mediated inhibition of virus replication and enhancement of IFN expression. PLoS Pathogens, 15(5), e1007756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald, M. E. , Fehr, A. R. , Athmer, J. , & Perlman, S. (2018). The coronavirus nucleocapsid protein is ADP‐ribosylated. Virology, 517, 62–68. 10.1016/j.virol.2017.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald, M. E. , Shaban, M. G. , Mackin, S. R. , Fehr, A. R. , & Perlman, S. (2020). Murine coronavirus infection activates the aryl hydrocarbon receptor in an indoleamine 2,3‐dioxygenase‐independent manner, contributing to cytokine modulation and proviral TCDD‐inducible‐PARP expression. Journal of Virology, 94(3), e01743‐19 10.1128/JVI.01743-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W.‐J. , Ni, Z.‐Y. , Hu, Y. , Liang, W.‐H. , Ou, C.‐Q. , He, J.‐X. , … Zhong, N.‐S. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382, 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman, G. J. , Larik, I. , Pennings, H. J. , Haenen, G. R. , Wouters, E. F. , & Bast, A. (2003). Systemic poly (ADP‐ribose) polymerase‐1 activation, chronic inflammation, and oxidative stress in COPD patients. Free Radical Biology & Medicine, 35(2), 140–148. 10.1016/S0891-5849(03)00237-5 [DOI] [PubMed] [Google Scholar]
- Hamahata, A. , Enkhbaatar, P. , Lange, M. , Yamaki, T. , Sakurai, H. , Shimoda, K. , … Traber, D. L. (2012). Administration of poly (ADP‐ribose) polymerase inhibitor into bronchial artery attenuates pulmonary pathophysiology after smoke inhalation and burn in an ovine model. Burns, 38(8), 1210–1215. 10.1016/j.burns.2012.08.021 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heer, C. D. , Sanderson, D. J. , Alhammad, Y. M. O. , Schmidt, M. S. , Trammell, S. A. J. , Perlman, S. , … Brenne, C. (2020). Coronavirus infection and PARP expression dysregulate the NAD metabolome: A potentially actionable component of innate immunity. bioRxiv. 10.1101/2020.1104.1117.047480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt, D. G. , & Lazo, J. S. (1992). Murine strain differences in acute lung injury and activation of poly (ADP‐ribose) polymerase by in vitro exposure of lung slices to bleomycin. American Journal of Respiratory Cell and Molecular Biology, 7(6), 645–651. 10.1165/ajrcmb/7.6.645 [DOI] [PubMed] [Google Scholar]
- Hoyt, D. G. , & Lazo, J. S. (1993). NAD depletion after in vitro exposure of murine lung slices to bleomycin. Biochemical Pharmacology, 46(10), 1819–1824. 10.1016/0006-2952(93)90588-N [DOI] [PubMed] [Google Scholar]
- Hu, B. , Wu, Z. , Hergert, P. , Henke, C. A. , Bitterman, P. B. , & Phan, S. H. (2013). Regulation of myofibroblast differentiation by poly (ADP‐ribose) polymerase 1. The American Journal of Pathology, 182(1), 71–83. 10.1016/j.ajpath.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet., 395(10223), 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagtap, P. , & Szabo, C. (2005). Poly (ADP‐ribose) polymerase and the therapeutic effects of its inhibitors. Nat.Rev.Drug Discov., 4(5), 421–440. 10.1038/nrd1718 [DOI] [PubMed] [Google Scholar]
- Janko, L. , Sari, Z. , Kovacs, T. , Kis, G. , Szanto, M. , Antal, M. , … Bai, P. (2020). Silencing of PARP2 blocks autophagic degradation. Cells, 9(2), 380 10.3390/cells9020380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayil, S. , Bhogaraju, S. , Bonn, F. , Shin, D. , Liu, Y. , Gan, N. , … Dikic, I. (2018). Insights into catalysis and function of phosphoribosyl‐linked serine ubiquitination. Nature, 557(7707), 734–738. 10.1038/s41586-018-0145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor, K. , Singla, E. , Sahu, B. , & Naura, A. S. (2015). PARP inhibitor, olaparib ameliorates acute lung and kidney injury upon intratracheal administration of LPS in mice. Molecular and Cellular Biochemistry, 400(1–2), 153–162. 10.1007/s11010-014-2271-4 [DOI] [PubMed] [Google Scholar]
- Karlberg, T. , Langelier, M. F. , Pascal, J. M. , & Schuler, H. (2013). Structural biology of the writers, readers, and erasers in mono‐ and poly (ADP‐ribose) mediated signaling. Molecular Aspects of Medicine, 34(6), 1088–1108. 10.1016/j.mam.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke, Y. , Wang, C. , Zhang, J. , Zhong, X. , Wang, R. , Zeng, X. , & Ba, X. (2019). The role of PARPs in inflammation‐ and metabolic‐related diseases: Molecular mechanisms and beyond. Cells, 8(9), 1047 10.3390/cells8091047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. S. , Challa, S. , Jones, A. , & Kraus, W. L. (2020). PARPs and ADP‐ribosylation in RNA biology: From RNA expression and processing to protein translation and proteostasis. Genes & Development, 34(5–6), 302–320. 10.1101/gad.334433.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. H. , Suk, M. H. , Yoon, D. W. , Kim, H. Y. , Jung, K. H. , Kang, E. H. , … Kang, K. H. (2008). Inflammatory and transcriptional roles of poly (ADP‐ribose) polymerase in ventilator‐induced lung injury. Critical Care, 12(4), R108 10.1186/cc6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouhpayeh, S. , Shariati, L. , Boshtam, M. , Rahimmanesh, I. , Mirian, M. , Zeinalian, M. , Salari‐jazi, A. , Khanahmad, N. , Damavandi, M. S. , Sadeghi, P. , & Khanahmad, H. (2020). The molecular story of COVID‐19; NAD+ depletion addresses all questions in this infection. Preprints, 10.20944/preprints202003.200346.v202001 [DOI]
- Kristeleit, R. , Shapiro, G. I. , Burris, H. A. , Oza, A. M. , LoRusso, P. , Patel, M. R. , … Shapira‐Frommer, R. (2017). A phase I–II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2‐mutated ovarian carcinoma or other solid tumors. Clinical Cancer Research, 23(15), 4095–4106. 10.1158/1078-0432.CCR-16-2796 [DOI] [PubMed] [Google Scholar]
- LaFargue, C. J. , Dal Molin, G. Z. , Sood, A. K. , & Coleman, R. L. (2019). Exploring and comparing adverse events between PARP inhibitors. The Lancet Oncology, 20(1), e15–e28. 10.1016/S1470-2045(18)30786-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledermann, J. , Harter, P. , Gourley, C. , Friedlander, M. , Vergote, I. , Rustin, G. , … Matulonis, U. (2012). Olaparib maintenance therapy in platinum‐sensitive relapsed ovarian cancer. The New England Journal of Medicine, 366(15), 1382–1392. 10.1056/NEJMoa1105535 [DOI] [PubMed] [Google Scholar]
- Leger, K. , Bar, D. , Savic, N. , Santoro, R. , & Hottiger, M. O. (2014). ARTD2 activity is stimulated by RNA. Nucleic Acids Research, 42(8), 5072–5082. 10.1093/nar/gku131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaudet, L. , Pacher, P. , Mabley, J. G. , Virag, L. , Soriano, F. G. , Hasko, G. , & Szabo, C. (2002). Activation of poly (ADP‐Ribose) polymerase‐1 is a central mechanism of lipopolysaccharide‐induced acute lung inflammation. Am.J.Respir.Crit Care Med., 165(3), 372–377. 10.1164/ajrccm.165.3.2106050 [DOI] [PubMed] [Google Scholar]
- Lin, S. Y. , Liu, C. L. , Chang, Y. M. , Zhao, J. , Perlman, S. , & Hou, M. H. (2014). Structural basis for the identification of the N‐terminal domain of coronavirus nucleocapsid protein as an antiviral target. Journal of Medicinal Chemistry, 57(6), 2247–2257. 10.1021/jm500089r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litton, J. K. , Rugo, H. S. , Ettl, J. , Hurvitz, S. A. , Gonçalves, A. , Lee, K. H. , … Blum, J. L. (2018). Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. The New England Journal of Medicine, 379(8), 753–763. 10.1056/NEJMoa1802905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, R. , Zhao, X. , Li, J. , Niu, P. , Yang, B. , Wu, H. , … Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. The Lancet, 395(10224), 565–574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarini, L. , Durante, M. , Lanzi, C. , Pini, A. , Boccalini, G. , Calosi, L. , … Mannaioni, G. (2017). HYDAMTIQ, a selective PARP‐1 inhibitor, improves bleomycin‐induced lung fibrosis by dampening the TGF‐β/SMAD signalling pathway. Journal of Cellular and Molecular Medicine, 21(2), 324–335. 10.1111/jcmm.12967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, P. , Liu, Y. , Qiu, L. , Liu, X. , Liu, D. , & Li, J. (2020). Tocilizumab treatment in COVID‐19: A single center experience. Journal of Medical Virology, 92(7), 814–818. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabley, J. G. , Horvath, E. M. , Murthy, K. G. , Zsengeller, Z. , Vaslin, A. , Benko, R. , … Szabo, C. (2005). Gender differences in the endotoxin‐induced inflammatory and vascular responses: Potential role of poly (ADP‐ribose) polymerase activation. The Journal of Pharmacology and Experimental Therapeutics, 315(2), 812–820. 10.1124/jpet.105.090480 [DOI] [PubMed] [Google Scholar]
- Mabley, J. G. , Jagtap, P. , Perretti, M. , Getting, S. J. , Salzman, A. L. , Virag, L. , … Szabo, C. (2001). Anti‐inflammatory effects of a novel, potent inhibitor of poly (ADP‐ribose) polymerase. Inflammation Research, 50(11), 561–569. 10.1007/PL00000234 [DOI] [PubMed] [Google Scholar]
- McGonagle, D. , Sharif, K. , O'Regan, A. , & Bridgewood, C. (2020). The role of cytokines including interleukin‐6 in COVID‐19 induced pneumonia and macrophage activation syndrome‐like disease. Autoimmunity Reviews, 19(6), 102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, P. , McAuley, D. F. , Brown, M. , Sanchez, E. , Tattersall, R. S. , & Manson, J. J. (2020). COVID‐19: Consider cytokine storm syndromes and immunosuppression. Lancet, 395(10229), 1033–1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza, M. R. , Monk, B. J. , Herrstedt, J. , Oza, A. M. , Mahner, S. , Redondo, A. , … Matulonis, U. A. (2016). Niraparib maintenance therapy in platinum‐sensitive, recurrent ovarian cancer. The New England Journal of Medicine, 375(22), 2154–2164. 10.1056/NEJMoa1611310 [DOI] [PubMed] [Google Scholar]
- Mohamed, J. S. , Hajira, A. , Pardo, P. S. , & Boriek, A. M. (2014). MicroRNA‐149 inhibits PARP‐2 and promotes mitochondrial biogenesis via SIRT‐1/PGC‐1α network in skeletal muscle. Diabetes, 63(5), 1546–1559. 10.2337/db13-1364 [DOI] [PubMed] [Google Scholar]
- Moore, K. , Colombo, N. , Scambia, G. , Kim, B. G. , Oaknin, A. , Friedlander, M. , … DiSilvestro, P. (2018). Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. The New England Journal of Medicine, 379(26), 2495–2505. 10.1056/NEJMoa1810858 [DOI] [PubMed] [Google Scholar]
- Moore, K. N. , Secord, A. A. , Geller, M. A. , Miller, D. S. , Cloven, N. , Fleming, G. F. , … Monk, B. J. (2019). Niraparib monotherapy for late‐line treatment of ovarian cancer (QUADRA): A multicentre, open‐label, single‐arm, phase 2 trial. The Lancet Oncology, 20(5), 636–648. 10.1016/S1470-2045(19)30029-4 [DOI] [PubMed] [Google Scholar]
- Morrow, D. A. , Brickman, C. M. , Murphy, S. A. , Baran, K. , Krakover, R. , Dauerman, H. , … Salzman, A. L. (2009). A randomized, placebo‐controlled trial to evaluate the tolerability, safety, pharmacokinetics, and pharmacodynamics of a potent inhibitor of poly (ADP‐ribose) polymerase (INO‐1001) in patients with ST‐elevation myocardial infarction undergoing primary percutaneous coronary intervention: Results of the TIMI 37 trial. Journal of Thrombosis and Thrombolysis, 27(4), 359–364. [DOI] [PubMed] [Google Scholar]
- Murakami, K. , Enkhbaatar, P. , Shimoda, K. , Cox, R. A. , Burke, A. S. , Hawkins, H. K. , … Traber, D. L. (2004). Inhibition of poly (ADP‐ribose) polymerase attenuates acute lung injury in an ovine model of sepsis. Shock, 21(2), 126–133. 10.1097/01.shk.0000108397.56565.4a [DOI] [PubMed] [Google Scholar]
- Murthy, P. , & Muggia, F. (2019). PARP inhibitors: Clinical development, emerging differences and the current therapeutic issues. Cancer Drug Resist, 2, 665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich‐Zugich, J. , Knox, K. S. , Rios, C. T. , Natt, B. , Bhattacharya, D. , & Fain, M. J. (2020). SARS‐CoV‐2 and COVID‐19 in older adults: What we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience, 42(2), 505–514. 10.1007/s11357-11020-00186-11350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oit‐Wiscombe, I. , Virag, L. , Soomets, U. , & Altraja, A. (2013). Increased DNA damage in progression of COPD: A response by poly (ADP‐ribose) polymerase‐1. PLoS ONE, 8(7), e70333 10.1371/journal.pone.0070333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza, A. M. , Tinker, A. V. , Oaknin, A. , Shapira‐Frommer, R. , McNeish, I. A. , Swisher, E. M. , … Kristeleit, R. S. (2017). Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high‐grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecologic Oncology, 147(2), 267–275. 10.1016/j.ygyno.2017.08.022 [DOI] [PubMed] [Google Scholar]
- Ozaydin, A. , Akbas, F. , Aksoy, F. , Yildirim, Y. S. , Demirhan, H. , Karakurt, F. , … Kanigur Sultuybek, G. (2014). Investigation of poly (ADP‐ribose) polymerase‐1 genetic variants as a possible risk for allergic rhinitis. Genetic Testing and Molecular Biomarkers, 18(1), 57–61. 10.1089/gtmb.2013.0363 [DOI] [PubMed] [Google Scholar]
- Pagano, A. , Métrailler‐Ruchonnet, I. , Aurrand‐Lions, M. , Lucattelli, M. , Donati, Y. , & Argiroffo, C. B. (2007). Poly (ADP‐ribose) polymerase‐1 (PARP‐1) controls lung cell proliferation and repair after hyperoxia‐induced lung damage. American Journal of Physiology. Lung Cellular and Molecular Physiology, 293(3), L619–L629. 10.1152/ajplung.00037.2007 [DOI] [PubMed] [Google Scholar]
- Pazzaglia, S. , & Pioli, C. (2019). Multifaceted role of PARP‐1 in DNA repair and inflammation: Pathological and therapeutic implications in cancer and non‐cancer diseases. Cells, 9(1), 41 10.3390/cells9010041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujade‐Lauraine, E. , Ledermann, J. A. , Selle, F. , Gebski, V. , Penson, R. T. , Oza, A. M. , … Pautier, P. (2017). Olaparib tablets as maintenance therapy in patients with platinum‐sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT‐Ov21): A double‐blind, randomised, placebo‐controlled, phase 3 trial. The Lancet Oncology, 18(9), 1274–1284. 10.1016/S1470-2045(17)30469-2 [DOI] [PubMed] [Google Scholar]
- Ramalingam, S. S. , Blais, N. , Mazieres, J. , Reck, M. , Jones, C. M. , Juhasz, E. , … Gorbunova, V. (2017). Randomized, placebo‐controlled, phase II study of veliparib in combination with carboplatin and paclitaxel for advanced/metastatic non‐small cell lung cancer. Clinical Cancer Research, 23(8), 1937–1944. 10.1158/1078-0432.CCR-15-3069 [DOI] [PubMed] [Google Scholar]
- Robson, M. , Im, S. A. , Senkus, E. , Xu, B. , Domchek, S. M. , Masuda, N. , … Conte, P. (2017). Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. The New England Journal of Medicine, 377(6), 523–533. 10.1056/NEJMoa1706450 [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Vargas, J. M. , Oliver‐Pozo, F. J. , & Dantzer, F. (2019). PARP1 and poly(ADP‐ribosyl)ation signaling during autophagy in response to nutrient deprivation. Oxidative Medicine and Cellular Longevity, 2019, 2641712 10.1155/2019/2641712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu, B. , Narota, A. , & Naura, A. S. (2020). Pharmacological inhibition of poly (ADP‐ribose) polymerase by olaparib, prevents acute lung injury associated cognitive deficits potentially through suppression of inflammatory response. European Journal of Pharmacology, 877, 173091 10.1016/j.ejphar.2020.173091 [DOI] [PubMed] [Google Scholar]
- Sarnaik, A. A. , Conley, Y. P. , Okonkwo, D. O. , Barr, T. L. , Fink, E. L. , Szabo, C. , … Clark, R. S. (2010). Influence of PARP‐1 polymorphisms in patients after traumatic brain injury. Journal of Neurotrauma, 27(3), 465–471. 10.1089/neu.2009.1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawalha, A. H. , Zhao, M. , Coit, P. , & Lu, Q. (2020). Epigenetic dysregulation of ACE2 and interferon‐regulated genes might suggest increased COVID‐19 susceptibility and severity in lupus patients. Clinical Immunology, 215, 108410 10.1016/j.clim.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber, V. , Ame, J. C. , Dolle, P. , Schultz, I. , Rinaldi, B. , Fraulob, V. , … de Murcia, G. (2002). Poly (ADP‐ribose) polymerase‐2 (PARP‐2) is required for efficient base excision DNA repair in association with PARP‐1 and XRCC1. The Journal of Biological Chemistry, 277(25), 23028–23036. 10.1074/jbc.M202390200 [DOI] [PubMed] [Google Scholar]
- Sethi, G. S. , Dharwal, V. , & Naura, A. S. (2017). Poly(ADP‐ribose)polymerase‐1 in lung inflammatory disorders: A review. Frontiers in Immunology, 8, 1172 10.3389/fimmu.2017.01172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi, G. S. , Sharma, S. , & Naura, A. S. (2019). PARP inhibition by olaparib alleviates chronic asthma‐associated remodeling features via modulating inflammasome signaling in mice. IUBMB Life, 71(7), 1003–1013. 10.1002/iub.2048 [DOI] [PubMed] [Google Scholar]
- Shi, Y. , Wang, Y. , Shao, C. , Huang, J. , Gan, J. , Huang, X. , … Melino, G. (2020). COVID‐19 infection: The perspectives on immune responses. Cell Death and Differentiation, 27, 1451–1454. 10.1038/s41418-41020-40530-41413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims, J. L. , Berger, S. J. , & Berger, N. A. (1983). Poly (ADP‐ribose) polymerase inhibitors preserve nicotinamide adenine dinucleotide and adenosine 5′‐triphosphate pools in DNA‐damaged cells: Mechanism of stimulation of unscheduled DNA synthesis. Biochemistry, 22(22), 5188–5194. 10.1021/bi00291a019 [DOI] [PubMed] [Google Scholar]
- Standiford, T. J. , & Ward, P. A. (2016). Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Translational Research, 167(1), 183–191. 10.1016/j.trsl.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher, E. M. , Lin, K. K. , Oza, A. M. , Scott, C. L. , Giordano, H. , Sun, J. , … McNeish, I. A. (2017). Rucaparib in relapsed, platinum‐sensitive high‐grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open‐label, phase 2 trial. The Lancet Oncology, 18(1), 75–87. 10.1016/S1470-2045(16)30559-9 [DOI] [PubMed] [Google Scholar]
- Tartier, L. , Spenlehauer, C. , Newman, H. C. , Folkard, M. , Prise, K. M. , Michael, B. D. , … de, M. G. (2003). Local DNA damage by proton microbeam irradiation induces poly (ADP‐ribose) synthesis in mammalian cells. Mutagenesis, 18(5), 411–416. 10.1093/mutage/geg015 [DOI] [PubMed] [Google Scholar]
- Toniati, P. , Piva, S. , Cattalini, M. , Garrafa, E. , Regola, F. , Castelli, F. , … Latronico, N. (2020). Tocilizumab for the treatment of severe COVID‐19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmunity Reviews, 19(7), 102568 10.1016/j.autrev.2020.102568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaninov, N. (2020). In the eye of the COVID‐19 cytokine storm. Nature Reviews. Immunology, 6(10), 20–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaschetto, R. , Kuiper, J. W. , Musters, R. J. , Eringa, E. C. , Della Corte, F. , Murthy, K. , … Plotz, F. B. (2010). Renal hypoperfusion and impaired endothelium‐dependent vasodilation in an animal model of VILI: The role of the peroxynitrite‐PARP pathway. Critical Care, 14(2), R45 10.1186/cc8932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virág, L. (2005). Poly(ADP‐ribosyl)ation in asthma and other lung diseases. Pharmacological Research, 52(1), 83–92. 10.1016/j.phrs.2005.02.012 [DOI] [PubMed] [Google Scholar]
- Virag, L. , Bai, P. , Bak, I. , Pacher, P. , Mabley, J. G. , Liaudet, L. , … Szabo, C. (2004). Effects of poly (ADP‐ribose) polymerase inhibition on inflammatory cell migration in a murine model of asthma. Med.Sci.Monit., 10(3), BR77–BR83. [PubMed] [Google Scholar]
- Wahlberg, E. , Karlberg, T. , Kouznetsova, E. , Markova, N. , Macchiarulo, A. , Thorsell, A. G. , … Weigelt, J. (2012). Family‐wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nature Biotechnology, 30(3), 283–288. 10.1038/nbt.2121 [DOI] [PubMed] [Google Scholar]
- Wang, G. , Huang, X. , Li, Y. , Guo, K. , Ning, P. , & Zhang, Y. (2013). PARP‐1 inhibitor, DPQ, attenuates LPS‐induced acute lung injury through inhibiting NF‐κB‐mediated inflammatory response. PLoS ONE, 8(11), e79757 10.1371/journal.pone.0079757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. X. , Ma, J. R. , Wang, S. Q. , Zeng, Y. Q. , Zhou, C. Y. , Ru, Y. H. , … Li, H. (2020). Utilizing integrating network pharmacological approaches to investigate the potential mechanism of Ma Xing Shi Gan Decoction in treating COVID‐19. European Review for Medical and Pharmacological Sciences, 24(6), 3360–3384. 10.26355/eurrev_202003_20704 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2020). “Solidarity” clinical trial for COVID‐19 treatments. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments (accessed 04/11/2020).
- Xiao, F. , Tang, M. , Zheng, X. , Liu, Y. , Li, X. , & Shan, H. (2020). Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology, 158, 1831–1833.e3. 10.1053/j.gastro.2020.1002.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, G. , Li, S. , Liu, X. , Gao, P. , Chen, X. , Wang, H. , … Zhang, F. (2019). PARP‐1 mediated cell death is directly activated by ZIKV infection. Virology, 537, 254–262. 10.1016/j.virol.2019.08.024 [DOI] [PubMed] [Google Scholar]
- Zaremba, T. , Thomas, H. D. , Cole, M. , Coulthard, S. A. , Plummer, E. R. , & Curtin, N. J. (2011). Poly (ADP‐ribose) polymerase‐1 (PARP‐1) pharmacogenetics, activity and expression analysis in cancer patients and healthy volunteers. The Biochemical Journal, 436(3), 671–679. 10.1042/BJ20101723 [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Wu, Z. , Li, J. W. , Zhao, H. , & Wang, G. Q. (2020). The cytokine release syndrome (CRS) of severe COVID‐19 and interleukin‐6 receptor (IL‐6R) antagonist tocilizumab may be the key to reduce the mortality. International Journal of Antimicrobial Agents, 55, 105954 10.1016/j.ijantimicag.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Wei, L. , Jiang, D. , Wang, J. , Cong, X. , & Fei, R. (2007). SARS‐CoV nucleocapsid protein induced apoptosis of COS‐1 mediated by the mitochondrial pathway. Artificial Cells, Blood Substitutes, and Immobilization Biotechnology, 35(2), 237–253. 10.1080/10731190601188422 [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Wang, X. , Ni, L. , Di, X. , Ma, B. , Niu, S. , … Reiter, R. J. (2020). COVID‐19: Melatonin as a potential adjuvant treatment. Life Sciences, 250, 117583 10.1016/j.lfs.2020.117583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F. , Yu, T. , Du, R. , Fan, G. , Liu, Y. , Liu, Z. , … Cao, B. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. Lancet, 395(10229), 1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, N. , Zhang, D. , Wang, W. , Li, X. , Yang, B. , Song, J. , … Tan, W. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine, 382(8), 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]


