Highlights
-
•
Radiation dose-escalation with intraoperative electron beam radiation therapy to the posterior resection margin and/or to residual disease is feasible with limited toxicity.
-
•
Preoperative therapy prolongs the interval to surgery and IOERT, allowing an improved selection of patients who are candidates for local treatment intensification.
-
•
Primary systemic therapy combined with chemoradiation allows to boost with IOERT in over 70% of patients with R0 surgical tumour beds.
-
•
Median survival time ranges from 19 to 35 months in electron boosted patients.
-
•
Overall survival at 5 years of over 30% is reported by contemporary expert IOERT institutions.
Keywords: Pancreatic cancer, Borderline, Intraoperative radiotherapy, IORT, IOERT, Electron beam, Pancreatic resection
Abstract
Radiation therapy (RT) is a valuable component of multimodal treatment for localized pancreatic cancer. Intraoperative radiation therapy (IORT) is a very precise RT modality to intensify the irradiation effect for cancer involving upper abdominal structures and organs, generally delivered with electrons (IOERT). Unresectable, borderline and resectable disease categories benefit from dose-escalated chemoradiation strategies in the context of active systemic therapy and potential radical surgery. Prolonged preoperative treatment may act as a filter for selecting patients with occult resistant metastatic disease. Encouraging survival rates have been documented in patients treated with preoperative chemoradiation followed by radical surgery and IOERT (>20 months median survival, >35% survival at 3 years). Intensive preoperative treatment, including induction chemotherapy followed by chemoradiation and an IOERT boost, appears to prolong long-term survival within the subset of patients who remain relapse-free for>2 years (>30 months median survival; >40% survival at 3 years). Improvement of local control through higher RT doses has an impact on the survival of patients with a lower tendency towards disease spread. IOERT is a well-accepted approach in the clinical scenario (maturity and reproducibility of results), and extremely accurate in terms of dose-deposition characteristics and normal tissue sparing. The technique can be adapted to systemic therapy and surgical progress. International guidelines (National Comprehensive Cancer Network or NCCN guidelines) currently recommend use of IOERT in cases of close surgical margins and residual disease. We hereby report the ESTRO/ACROP recommendations for performing IOERT in borderline-resectable pancreatic cancer.
1. Introduction
Pancreatic cancer is the seventh leading cause of cancer death worldwide [1]. Although significant improvements in overall survival rates have been observed in the last three decades, overall outcome remains poor [2]. Multimodality therapy including preoperative and adjuvant systemic treatment with RT components are needed for disease that is locally advanced or borderline resectable on presentation. Surgery is a curative element of therapy, although few patients present with resectable disease [3], [4].
For locally advanced disease including borderline imaging resectability, preoperative treatment with systemic agents and/or external beam radiotherapy (EBRT) (18 studies published from 1966 to 2015, 959 patients analysed) reported an objective response in 31.5%; 65.3% underwent resection (57.4% R0) with a median survival of 17.9 months (25.9 months for resected patients) [5]. The potential of preoperative therapy in borderline resectable patients has been recently updated [6]. In the era of preoperative FOLFIRINOX (5-Fluorouracil, irinotecan and oxaliplatin), the meta-analysis data for patients with borderline pancreatic cancer (13 studies, 253 patients) shows a tendency towards favourable resection rates of 39.4% and R0 specimens of 63.5% [7]. International pooled analysis (518 patients) has indicated that postoperative RT significantly improves survival: 23.0 months with doses of>45 Gy with conventional fractionation vs 13.0 months with doses of less than 45 Gy with conventional fractionation [8].
Clinical results of intraoperative radiation therapy (IORT) using high-energy electron beams (IOERT) have been consistently reported in the last four decades [9], [10]. IOERT used as a boost strategy (integrated for a dose-escalation multimodality approach) or as the only RT component was tested for localized non-resected, borderline or post-resection pancreatic cancer [11], [12]. Favourable improvement in local control has been described in cohorts treated with extended resection through the addition of intraoperative irradiation [13], [14]. In this decade, IOERT is considered a risk-adapted methodology for maximizing loco-regional control as a safe, accurate and efficient radiation dose-escalation technique [15], [16], [17].
In this study, the ESTRO IORT Task Force working group reports recommendations for performing in resected and in borderline resectable pancreatic cancer underwent resection after preoperative treatment (borderline-resected). These recommendations aim to define clinical indications, patient selection and technical aspects in a multidisciplinary setting in order to standardize treatment modalities across centres already using IOERT, and to help institutions that intend to start IOERT programmes for pancreatic cancer.
2. Evidence review and update
We performed a retrospective bibliographic review of time-period, treatment strategies, disease characteristics and clinical results reported, including an intraoperative irradiation component, typically with electrons (IOERT) between 1981 and 2016. Table 1 contains data evaluated in 31 reports on post-resection disease status (1,435 patients analysed) [12], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44].
Table 1.
Chronologic data analysis from a 35 years literature review period on IORT for Pancreatic cancer after surgical resection (31 articles, 1.435 patients).
| Treatment Characteristics | 1981–1989 | 1990–1999 | 2000–2009 | 2010–2018 |
|---|---|---|---|---|
| Resected | ||||
| ▪ #Studies | 5 | 12 | 8 | 6 |
| ▪ #Patients | 46 | 257 | 611 | 521 |
| References | [18], [19], [20], [21], [28] | [14], [22], [23], [24], [29], [30], [31], [32], [33], [34], [35], [36] | [25], [26], [37], [38], [39], [41], [42], [43] | [12], [15], [16], [17], [27], [44] |
| IORT | ||||
| ▪ Dose range (Gy) | 10–40 | 10–30 | 7.5–25 | 10–30 |
| ▪ Mean dose (Gy) | 25 | 15 | 15 | 15 |
| ▪ Electron beam | 100% | 100% | 100% | 75% |
| ▪ 250 kV | – | – | – | 25% |
| EBRT delivered | ||||
| > 50% patients | 75% | 39% | 75% | 50% |
| Adjuvant CT | ||||
| > 50% pts | 0% | 70% | 100% | 60% |
| Median Survival | ||||
| ▪ Range (months) | 5–12.6 | 10–19 | 9–28 | 19–35 |
| ▪ Mean (months) | 9.5 | 15 | 15 | 23 |
IORT: IntraOperative Radiation Therapy; EBRT: external beam radiotherapy; CT: chemotherapy; Gy: Gray; kV: kilovoltage
Table 2 summarizes contemporary clinical results [15], [16], including preoperative induction chemotherapy, chemo-radiation and surgical exploration (with or without resection, with or without IOERT) in unresectable or borderline resectable disease.
Table 2.
Institutional contemporary experiences regarding IOERT component in borderline resectable disease treated with preoperative chemoradiation with or without FOLFIRINOX induction.
| Parameter | Mayo Clinic 2013[16] | MGH 2018[15] |
|---|---|---|
| Period of analysis | 2002–2010 | 2010–2015 |
| Initial local status: | ||
| ▪ Resected/Borderline | 11 (35%) | 8 (12%) |
| ▪ Median T size | – | 3.6 cm |
| Preoperative therapy | ||
| ▪ Chemo-radiation | 31* | 68* |
| ▪ Induction FOLFIRINOX | – | 68* |
| Resected | ||
| ▪ # patients | 17 (55%) | 41 (60%) |
| ▪ R0 | 11 (65%) | 19 (46%) |
| ▪ R1 | 5 (29%) | 16 (39%) |
| ▪ R2 | 1 (6%) | 6 (15%) |
| IOERT | ||
| ▪ # patients | 14 of 17 (79%) | 22 of 41 (54%) |
| ▪ Dose range | 10–15 Gy | 8–13 Gy |
| Outcomes for resection + IOERT | ||
| ▪ Local control | 94% | 73% |
| ▪ Median OS | 23 months | 35 months |
| ▪ 3 years OS | + 40% | – |
MGH: Massachusetts General Hospital, IOERT: IntraOperative Electron Radiation Therapy, R0: complete resection of the tumour or complete remission, R1: microscopic residual tumour, R2: macroscopic residual tumour, LC: Local control, OS: Overall Survival, * Entire group
In 2017, Krempien et al. published an IOERT review on pancreatic cancer with a selective update of post-resected status results [11].
3. Pre-treatment investigations
Studies required for candidate selection include the following:
-
1.
Pathology of adenocarcinoma
-
2.
History and physical examination
-
3.
American Society of Anaesthesiologists (ASA) score
-
4.
Conventional blood tests
-
5.
CA 19.9
-
6.
Abdominal Multidetector computed tomography angiography
-
7.
Esophago-gastric duodenoscopy and Endoscopic ultrasonography
-
8.
Chest computed tomography (CT)
Potential supportive actions to be considered preoperatively include the following:
-
1.
Self-expanding stent
-
2.
Gastro-jejunostomy
-
3.
Neurolysis
Additional studies in high-risk patients, as clinically indicated, include the following:
-
1.
Magnetic Resonance Imaging (MRI) / magnetic resonance cholangiopancreatography
-
2.
Positron Emission Tomography (PET)-CT
-
3.
Laparoscopy
4. Pre-treatment imaging for clinical staging
Accurate diagnosis, clinical staging and treatment of pancreatic cancer require extensive interdisciplinary interaction and cooperation between specialties (diagnostic radiology, interventional upper endoscopy, nuclear medicine, surgery, medical oncology, radiation oncology). Accurate clinical staging is based on angiographic high-quality (helical) multidetector computed tomography (CT) with advanced volumetric techniques to accurately define the relationship of the tumour to the celiac axis and superior mesenteric vessels in three dimensions. In the absence of extrapancreatic disease, the relationship of the low-density tumour mass to the superior mesenteric artery (SMA) and celiac axis is the main focus of preoperative imaging studies. Endoscopic ultrasound-guided needle biopsy is a preferred method of diagnosis. Endoscopic retrograde cholangiopancreatography may be of use in decompression of the biliary tract in patients with jaundice and pruritus. Local tumour resectability is most accurately assessed by preoperative imaging studies: intraoperative exploration is an inadequate method to assess critical tumour–vessel relationships [45], [46]. Objective, reproducible radiographic criteria define potentially resectable disease as the absence of extrapancreatic disease, absence of superior mesenteric vein (SMV) or portal vein encasement, abutment or distortion, or associated thrombi, and presence of a patent SMV–portal vein confluence and distinct fat planes around the SMA, celiac axis and hepatic artery [47]. Pre-treatment staging to exclude patients with locally advanced disease is crucial to allow for accurate interpretation of results from studies examining the value of multimodality therapy in patients with pancreatic cancer. Borderline resectable tumours, which may benefit from preoperative therapy, include tumours with abutment or encasement of the SMV/portal vein without arterial involvement, in which sufficient vessel is present proximally and distally to permit resection and venous reconstruction, gastroduodenal artery encasement without extension to the celiac axis and with or without abutment or minor encasement of the hepatic artery, abutment of less than 180° of the SMA [48]. The margin most frequently reported as positive in patients who undergo pancreaticoduodenectomy is along the SMV or proximal SMA. IOERT following pancreatic resection should accurately document the pathologic status of the retroperitoneal margin of resection. PET-CT at initial staging contributes complementary information on potential distant metastasis and the metabolic profile of the primary lesion [49].
5. Patient selection for IOERT
All patients diagnosed with localized pancreatic cancer have to be extensively evaluated and discussed at a multidisciplinary tumour board (MTB) for defining the optimal multimodal treatment strategy, including exploratory laparotomy, with or without resection, and IOERT.
A significant proportion of unresectable or borderline resectable patients will be advised to undergo preoperative strategies (including a preoperative chemoradiation component) and should be restaged before laparotomy in terms of performance status, imaging and CA19.9 evolution.
Patients amenable to the multidisciplinary approach including IOERT should have a good performance status (ECOG less than 2) and without distant metastases. The strongest recommendation exists for patients with clinical stage > IA (UICC TNM, 2016).
Table 3 reports patient selection for IOERT: disease status, treatment sequence and radiation dose recommendations.
Table 3.
Patient selection for IOERT: disease, treatment sequence and radiation dose recommendations.
| Disease Status | |
|---|---|
| Clinical setting | Borderline resected pancreatic cancer |
| Indications | Borderline/resected |
| Stage | > IA (UICC TNM, 2016) |
| Treatment | |
| Preoperative chemoradiation followed by resection + IOERT boost | |
| Radiotherapy Dose | |
| IOERT boost | 10 to 12.5 Gy for negative resection margins (R0) 12.5 to 15 Gy for microscopic positive resection margins (R1) 15 to 20 Gy for macroscopic or gross residual tumor (R2) |
| 3D-CRT or IMRT | 45–50.4 Gy (in 1.8–2 Gy per fraction) |
6. External beam radiation therapy
For patients with borderline-resectable cancers, pre-operative EBRT plus concurrent chemotherapy is preferably given prior to exploratory laparotomy and possible surgical resection/IOERT. External-beam radiation therapy (EBRT) is delivered using 3-dimensional conformal irradiation (3D-CRT) or intensity-modulated irradiation (IMRT), daily, over a period of 5–6 weeks at a dose of 45.0–50.4 Gy in 1.8 daily fractions, together with 5-FU, capecitabine or institutional chemo-radiation regimes. IMRT shows dosimetric advantages, sparing renal and liver parenchyma, stomach and small bowel. The planning target volume (PTV) takes into account organ motion and encompassing the clinical target volume (CTV) that includes areas consisting of the tumour itself and areas at risk of tumour involvement and occult (at-risk) nodal metastases. Nodal target volumes for tumours in the head of the pancreas include the pancreaticoduodenal, peripancreatic, porta hepatis, celiac and suprapancreatic nodes. The portion of the duodenal loop at risk of involvement with extrapancreatic tumour extension is also included. For lesions involving the body and tail of the pancreas, the suprapancreatic, celiac and splenic hilar nodes should be included in the CTV; inclusion of more medially placed lymph nodes (pancreaticoduodenal and porta hepatis) may be optional, depending on the ability to spare normal organs and structures. Normal tissue tolerances should be carefully respected. Details regarding dose-volume histogram parameters for treatment of pancreatic malignancies with RT have been recently updated (50). The dose limits of the kidneys, liver, stomach, small bowel and spinal cord will influence the selection of beam arrangement. In situations where IOERT has been administered or is intended to be administered to tumour or tumour bed located in proximity of vertebral column, the spinal-cord dose should be limited to 35.0–40.0 Gy. In the postoperative setting, similar planning principles can be used, with the operative bed and areas of potential residual tumour or microscopic extension taking the place of the initial gross tumour volume in treatment planning.
7. Surgical procedure
7.1. Surgical factors and tumour resection (pancreaticoduodenectomy; distal pancreatectomy)
Pancreaticoduodenectomy involves the excision of the pancreatic head, duodenum, gallbladder and bile duct, with or without removal of the gastric antrum. Access to the peritoneal cavity is through a longitudinal midline incision or a bilateral subcostal incision. Once in the abdominal cavity, all intra-abdominal organs and peritoneal surfaces are carefully inspected and palpated to exclude distant metastatic disease. Any suspicious lesions should be biopsied and sent for frozen-section examination, as presence of distant metastasis is a contraindication to proceeding with resection. A wide Kocher manoeuvre is performed lifting all lymphatic tissue over the medial aspect of the right kidney, inferior vena cava and left renal vein. The gastrocolic ligament is divided, with special attention to preserving the gastroepiploic arcade if pyloric preservation is being entertained. The neck of the pancreas is then carefully dissected off the SMV. Dissection of the porta hepatis is usually initiated by excising the common hepatic artery lymph node to facilitate exposure. The gastroduodenal and right gastric artery are identified, legated and divided. The superior portion of the pancreatic neck is dissected off the portal vein. Cholecystectomy is then performed and the common hepatic duct is divided. The gastric antrum or duodenum is divided using a liner gastrointestinal stapler. The jejunum is then transected approximately 10 cm from the ligament of Treitz with subsequent mobilization of its mesentery, as well as mobilization of the third and fourth portions of the duodenum. The pancreatic neck is transected. The pancreatic head and uncinate process are now dissected from the portal vein and SMV by legating and dividing the often-multiple venous tributaries that are encountered. Vascular resection of the SMV–portal vein confluence using either lateral venectomy or segmental venous resection and reconstruction should be performed when there is no tissue plane between the tumour and SMV and portal vein. Medial retraction of the SMV–portal vein confluence and the SMA is identified. All of the soft tissue along the right lateral aspect of the SMA should be excised. Special attention should be paid to this step, given the high incidence of local recurrence. In spite of all efforts, a microscopically positive margin will occur in between 10% and 20% of cases due to perineural invasion along the mesenteric plexus at the SMA origin and microscopic lymphatic spread beyond the extent of the palpable tumour. Prior to obtaining frozen-section histologic examination of the surgical margins, the specimen is appropriately oriented and the areas in question are identified. Reconstruction after pancreaticoduodenectomy have to be start after IOERT procedure to avoid irradiation of anastomotic structures (see below). Reconstruction will be start with the pancreaticojejunostomy. A retrocolic end-to-side duct-to-mucosa technique using interrupted sutures is recommended. Then, distal to the pancreaticojejunostomy, the hepaticojejunostomy is completed in a single layer using either interrupted or running sutures, depending on the calibre of the common hepatic duct. An antecolic, end-to-side duodenojejunostomy (or gastrojejunostomy if pyloric preservation has not been used) in two layers is then completed approximately 40–50 cm from the hepaticojejunostomy. The abdomen is then irrigated copiously prior to placing surgical drains and performing abdominal closure. Perhaps one of the most debated technical aspects of the pancreaticoduodenectomy is the extent of the associated lymphadenectomy (standard vs extended). A standard lymphadenectomy (standard pancreaticoduodenectomy) commonly refers to the resection of gastric and pyloric nodes, nodes to the right of the hepatoduodenal ligament, anterior and posterior pancreaticoduodenal nodes, nodes to the right of the SMA and nodes anterior to the common hepatic artery. An extended lymphadenectomy (extended pancreaticoduodenectomy) includes the skeletonization of the common and proper hepatic arteries, celiac axis nodes, all nodes to the left and right of the hepatoduodenal ligament, circumferential skeletonization of the SMA between the aorta and the inferior pancreaticoduodenal artery, all nodes in the anterolateral aspect of the aorta and the inferior vena cava, in continuity with Gerota’s fascia, between the celiac axis and the inferior mesenteric artery.
8. IOERT procedure: Post-resection
8.1. IOERT: Methods and techniques of treatment
IOERT for pancreatic cancer has predominantly been delivered with megavoltage electrons (IOERT) produced by a medical linear accelerator (Fig. 1) [50]. Although brachytherapy and orthovoltage treatment have been described, the data on efficacy is uncertain. Therefore, these recommendations will focus IOERT. The electron beam energy and dose of IOERT are determined by the resection status and geometry of the treated field. Bolus is not recommended due to the fact that intraoperative fluid is present after tissue manipulation and resection. Protections inside the tumour bed are not recommended too, due to the dosimetric uncertainties introduced by such action. The best protection is mechanical retraction for temporary displacement of dose-sensitive structures at risk. Surgical retractors for large upper abdominal interventions are most helpful for properly exposing the radiation target and for expediting IOERT applicator positioning, displacement of normal tissue and iconographic documentation of the final pre- and post-intraoperative irradiation assemblage.
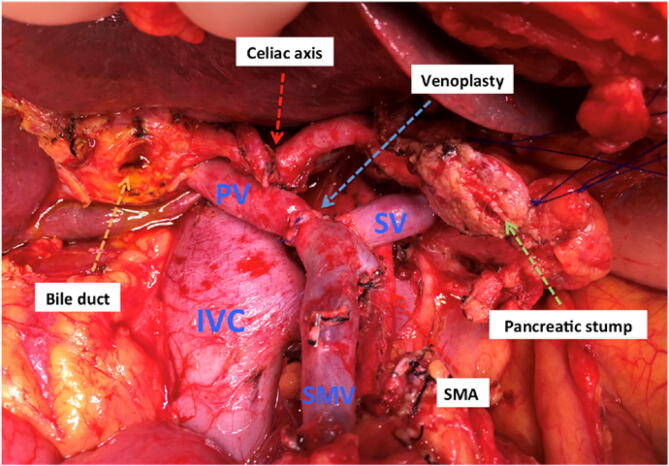
Fig. 1.
A post-pancreatectomy tumour bed area including the description of remaining anatomical structures. SMA: superior mesenteric artery.
9. Radiation target definition
After marginal resection of borderline resectable or resectable lesions, the tumour bed contains retroperitoneal soft tissue, vascular structures (portal vein, superior mesenteric artery and vein, aorta), the prevertebral ligament and the anterior part of the vertebral bone (Fig. 1).
The sectioned bile duct, the pancreatic remnant, colon and stomach are excluded from the IOERT field and should be under visual control and mechanically retracted. The upper pole of the right kidney can also be controlled and displaced by palpation. Under appropriate haemostasis, intraoperative fluid is not a limiting factor for selection of electron beam energy as long as the fluid level covering the target volume is stable. The target at risk should include the whole circumferences of the mentioned vascular structures and the post-resected retroperitoneal surface with a safety margin, which can be adequately treated with lower-energy electrons in the 9–12 MeV range. Intraoperatively, the radiation oncologist and surgeon consult regarding the retroperitoneal area at risk of residual tumour after maximal resection, and the volume at risk is encompassed within a field defined by an IOERT applicator with a margin of at least 1–2 cm laterally, covering the PTV including anatomic, dosimetric and geometric uncertainties; in depth, a margin of 0,1–0,5cm margin should allow for penetration uncertainties. The post-pancreatectomy tumour bed is appropriately encompassed by applicators in the range of 7 to 10 cm. For larger field sizes, consider the use of bevelled applicators; field abutments should be discouraged. Fig. 2 illustrates an IOERT applicator in position for treatment of a tumour bed post-pancreatectomy.
Fig. 2.
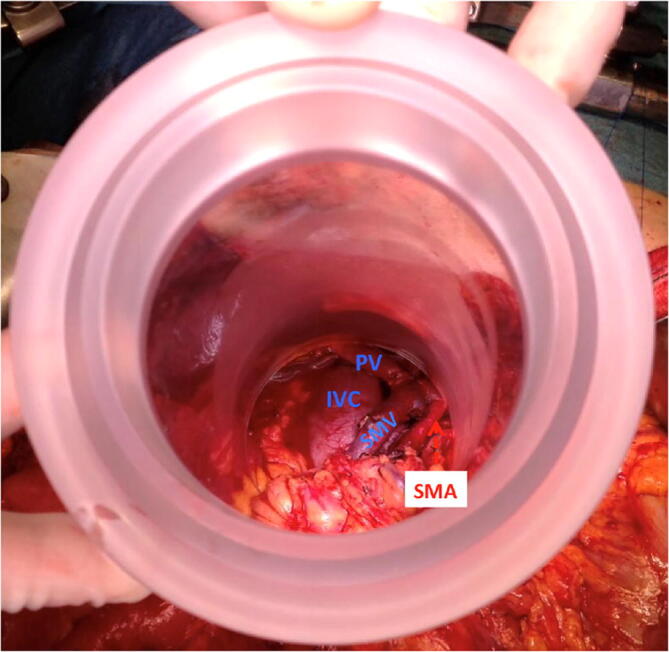
IOERT applicator positioned encompassing the tumour bed (vascular structures and retroperitoneal tissue margin) excluding from the radiation beam: pancreatic stump, bile duct, liver and transverse colon. SMA: superior mesenteric artery.
The whole process can be summarized as follows:
-
1.
Tumour bed definition/assessment: surgical margin status (inspection of the surgical field and the posterior aspect of the surgical specimen; optional frozen section pathology). Perivascular tissue characteristics in borderline lesions.
-
2.
Normal uninvolved tissue to be excluded (mobilized out) from the IOERT radiation volume: bile duct (sections); pancreatic remnant (sectioned if it exists); stomach; colon; small bowel; liver; upper pole of right kidney.
-
3.
Normal tissue at risk to be included in the radiation target volume: circumferential vasculature structures (inferior cava vein; portal vein; superior mesenteric artery and vein, aorta; legated left gastric artery); lymphatic and retroperitoneal soft tissue; prevertebral ligament.
Such decisions should be agreed at the time of IOERT.
10. Applicator selection
The applicator selection and adaptation to the post-surgical bed at risk should consider the following technical elements of decisions:
-
1.
Size (diameter): in the range of 1 to 2 cm larger than the original maximal dimension of the tumour (T) in the staging imaging studies, including the area at risk or positive margins. Availability of applicators with a diameter of 5 to 10 cm provides a safe range.
-
2.
Bevel angle: to appropriately encompass the surgical bed the anatomical configuration of the right sided head of the pancreas surgical bed, 15° to 30° bevel angles are recommended. The same criteria apply to primaries in the tail of the pancreas (opposite direction). Dominant lesions in the body of the pancreas are properly encompassed with 0° to 15° angles.
11. IOERT irradiation
IOERT irradiation consists of the following:
-
1.
Electron energy selection: 90% isodose should encompass in depth the full circumference of vessels involved, in contact or at risk, together with retroperitoneal tissue and prevertebral ligament. The estimations of this distance can be made using real-time intraoperative measurements, together with data obtained from the presurgical CT scan. Meticulous haemostasis and intra-abdominal surgical fluid are relevant for electron energy selection. In the event of fluid instability at the radiation target, a superior electron energy level selection is an alternative.
-
2.
Dose selection (single fraction boost component): resection specimens at low risk after favourable dissection procedure doses of 10 to 12.5 Gy are recommended. Specimen with close or suspected/confirmed cancer involved margins doses in the range from 12.5 to 15 Gy are recommended. After laborious vascular and/or soft tissue dissection with suspected residual cancer, 15 to 20 Gy should be considered, even in the event of vascular anastomosis.
12. Dose prescription
For tumours resected without identifiable residual gross tumour volume, doses in the range of 10.0–12.5 Gy are applied. Depending on the extent of suspected residual microscopic malignant disease, this range may be 12.5–15 Gy. For gross residual disease, doses of 15–20 Gy have been employed. During surgery, care must be taken to accurately identify the depth of the spinal cord beneath the IOERT field using anatomic landmarks and a review of preoperative CT imaging. A library of predefined isodose curve distribution for a range of IOERT applicator diameters, bevel shapes and electron beam energies has to be available for intraoperative consultation (Fig. 3). The electron energy should be chosen to adequately encompass the target tissues within the 90% isodose curves, with attention to the spinal cord dose contribution.
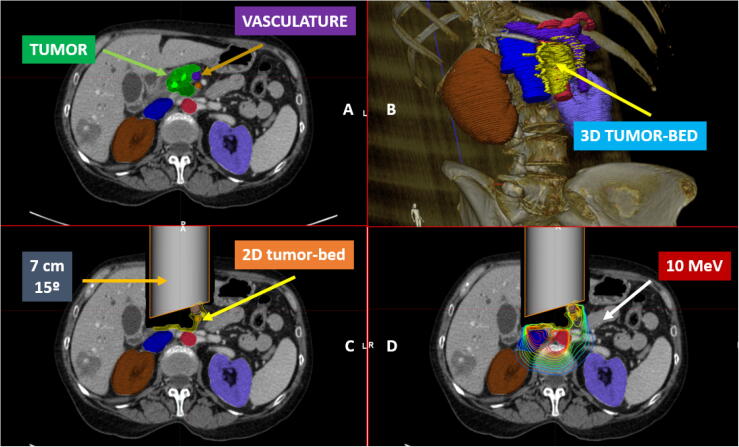
Fig. 3.
A virtual 2D simulation (7 cm diameter applicator, 15° bevel angle) and dosimetric representation (10 MeV electron beam) of an IOERT post-pancreatectomy procedure: A. CT contoured including tumour, adjacent organs at risk and upper abdominal vasculature, B. Post resected tumour bed estimation (3D): retroperitoneal tissue and vasculature, C. Applicator positioning: 7 cm diameter, 15 ° bevel angle and D. Isodose distribution: 10 MeV electron beam.
13. Treatment delivery
Before dose delivery, the appropriate physical and dosimetrical parameters (electron energy, applicator size, length and bevel angle, monitor units, bolus choice, should be checked by a medical physicist as well as by the physician (radiation oncologist) in a four-eyes principle. Usually, a medical physicist or a radiation therapist (RTT) aligns the gantry to the applicator for soft-docking system and enters the data in the control console. During irradiation (approximately 1–2 min, depending on dose and dose rate), nobody is allowed to stay in the operation room except the patient for radiation protection purposes.
14. Applicator removal
After IOERT irradiation, the surgeon or radiation oncologist performs applicator removal with the assistance of a surgical assistant/nurse.
Special attention should be paid during this step to avoid traumatization of surrounding tissues and possible bleeding. In the event of bleeding during irradiation, blood should first be aspirated in order to clearly visualize the end of the collimator in contact with the patient's tissues and allow for a safe manoeuvre.
15. Recording and reporting
Clinical and dosimetry forms should be filled out with all relevant patient, tumour and treatment parameters. Clinical data should include demographic data, performance status, symptoms and serum tests, including CA19-9, comorbidities and Charlson comorbidity index. Tumour-related data should include imaging studies, a biopsy report, clinical and pathological stage, grading and possible biomolecular studies. Treatment data should include bile-duct permeability manoeuvres, preoperative treatments, the surgical report and main characteristics of the IOERT procedure, including applicator diameter, bevel angle, bolus, beam energy, dose prescription and duration of the procedure. In vivo dosimetry is strongly recommended as a quality-assurance procedure. Radiation target contents should be described: organs and structures included in the radiation beam. Radiation protection of normal uninvolved tissues: description of temporary mobilization or intra-field customized protection. The documentation of the radiation target, adjacent - if necessary mobilized - normal tissue and used shielding should ideally include drawings or photos.
Preoperative MRI and CT-scans can be obtained to identify the primary tumour, regional lymph nodes and critical organs in order to design a provisional treatment plan. No fully reliable treatment planning systems currently exist for intraoperative irradiation; however, the availability of preoperative images may help with identifying anatomical structures to guide the positioning of the collimator. Whenever obtained, all these imaging data should be included in the patient's final documentation. Additionally, clinical photographs of the applicator positioning and surface anatomy in the IOERT target volume are recommended.
Intraoperative ultrasound can also be helpful in some cases to verify tumour size in depth and the location of critical structures such as kidneys and major vessels.
The final documentation of the IOERT procedure should also include the surgical notes and the anaesthesiology report.
Table 4 contains a summary of the relevant IOERT parameters for local treatment.
Table 4.
Reporting parameters for IORT electrons beam procedures in resected pancreatic cancer.
| Iort Parameters | |
|---|---|
| Target volume description |
|
| IORT factors |
|
| Integrated pre-IORT treatment factors |
|
16. Recommendation on patient care:
16.1. Care during the course of IOERT
The sterile field should be guaranteed throughout the IOERT procedure. Before irradiation, sterile drapes should cover the surgical bed and the part of the applicator inside and near the surgical bed. In some cases, an aspiration drain may be useful to avoid bleeding outside the surgical bed. The patient should be carefully observed by means of a camera during irradiation and vital parameters should be monitored and be visible from outside the operating room. In the event of an emergency, irradiation should be stopped immediately and nurses, anaesthesiologists and surgeons should be prepared to enter the operating room at any time immediately after cessation of irradiation.
17. Post-treatment patient care and follow-up
Patients treated with IOERT for pancreatic tumours require thorough care, as after any other surgical procedure for pancreatic tumours. All vital and clinical parameters should be monitored in the days following the procedure, and special attention should be paid to blood tests, including renal and liver functions, bowel movements and onset of new symptoms and signs.
After IOERT for pancreatic-head tumours, the risk of duodenal radiation damage, even with bleeding, should be taken into account. In the event of persistent pain and anaemia with faecal occult blood, medical therapy and endoscopy/surgical procedures should be considered.
In the case of unresected or partially resected lesions, the risk of bleeding related to haemorrhage from arteries and veins encased by the tumour tissue that undergoes necrosis should be carefully considered. In the event of significant bleeding, re-operation aiming at haemostasis should be considered where possible.
After the IOERT procedure, alone or combined with surgical resection, the patient may receive further treatments, including postoperative RT and adjuvant systemic treatment. Therefore, the follow-up schedule starts after treatment completion and usually does not substantially differ from that of pancreatic cancer treated without IOERT. During imaging studies, special attention should be paid to any tissue potentially involved in the IOERT volume such as the duodenum and major vessels.
18. Adverse effects
IOERT in the setting of post-resection pancreatic cancer is typically well tolerated. In the European pooled analysis, acute treatment toxicity was minimal and limited to grade 2. Surgical adverse events consisted of pancreatic fistula in 27%, delayed gastric emptying in 22%, haemorrhage in 18%, repeat laparotomy in 15%, abdominal abscess in 14%, sepsis in 3%, and perioperative mortality in 2% [42]. Large animal experimental studies have analysed the pathologic response of the pancreas and duodenum [51]. Clinical and experimental data of upper abdominal tissues and organs support good tolerance to a 10-Gy to 20-Gy IOERT boost plus 50-Gy external beam RT (conventional fractionation), including post-resection vascular anastomosis [52], [53].
19. Conclusions and future directions
Long-term survival and disease control are achievable in selected patients with borderline-resectable pancreas cancer, with a tendency towards improved survival reported for patients who underwent resection after intensive-induction systemic and locoregional full-dose chemoradiation. IOERT as part of a multimodality treatment option for borderline or resected pancreatic cancer has been shown to promote high local control at the site of the primary tumour without a significant increase in treatment toxicity. With advances in the ability of systemic therapy to treat occult systemic metastases, the importance of sustained long-term local and regional control is increasing interest in the expansion of IOERT.
Strategies for selecting appropriate patients for aggressive local therapy in resected and borderline-resectable settings will advance through improvements in imaging, biomarkers and genetics, as well as through the timing of when to administer IOERT and/or resection. In-vivo-dosimetry and intra-operative imaging should be encouraged to improve the accuracy, reproducibility and documentation and provide data for evaluation and tailoring of IOERT.
IOERT is a risk-adaptable technique in the era of personalized oncology [54]. New opportunities for systemic or regional therapy (intrahepatic and intraperitoneal), targeted therapies, vaccines and immunotherapy should be evaluated in an attempt to improve systemic disease control. As improvements are being made in distant disease control, the benefit of improved local control with regimens including IOERT may become even more decisive [55], [56].
20. Disclosures
Jose M. Asencio
-
•
Is member of the IOeRT Consortium, established on 21 December 2019, supported by Sordina IORT Technologies spa
Felipe A. Calvo:
-
•
Is member of the IOeRT Consortium, established on 21 December 2019, supported by Sordina IORT Technologies spa
Philip Poortmans
-
•
Is member of the IOeRT Consortium, established on 21 December 2019, supported by Sordina IORT Technologies spa
-
•
Is medical advisor of Sordina IORT Technologies spa, starting from 1 April 2020 on
Marco Krengli
-
•
Received travel grants from IntraOp Medical Corporation, CA, USA
Acknowledgments
Acknowledgements
Intraoperative radiotherapy (IORT) is a multidisciplinary oncological activity requiring a close collaboration of team members, using optimal tools and techniques. The authors of this guideline acknowledge the remarkable contribution of all the health professionals involved in the care of patients who are candidates for IORT procedures.
The authors are grateful to Dr. Javier Serrano of Hospital Gregorio Marañon for his assistance on the manuscript development.
Authors are grateful to the ESTRO/ACROP reviewers Wim Dries, Philipp Scherer, Dirk Verellen, Alexandra Stewart and Antonino De Paoli for their useful and constructive comments.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Cloyd J.M., Katz M.H., Prakash L., Varadhachary G.R., Wolff R.A., Shroff R.T. Preoperative therapy and pancreatoduodenectomy for pancreatic ductal adenocarcinoma: a 25-year single-institution experience. J Gastrointest Surg. 2017;21:164–174. doi: 10.1007/s11605-016-3265-1. [DOI] [PubMed] [Google Scholar]
- 3.Bouvier A.M., Bossard N., Colonna M., Garcia-Velasco A., Carulla M., Manfredi S. EUROCARE-5 working group. Trends in net survival from pancreatic cancer in six European Latin countries: results from the SUDCAN population-based study. Eur J Cancer Prev. 2017;26:S63–S69. doi: 10.1097/CEJ.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 4.Ahola R., Siiki A., Vasama K., Vornanen M., Sand J., Laukkarinen J. Patients with resected, histologically re-confirmed pancreatic ductal adenocarcinoma (PDAC) can achieve long-term survival despite T3 tumour or nodal involvement. The Finnish Register Study 2000–2013. Pancreatology. 2017;17:822–826. doi: 10.1016/j.pan.2017.07.192. [DOI] [PubMed] [Google Scholar]
- 5.Tang K., Lu W., Qin W., Wu Y. Neoadjuvant therapy for patients with borderline resectable pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. Pancreatology. 2016;16:28–37. doi: 10.1016/j.pan.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Zhan H.X., Xu J.W., Wu D., Wu Z.Y., Wang L., Hu S.Y. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med. 2017;6:1201–1219. doi: 10.1002/cam4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrelli F., Coinu A., Borgonovo K., Cabiddu M., Ghilardi M., Lonati V. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas. 2015;44:515–521. doi: 10.1097/MPA.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 8.De Filippo L., Mattiucci G.C., Morganti A.G., Falconi M., Van Stiphout R.G.P.M., Alfieri S. The impact of dose on survival in adjuvant chemoradiation pancreatic cancer. Radiother Oncol. 2017;suppl1:S330. [Google Scholar]
- 9.Czito B.G., Calvo F.A., Haddock M.G., Palta M., Willett C.G. Intraoperative irradiation. In: Gunderson L.L., Tepper J.E., editors. Clinical Radiation Oncology. Elsevier Saunders; Philadelphia: 2016. pp. 325–340. [Google Scholar]
- 10.Sole C.V., Calvo F.A., Ferrer C., Pascau J., Marsiglia H. Bibliometrics of intraoperative radiotherapy: analysis of technology, practice and publication tendencies. Strahlenther Onkol. 2014;190:1111–1116. doi: 10.1007/s00066-014-0695-0. [DOI] [PubMed] [Google Scholar]
- 11.Krempien R., Roeder F. Intraoperative radiation therapy (IORT) in pancreatic cancer. Radiat Oncol. 2017;12:8–14. doi: 10.1186/s13014-016-0753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa K1, Karasawa K, Ito Y, Ogawa Y, Jingu K, Onishi H, Aoki S, Wada H, Kokubo M, Etoh H, Kazumoto T, Takayama M, Negoro Y, Nemoto K, Nishimura Y. Intraoperative radiotherapy for resected pancreatic cancer: a multi-institutional retrospective analysis of 210 patients, Int J Radiat Oncol Biol Phys 2010;77:734-42 [DOI] [PubMed]
- 13.Hiraoka T. Extended radical resection of cancer of the pancreas with intraoperative radiotherapy. Baillieres Clin Gastroenterol. 1990;4:985–993. doi: 10.1016/0950-3528(90)90031-b. [DOI] [PubMed] [Google Scholar]
- 14.Hiraoka T., Uchino R., Kanemitsu K. Combination of intraoperative radiation with resection of cancer of the pancreas. Int J Pancreatol. 1990;7:201–207. doi: 10.1007/BF02924238. [DOI] [PubMed] [Google Scholar]
- 15.Keane Florence K., Wo Jennifer Y., Ferrone Cristina R., Clark Jeffrey W., Blaszkowsky Lawrence S., Allen Jill N., Kwak Eunice L., Ryan David P., Lillemoe Keith D., Fernandez-del Castillo Carlos, Hong Theodore S. Intraoperative Radiotherapy in the Era of Intensive Neoadjuvant Chemotherapy and Chemoradiotherapy for Pancreatic Adenocarcinoma. Am J Clin Oncol. 2018;41(6):607–612. doi: 10.1097/COC.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 16.Ashman J.B., Moss A.A., Rule W.G., Callister M.G., Reddy K.S., Mulligan D.C. Preoperative chemoradiation and IOERT for unresectable or borderline resectable pancreas cancer. J Gastrointest Oncol. 2013;4:352–360. doi: 10.3978/j.issn.2078-6891.2013.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvo F.A., Sole C.V., Atahualpa F., Lozano M.A., Gomez-Espi M., Calin A. Chemoradiation for resected pancreatic adenocarcinoma with or without intraoperative radiation therapy boost: Long-term outcomes. Pancreatology. 2013;13:576–582. doi: 10.1016/j.pan.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Manabe T., Baba N., Nonaka A. Combined treatment using radiotherapy for carcinoma of the pancreas involving the adjacent vessels. Int Surg. 1988;73:153–156. [PubMed] [Google Scholar]
- 19.Nishimura A., Sakata S., Iida K. Evaluation of intraoperative radiotherapy for carcinoma of the pancreas: prognostic factors and survival analyses. Radiat Med. 1988;6:85–91. [PubMed] [Google Scholar]
- 20.Cromack D.T., Maher M.M., Hoekstra H. Are complications in intraoperative radiation therapy more frequent than in conventional treatment? Arch Surg. 1989;124:229–234. doi: 10.1001/archsurg.1989.01410020103017. [DOI] [PubMed] [Google Scholar]
- 21.Gilly F., Romestaing P., Gerard J. Experience of three years with intra-operative radiation therapy using the Lyon intra-operative device. Int Surg. 1990;75:84–88. [PubMed] [Google Scholar]
- 22.Calvo F., Santos M., Abuchaibe O. Intraoperative radiotherapy in gastric and pancreatic carcinoma: a European experience. Front Radiat Ther Oncol. 1991;25:270–273. doi: 10.1159/000429598. [DOI] [PubMed] [Google Scholar]
- 23.Dobelbower R., Konski A., Merrick H. Intraoperative electron beam radiation therapy (IOEBRT) for carcinoma of the exocrine pancreas. Int J Radiat Oncol Biol Phys. 1991;20:113–119. doi: 10.1016/0360-3016(91)90146-u. [DOI] [PubMed] [Google Scholar]
- 24.Fossati V., Cattaneo G., Zerbi A. The role of intraoperative therapy by electron beam and combination of adjuvant chemotherapy and external radiotherapy in carcinoma of the pancreas. Tumouri. 1995;81:23–31. doi: 10.1177/030089169508100106. [DOI] [PubMed] [Google Scholar]
- 25.Ihse I., Andersson R., Ask A. Intraoperative radiotherapy for patients with carcinoma of the pancreas. Pancreatology. 2005;5:438–442. doi: 10.1159/000086546. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor J., Sause W., Hazard L. Survival after attempted surgical resection and intraoperative radiation therapy for pancreatic and periampullary adenocarcinoma. Int J Radiat Oncol Biol Phys. 2005;63:1060–1066. doi: 10.1016/j.ijrobp.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Jingu K., Tanabe T., Nemoto K. Intraoperative radiotherapy for pancreatic cancer: 30-year experience in a single institution in Japan. Int J Radiat Oncol Biol Phys. 2012;83:e507–e511. doi: 10.1016/j.ijrobp.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Hoekstra H.J., Restrepo C., Kinsella T.J., Sindelar W.F. Histopathological effects of intraoperative radiotherapy on pancreas and adjacent tissues: a postmortem analysis. J Surg Oncol. 1988;37:104–108. doi: 10.1002/jso.2930370208. [DOI] [PubMed] [Google Scholar]
- 29.Ozaki H., Kinoshita T., Kosuge T., Egawa S., Kishi K. Effectiveness of multimodality treatment for resectable pancreatic cancer. Int J Pancreatol. 1990;7:195–200. doi: 10.1007/BF02924237. [DOI] [PubMed] [Google Scholar]
- 30.Gotoh M., Monden M., Sakon M. Intraoperative irradiation in resected carcinoma of the pancreas and portal vein. Arch Surg. 1992;127:1213–1215. doi: 10.1001/archsurg.1992.01420100071012. [DOI] [PubMed] [Google Scholar]
- 31.Johnstone P., Sindelar W. Patterns of disease recurrence following definitive therapy of adenocarcinoma of the pancreas using surgery and adjuvant radiotherapy: correlations of a clinical trial. Int J Radiat Oncol Biol Phys. 1993;27:831–834. doi: 10.1016/0360-3016(93)90456-6. [DOI] [PubMed] [Google Scholar]
- 32.Zerbi A., Fossati V., Parolini D. Intraoperative radiation therapy adjuvant to resection in the treatment of pancreatic cancer. Cancer. 1994;73:2930–2935. doi: 10.1002/1097-0142(19940615)73:12<2930::aid-cncr2820731209>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 33.Staley C.A., Lee J.E., Cleary K.R. Preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for adenocarcinoma of the pancreatic head. Am J Surg. 1996;171:118–125. doi: 10.1016/S0002-9610(99)80085-3. [DOI] [PubMed] [Google Scholar]
- 34.Farrell T., Barbot D., Rosato F. Pancreatic resection combined with intraoperative radiation therapy for pancreatic cancer. Cancer. 1997;226:66–69. doi: 10.1097/00000658-199707000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coquard R., Ayzac L., Gilly F.-N. Intraoperative radiotherapy in resected pancreatic cancer: feasibility and results. Radiother Oncol. 1997;44:271–275. doi: 10.1016/s0167-8140(97)00107-2. [DOI] [PubMed] [Google Scholar]
- 36.Sindelar W.F., Kinsella T.J. Studies of intraoperative radiotherapy in carcinoma of the pancreas. Ann Oncol. 1999;10(suppl4):226–230. [PubMed] [Google Scholar]
- 37.Reni M., Panucci M., Ferreri A. Effect on local control and survival of electron beam intraoperative Irradiation for resectable pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2001;50:651–658. doi: 10.1016/s0360-3016(01)01470-5. [DOI] [PubMed] [Google Scholar]
- 38.Alfieri S., Morganti A.G., Di Giorgio A., Valentini V., Bossola M., Trodella L. Improved survival and local control after intraoperative radiation therapy and postoperative radiotherapy: a multivariate analysis of 46 patients undergoing surgery for pancreatic head cancer. Arch Surg. 2001;136:343–347. doi: 10.1001/archsurg.136.3.343. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto A., Matsumoto G., Tsuruta K. Intraoperative radiation therapy for pancreatic adenocarcinoma: the Komagome hospital experience. Pancreas. 2004;28:296–300. doi: 10.1097/00006676-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Takamori H., Hiraoka T., Kanemitsu K., Tsuji T., Hamada C., Baba H. Identification of prognostic factors associated with early mortality after surgical resection for pancreatic cancer–under-analysis of cumulative survival curve. World J Surg. 2006;30:213–218. doi: 10.1007/s00268-005-7899-5. [DOI] [PubMed] [Google Scholar]
- 41.Nakagohri T., Kinoshita T., Konishi M., Takahashi S., Tanizawa Y. Clinical results of extended lymphadenectomy and intraoperative radiotherapy for pancreatic adenocarcinoma. Hepatogastroenterology. 2007;54:564–569. [PubMed] [Google Scholar]
- 42.Valentini V., Calvo F., Reni M. Intra-operative radiotherapy (IORT) in pancreatic cancer: joint analysis of the ISIORT-Europe experience. Radiother Oncol. 2009;9:54–59. doi: 10.1016/j.radonc.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 43.Showalter T.N., Rao A.S., Rani Anne P., Rosato F.E., Rosato E.L., Andrel J. Does intraoperative radiation therapy improve local tumour control in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma? A propensity score analysis. Ann Surg Oncol. 2009;16:2116–2122. doi: 10.1245/s10434-009-0498-1. [DOI] [PubMed] [Google Scholar]
- 44.Bachireddy P., Tseng D., Horoschak M. Orthovoltage intraoperative radiation therapy for pancreatic adenocarcinoma. Radiat Oncol. 2010;5:105–107. doi: 10.1186/1748-717X-5-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuhrman G.M., Charnsangavej C., Abbruzzese J.L. Thin-section contrast-enhanced computed tomography accurately predicts the resectability of malignant pancreatic neoplasms. Am J Surg. 1994;167:104–113. doi: 10.1016/0002-9610(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 46.Robinson E.K., Lee J.E., Lowy A.M., Fenoglio C.J., Pisters P.W.T., Evans D.B. Reoperative pancreaticoduodenectomy for periampullary carcinoma. Am J Surg. 1996;172:432–438. doi: 10.1016/S0002-9610(96)00218-8. [DOI] [PubMed] [Google Scholar]
- 47.Spitz F.R., Abbruzzese J.L., Lee J.E. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15:928–937. doi: 10.1200/JCO.1997.15.3.928. [DOI] [PubMed] [Google Scholar]
- 48.Callery M., Chang K., Fishman E., Talamonti M., William Traverso L., Linehan D. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 49.Chang J.S., Choi S.H., Lee Y., Kim K.H., Park J.Y., Song S.Y. Clinical usefulness of 18F-fluorodeoxyglucose-positron emission tomography in patients with locally advanced pancreatic cancer planned to undergo concurrent chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2014;90:126–133. doi: 10.1016/j.ijrobp.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 50.Miller R.C., Valentini V., Moss A., D’Agostino G.R., Callister M.D., Hong T.S. Pancreas cancer. In: Gunderson L.L., Willett C.G., Calvo F.A., Harrison L.B., editors. Intraoperative irradiation: techniques and results. Humana Press, Springer; New York: 2011. pp. 249–272. [Google Scholar]
- 51.Ahmadu-Suka F., Gillette E.L., Withrow S.J., Husted P.W., Nelson A.W., Whiteman C.E. Pathologic response of the pancreas and duodenum to experimental intraoperative irradiation. Int J Radiat Oncol Biol Phys. 1988;14:1197–1204. doi: 10.1016/0360-3016(88)90398-7. [DOI] [PubMed] [Google Scholar]
- 52.Evans D.B., Lee J.E., Leach S.D., Fuhrman G.M., Cusack J.C., Jr, Rich T.A. Vascular resection and intraoperative radiation therapy during pancreaticoduodenectomy: rationale and technique. Adv Surg. 1996;29:235–262. [PubMed] [Google Scholar]
- 53.Vujaskovic Z., Willett C.G., Tepper L.E., Kinsella T.J., Gunderson L.L. Normal tissue tolerance to IOERT, EBRT or both: animal and clinical studies. In: Gunderson L.L., Willett C.G., Calvo F.A., Harrison L.B., editors. Intraoperative irradiation: techniques and results. Humana Press, Springer; New York: 2011. pp. 119–140. [Google Scholar]
- 54.Calvo F.A. Intraoperative irradiation: precision medicine for quality cancer control promotion. Radiat Oncol. 2017;12:36–37. doi: 10.1186/s13014-017-0764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calvo F.A., Valentini V. Radiotherapy for pancreatic cancer: systematic nihilism or intraoperative realism. Radiother Oncol. 2008;87:314–317. doi: 10.1016/j.radonc.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 56.Li Y., Feng Q., Jin J., Shi S., Zhang Z., Che X. Experts' consensus on intraoperative radiotherapy for pancreatic cancer. Cancer Lett. 2019;1(449):1–7. doi: 10.1016/j.canlet.2019.01.038. [DOI] [PubMed] [Google Scholar]